Introduction
The results of clinical trials (1,2) have
suggested that chloroquine (CQ) is a possible adjuvant therapy for
glioblastoma. We previously observed that mefloquine (MQ) was more
potent than CQ in killing glioblastoma cells in
vitro(3) and that it may prove
more efficacious than CQ as a chemotherapeutic agent in hormone
receptor-positive and -negative breast cancer cell lines (4). MQ is a prophylactic antimalarial drug
that is also used for malaria chemotherapy. MQ is well-tolerated at
prophylactic dosages, making it an optimal choice for the majority
of travelers. MQ remains a valuable drug for prophylaxis and the
treatment of the majority of patients (5). Previous studies have indicated that
males have a superior tolerance to MQ compared with females
(6). Krudsood et al(7) reported that MQ caused a blood plasma
concentration of 5,796 ng/ml (15.35 μM) in a clinical study
on Plasmodium falciparum-infected adults. Dow et
al(8) noted that higher blood
levels of MQ were reached under therapeutic regimens (2.1–23
μM) rather than in prophylaxis (3.8 mM) (9,10).
PC3 cells are isolated from bones with metastatic
carcinoma of the prostate. However, p53-null PC3 cells (11,12)
are androgen-independent and proliferate normally in
androgen-deprived media. In the present study, the cytotoxic
efficacy of the quinoline analog, MQ, was investigated in the most
commonly used prostate cancer (PCa) cell line, PC3, to determine
whether MQ possesses anticancer effects at physiologically relevant
concentrations in vitro and potential therapeutic efficacy
in vivo.
Materials and methods
Cell culture
The human androgen-independent PC3 cell line was
maintained in Dulbecco’s modified Eagle’s medium (DMEM) and
supplemented with 10% fetal bovine serum. PCa cells were
continuously cultured in a standard cell culture medium with 2 mM
L-glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin,
in a humidified atmosphere of 5% CO2. The study was
approved by the Ethics Committee of Taipei Medical University, Wan
Fang Hospital, Cancer Center, Taipei, Taiwan.
Cell viability assay
The PC3 cells were seeded on 96-well plates, then
assayed by sulphorhodamine-B (SRB) staining. The SRB staining
process was the same as described previously (13). Absorbance was measured at 570 nm
using an ELISA reader.
Cell cycle analysis
The cell cycle was assayed by propidium iodide (PI)
staining, followed by Cytomics FC500 Flow Cytometer CXP analysis.
Cell cycle profiles were then determined using the CXP analysis
software (Beckman Coulter Inc., Miami, FL, USA).
Western blot analysis
The MQ-treated cells (or the untreated for control)
were harvested for western blot analysis. We treated PC3 cells with
10 mM MQ for 24 h. The indicated intervals are showm on Fig 3B. We analyzed cell lysates with
western blotting for GAPDH, p21 and cyclin D1. The data is shown
for the indicated intervals. Antibodies against GAPDH, p21 and
cyclin D1 were purchased from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA).
Flow cytometric assessment of cell death
using annexin V/PI assay
A commercially available annexin V apoptosis
detection kit (Beckman Coulter Inc.) and flow cytometry were used
to identify the annexin V-binding cells. The stained cells were
analyzed using a Cytomics FC500 Flow Cytometer CXP. A count of
∼10,000 events was collected for each sample. The percentage
distributions of dead cells were calculated using CXP analysis
software (Beckman Coulter Inc.).
Intracellular reactive oxygen species
(ROS) assays
To detect ROS formation, a 5 μM
non-fluorescent probe, 2′,7′-dichlorofluorescein-diacetate
(DCFH-DA), was used as a sensitive intracellular probe. The
oxidation of DCFH by ROS was determined by measuring the mean
fluorescence intensity of DCFH with flow cytometry (FC500).
Mouse experiment
Six-week-old male C57BL/6J mice were subcutaneously
implanted with 2×106 PC3 cells on the left flank, above
the hind limb, on day 1. The mice were then randomly divided into
two groups. The mice (control, n=4; MQ-treatment, n=4) weighed
24.0±0.6 g in the control group and 24.0±0.9 g in the treatment
group. On days 32, 36, 39 and 43, the control group was treated
with 200 μl phosphate-buffered saline (PBS; 1% DMSO), while
the treatment group was treated with 200 μg MQ per 25 g of
body weight (1% DMSO in PBS/200 μl/mouse) by intraperitoneal
(IP) injection. Body weights were measured on days 32, 39, 43 and
47, and survival was monitored continuously between days 1 and 51.
The data of the surviving mice were consequently analyzed (n=4 per
group).
