Introduction
Mucinous tubular and spindle cell carcinoma of the
kidney (MTSCC-K) is a rare renal epithelial tumor, believed to be a
type of low-grade malignant tumor. The precise origin is unclear
certain researchers have hypothesised that it originates from the
loop of Henle or the distal tubule, however the majority of
researchers hypothesise that its origin is in the distal tubule
(1). MTSCC-K is difficult to
pathologically differentiate from high-grade malignant renal
tumors, including collecting duct carcinoma and sarcomatoid
carcinoma. It was first reported in 1997 by MacLennan et al
and was known as a ‘low-grade collecting duct carcinoma’ (2). As the number of MTSCC-K cases have
increased continuously, this special type of kidney tumor has been
described as ‘a unique group of renal neoplasms composed of
cytologically low-grade cells organized in tubules and spindled
cords and set in an abundant extracellular mucinous matrix’
(3,4). In 2004, the World Health Organization
recognized the tumor as a specific entity and officially named it
‘MTSCC-K’ (5). MTSCC-K has a
relatively good patient prognosis when compared with other
malignant renal tumors (6). The
present study analyzed the clinical results of a patient who
presented to the First Hospital of Jilin University (Changchun,
China) suffering from MTSCC-K, and performed a review of the
relevant literature, to increase understanding of the tumor.
Additionally the purpose of this study was to raise awareness of
this tumor type for clinicians and pathologists in order to
decrease the rate of misdiagnosis.
Case report
Clinical results
A 60-year-old female presented to the First Hospital
of Jilin University suffering from lumbodorsalgia on the right side
for approximately one month, without gross hematuria and fever.
Laboratory tests revealed no significant abnormalities in renal
function, routine blood tests or urine routine. Imaging examination
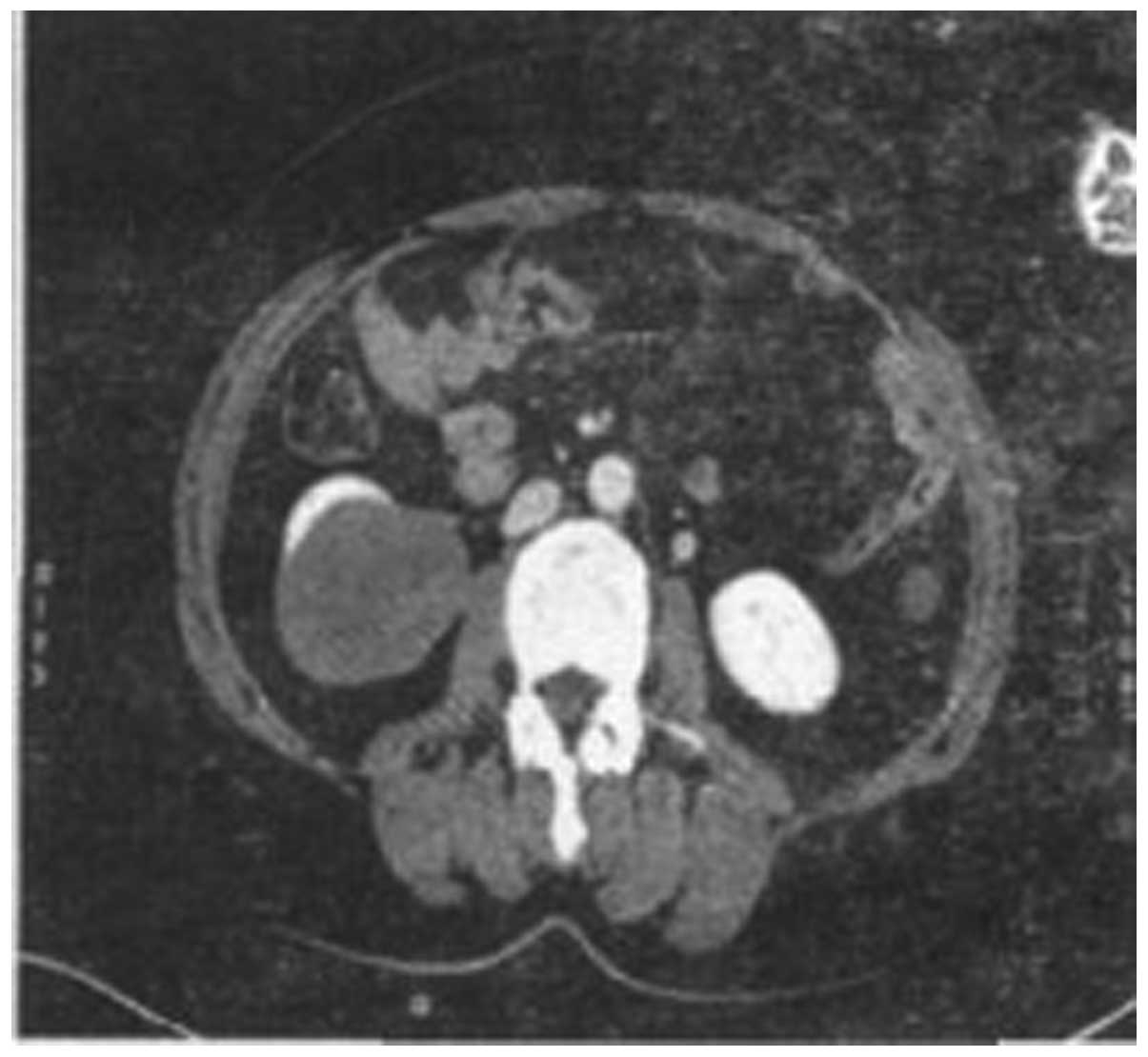
by abdominal computed tomography (CT) scan revealed a ~6.5×5.0-cm
solid mass in the inferior pole of the right kidney. The tumor was
well-circumscribed and protruding outside the renal contour, and no
clear enhancement was identified in the arterial phase (Fig. 1). However, marginal uneven
enhancement was observed in the venous phase (Fig. 2). No metastasis was identified to
the retroperitoneal lymph node, abdominal organs or lungs. The
patient provided written informed consent.