Statistical analysis
Error bars represent the standard error of the mean
(SEM) from independent triplicates (n=3). All data are expressed as
the mean ± SEM. Sigma Plot 2001 software was used for the
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of MQ on the proliferation of PC3
cells
The PC3 cells were studied for their sensitivity to
MQ using SRB staining in vitro. MQ exhibited an
IC50 of <20 μM (Fig. 1A). The results showed that the
IC50 value of MQ was around the clinically achievable
concentrations for the PC3 cells. The data revealed that 10
μM MQ achieved the IC50 at 24 h in one treatment,
with no further cytotoxicity at 48 and 72 h exposure. It appeared
that the drug was depleted rapidly by the intercellular metabolism.
Additionally, 40 μM MQ possessed a high cytotoxicity that
caused ∼30% cell death following 60 min of treatment (Fig. 1B).
Effects of MQ on non-apoptotic cell death
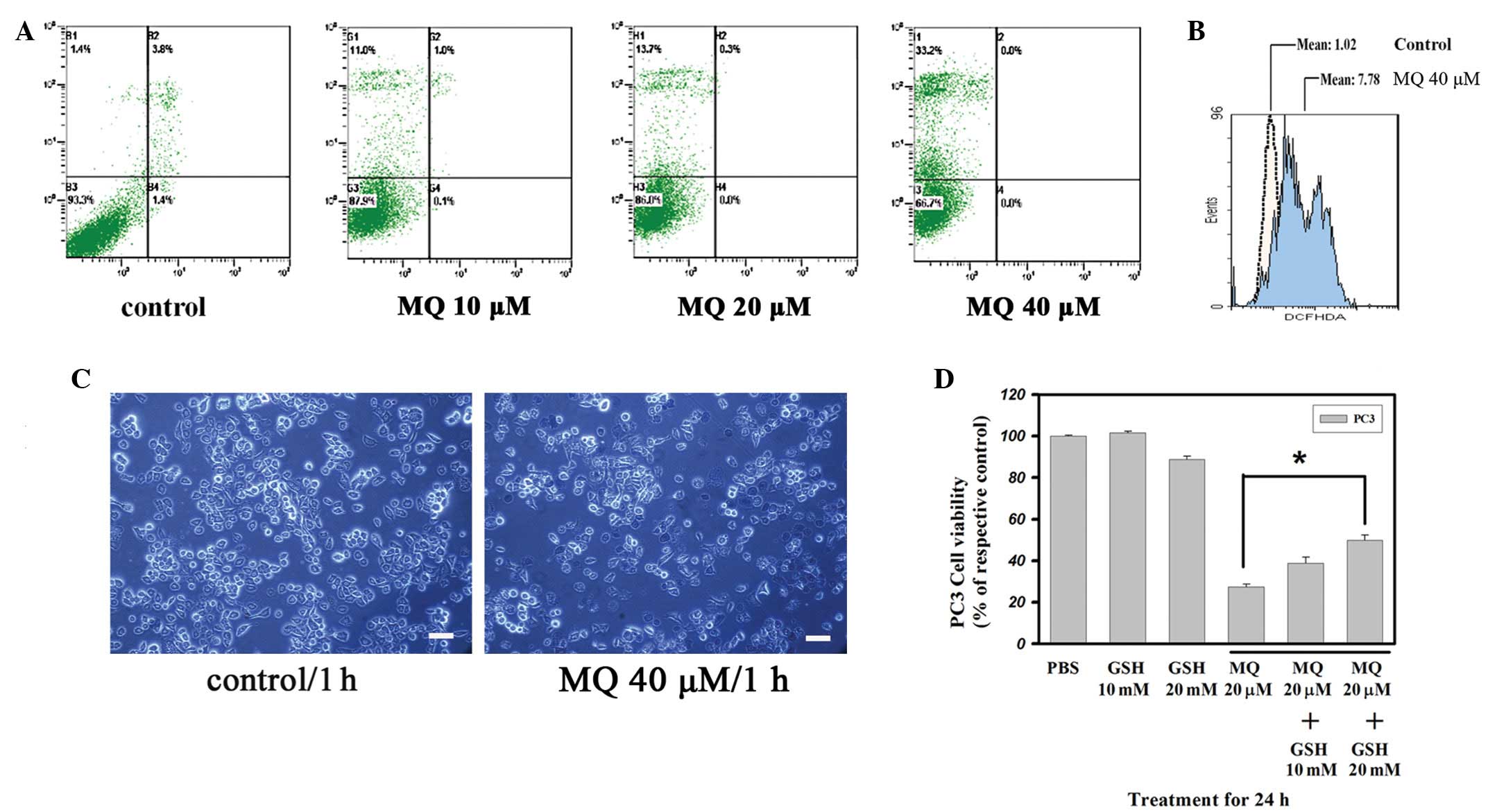
in PCa cells
The levels of annexin V/PI staining were analyzed
following the treatment with MQ for 1 h in the PC3 cells (Fig. 2A). The number of annexin V-stained
cells in the 10–40 μM MQ-treated PC3 cells did not increase
after 1 h. However, the number of PI stain-positive cells treated
with 10 μM MQ increased from 1.4 to 11.0% in the PC3 cells
(20 μM MQ, 13.7%). Furthermore, MQ rapidly reduced the
population of viable PC3 cells following MQ treatment at 40
μM. The proportion of dead cells among the PC3 cells treated
with 40 μM MQ increased to 33.2%. Higher doses of MQ did not
increase the number of annexin V-stained cells compared with PI
stain-positive cells. Furthermore, numerous trypan blue
stain-positive cells appeared in the group treated with 40
μM MQ (Fig. 2C). The data
indicated that MQ rapidly caused an increase in the rate of
non-apoptotic cell death.
Effect of MQ on ROS in PC3 cells
Since MQ has a rapid effect on cytotoxicity, the
alterations to the levels of ROS were analyzed (Fig. 2B). The data showed that treatment
with 40 μM MQ for 1 h caused PC3 intracellular ROS (DCFH)
levels to increase significantly to levels 7.6-fold higher than
those of the untreated cells. To demonstrate the role of ROS in
MQ-induced anticancer effects, cell viability was detected in the
PC3 cells pretreated with glutathione (GSH). The MQ-induced
anticancer effects were significantly antagonized in the GSH
pretreated cells (Fig. 2D),
indicating that MQ-mediated cytotoxicity may be quenched by the
antioxidant GSH. These data may also suggest that increased ROS
generation is essential in MQ-mediated cell death.
Effect of MQ on cell cycle regulation in
PC3 cells
Using PI staining by flow cytometry, the effect of
the MQ treatment on cell cycle regulation was examined (Fig. 3A). The 10 μM MQ treatment for
24 h caused no significant alteration to the proportion of
sub-G1 PC3 cells, however the MQ treatment significantly
induced G1 cell-cycle arrest and reduced the proportion
of cells in the S and G2/M phases. Higher doses of MQ
(20 μM) did not increase the number of sub-G1
cells in the PC3 cells, but enhanced the G1 accumulation
from 49.1% (control) to 68.2%. In the western blot analysis
(Fig. 3B), 10 μM MQ caused
an accumulation of the G1 phase marker, the cyclin D1
protein, thus indicating the involvement of p21 upregulation.
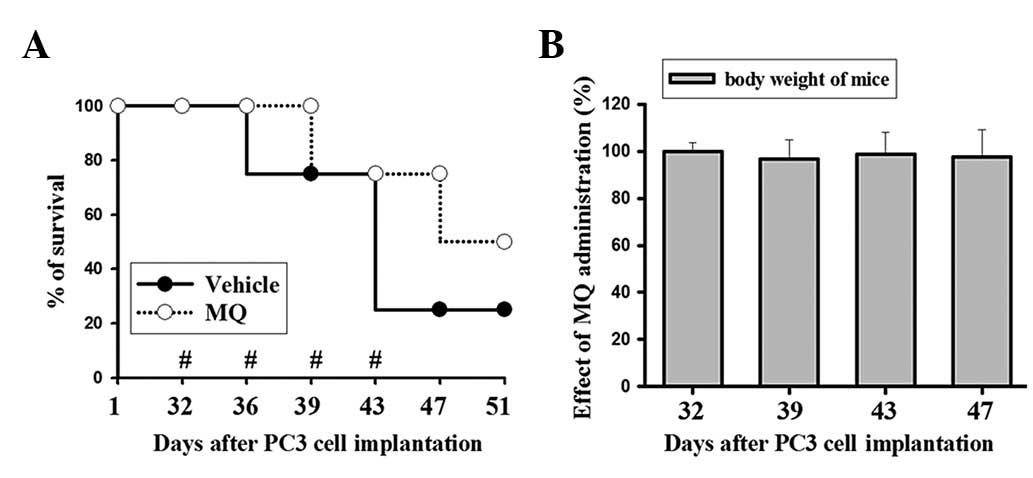
Potential therapeutic efficacy of MQ in
an animal model
The potential therapeutic efficacy of MQ is shown in
Fig. 4A. The survival of mice in
the MQ group was prolonged compared with those of the
vehicle-control group. The MQ-treated group had an increased
lifespan and 75% of the mice were still alive at 47 days subsequent
to the cancer cell implantation. Moreover, 50% of the mice were
still alive at 51 days in the MQ-treated group. By contrast, only
25% of the mice in the control group survived to 47 days post-tumor
implantation. MQ treatment improved the survival rate of the mice
with tumors. The body weights were measured to evaluate the MQ
toxicity caused by the IP injection with 200 μg MQ per 25 g
of mouse weight. The results showed no significant alterations in
the body weights during the MQ administration period between days
32 and 47 (Fig. 4B). Additionally,
as the mice died rapidly after losing control of tumor growth in
the control group, the mean tumor size among the survivors was not
significantly different between the two groups.
Discussion
The mechanism of action of MQ was investigated in
androgen-independent PCa cells as a model of aggressive PCa in the
present study. The PC3 cells were sensitive to the anticancer
effects of MQ at ∼10 μM. The results showed that the
IC50 value of MQ was at clinically achievable
concentrations for the PC3 cells. However, treatment with MQ for 24
h induced G1 phase cell-cycle arrest in p53-null PC3
cells, although MQ did not induce cell apoptosis. The accumulation
of the G1 phase marker, the cyclin D1 protein, was
consistent with an increased proportion of cells in the
G1 phase of the cell cycle. The derived results revealed
that MQ-mediated G1 cell-cycle arrest was
p53-independent. By contrast, an increase in p21 was induced in the
PC3 cells. The biological functions of p21 regulation have been
shown to be emphasized in p53-independent tumor suppressor
activities against cancer (14),
suggesting that p21 is crucial for MQ-mediated G1
cell-cycle arrest in p53-null PC3 cells and that MQ has marked
anticancer capability at physiologically achievable concentrations
in vitro. The present study also investigated the
cytotoxicity of MQ in PC3 cells. Cell death rapidly occurred within
1 h of a 10–40 μM MQ exposure, as measured by an annexin
V/PI double-staining assay. Consistent with the results of the
PI-staining, a significant quantity of trypan blue stain-positive
cells was observed in the group treated with 40 μM MQ.
Moreover, the ratio of non-apoptotic cell death in the annexin V/PI
double-staining assay was consistent with the SRB cell viability
data for the 40 μM MQ treatment. The results also revealed
that no significant early apoptosis was caused by MQ, even at a
concentration of 40 μM.
Evidence suggests that ROS may be used as a
therapeutic modality to kill cancer cells. Previous studies have
indicated that MQ treatment induces a decreased concentration of
GSH that is dependent on the increase in oxidative stress in
primary rat cortical neurons (15).
ROS generation is an early molecular event that precedes cell death
(16). In certain types of
necrosis, an increase in the overproduction of ROS mediates cell
death (17). A previous study has
shown that ROS cause oxidative stress-induced necrotic cell death
(18). In the present study,
necrotic cell death was accompanied by significant ROS generation,
as shown in Fig. 2. This suggested
that the MQ treatment mediated the intracellular ROS, allowing the
non-apoptotic cell death of the PC3 cells. Pre-treatment of the PC3
cells with GSH abrogated ROS induction by MQ and significantly
protected the PC3 cells against MQ-induced anti-cancer effects.
These results support the hypothesis that MQ induces ROS
generation, which subsequently affects cancer cell
proliferation.
Moreover, the present study investigated the
potential therapeutic efficacy of MQ in mice (Fig. 4). The results revealed that MQ may
contribute to anticancer efficacy by prolonging the survival time
of those treated. The MQ-treated group survived longer than the
mice of the control group. This suggests a novel strategy that
offers a potential new therapy for the treatment of PCa. The
results demonstrated that MQ was effective at prolonging the
survival time of mice. Although the details remain unclear, the
observations of the present study are an initial attempt toward
identifying novel treatments for PCa. MQ may be a promising regimen
of cancer therapy or tertiary chemoprevention in the future.
Effective and less toxic treatments are the goal of
cancer therapy. Since current treatments for advanced PCa are
limited, the anticancer effects of MQ provide an attractive target
for study. The present results indicate that MQ may be a potential
candidate for clinical trials of its cancer therapy applications in
the future. Future research may determine whether MQ is able to
further synergize with traditional chemotherapy drugs or
radiotherapy in the treatment of PCa.
Abbreviations:
|
MQ
|
mefloquine
|
|
PCa
|
prostate cancer
|
|
ROS
|
reactive oxygen species
|
|
GSH
|
glutathione
|
Acknowledgements
The present study was supported by the
Research Fund of the Center of Excellence for Cancer Research,
Taipei Medical University, Taipei, Taiwan (Project No.
DOH101-TD-C-111-008 and Grant Nos. (NSC 100-2325-B-038-006, NSC
100-2314-B-038-039) awarded by the National Science Council. This
research was also supported by the Wan Fang Hospital
(101-wf-eva-21).
References
|
1
|
Sotelo J, Briceño E and López-González MA:
Adding chloroquine to conventional treatment for glioblastoma
multiforme: a randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 144:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Briceño E, Reyes S and Sotelo J: Therapy
of glioblastoma multiforme improved by the antimutagenic
chloroquine. Neurosurg Focus. 14:e32003.PubMed/NCBI
|
|
3
|
Geng Y, Kohli L, Klocke BJ and Roth KA:
Chloroquine-induced autophagic vacuole accumulation and cell death
in glioma cells is p53 independent. Neuro Oncol. 12:473–481.
2010.PubMed/NCBI
|
|
4
|
Sharma N, Thomas S, Golden EB, Hofman FM,
Chen TC, Petasis NA, Schönthal AH and Louie SG: Inhibition of
autophagy and induction of breast cancer cell death by mefloquine,
an anti-malarial agent. Cancer Lett. 326:143–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor WR and White NJ: Antimalarial drug
toxicity: a review. Drug Saf. 27:25–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
ter Kuile FO, Nosten F, Thieren M,
Luxemburger C, Edstein MD, Chongsuphajaisiddhi T, Phaipun L,
Webster HK and White NJ: High-dose mefloquine in the treatment of
multidrug-resistant falciparum malaria. J Infect Dis.
166:1393–1400. 1992.
|
|
7
|
Krudsood S, Looareesuwan S, Wilairatama P,
Leowattana W, Tangpukdee N, Chalermrut K, Ramanathan S, Navaratnam
V, Olliaro P, Vaillant M, Kiechel JR and Taylor WR: Effect of
artesunate and mefloquine in combination on the Fridericia
corrected QT intervals in Plasmodium falciparum infected
adults from Thailand. Trop Med Int Health. 16:458–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dow GS, Caridha D, Goldberg M, Wolf L,
Koenig ML, Yourick DL and Wang Z: Transcriptional profiling of
mefloquine-induced disruption of calcium homeostasis in neurons in
vitro. Genomics. 86:539–550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simpson JA, Price R, ter Kuile F,
Teja-Isavatharm P, Nosten F, Chongsuphajaisiddhi T, Looareesuwan S,
Aarons L and White NJ: Population pharmacokinetics of mefloquine in
patients with acute falciparum malaria. Clin Pharmacol Ther.
66:472–484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kollaritsch H, Karbwang J, Wiedermann G,
Mikolasek A, Na-Bangchang K and Wernsdorfer WH: Mefloquine
concentration profiles during prophylactic dose regimens. Wien Klin
Wochenschr. 112:441–447. 2000.PubMed/NCBI
|
|
11
|
Bajgelman MC and Strauss BE: The DU145
human prostate carcinoma cell line harbors a temperature-sensitive
allele of p53. Prostate. 66:1455–1462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isaacs WB, Carter BS and Ewing CM:
Wild-type p53 suppresses growth of human prostate cancer cells
containing mutant p53 alleles. Cancer Res. 51:4716–4720.
1991.PubMed/NCBI
|
|
13
|
Keepers YP, Pizao PE, Peters GJ, van
Ark-Otte J, Winograd B and Pinedo HM: Comparison of the
sulforhodamine B protein and tetrazolium (MTT) assays for in vitro
chemosensitivity testing. Eur J Cancer. 27:897–900. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hood JE, Jenkins JW, Milatovic D, Rongzhu
L and Aschner M: Mefloquine induces oxidative stress and
neurodegeneration in primary rat cortical neurons. Neurotoxicology.
31:518–523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Stefano A, Frosali S, Leonini A,
Ettorre A, Priora R, Di Simplicio FC and Di Simplicio P: GSH
depletion, protein S-glutathionylation and mitochondrial
transmembrane potential hyperpolarization are early events in
initiation of cell death induced by a mixture of isothiazolinones
in HL60 cells. Biochim Biophys Acta. 1763:214–225. 2006.PubMed/NCBI
|
|
17
|
Skulachev VP: Bioenergetic aspects of
apoptosis, necrosis and mitoptosis. Apoptosis. 11:473–485. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi K, Kim J, Kim GW and Choi C:
Oxidative stress-induced necrotic cell death via
mitochondria-dependent burst of reactive oxygen species. Curr
Neurovasc Res. 6:213–222. 2009. View Article : Google Scholar : PubMed/NCBI
|