Surgical procedures
The patient was placed under general anesthesia and
underwent laparoscopic radical resection of the right kidney
(surgical excision of the right kidney, perirenal fat, a section of
the ureter over and the right adrenal gland).
Macroscopy
Dissection of the specimen revealed that the tumor
was well-circumscribed, solid and off-white, measuring ~7.0×6.5×6.5
cm. No areas of hemorrhage or necrosis were identified in the
tumor. In addition, no invasion of the renal pelvis, renal sinus or
perirenal fat was identified.
Microscopy
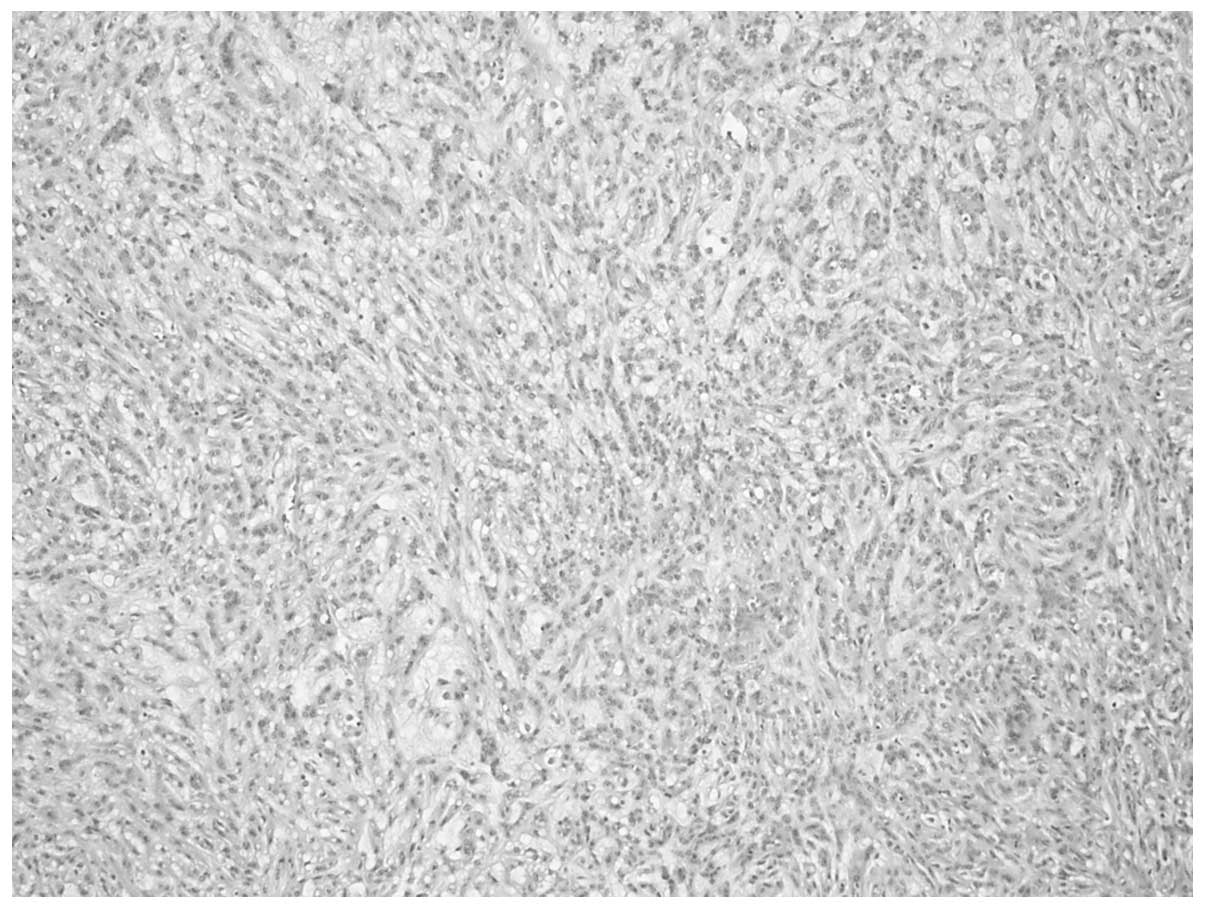
The tumor was composed of small, elongated cords or
tubules, in a tightly packed arrangement (Fig. 3). Myxoid stroma was shown to be
interspersed among the tubular cells (Fig. 4), and appeared to exhibit slender
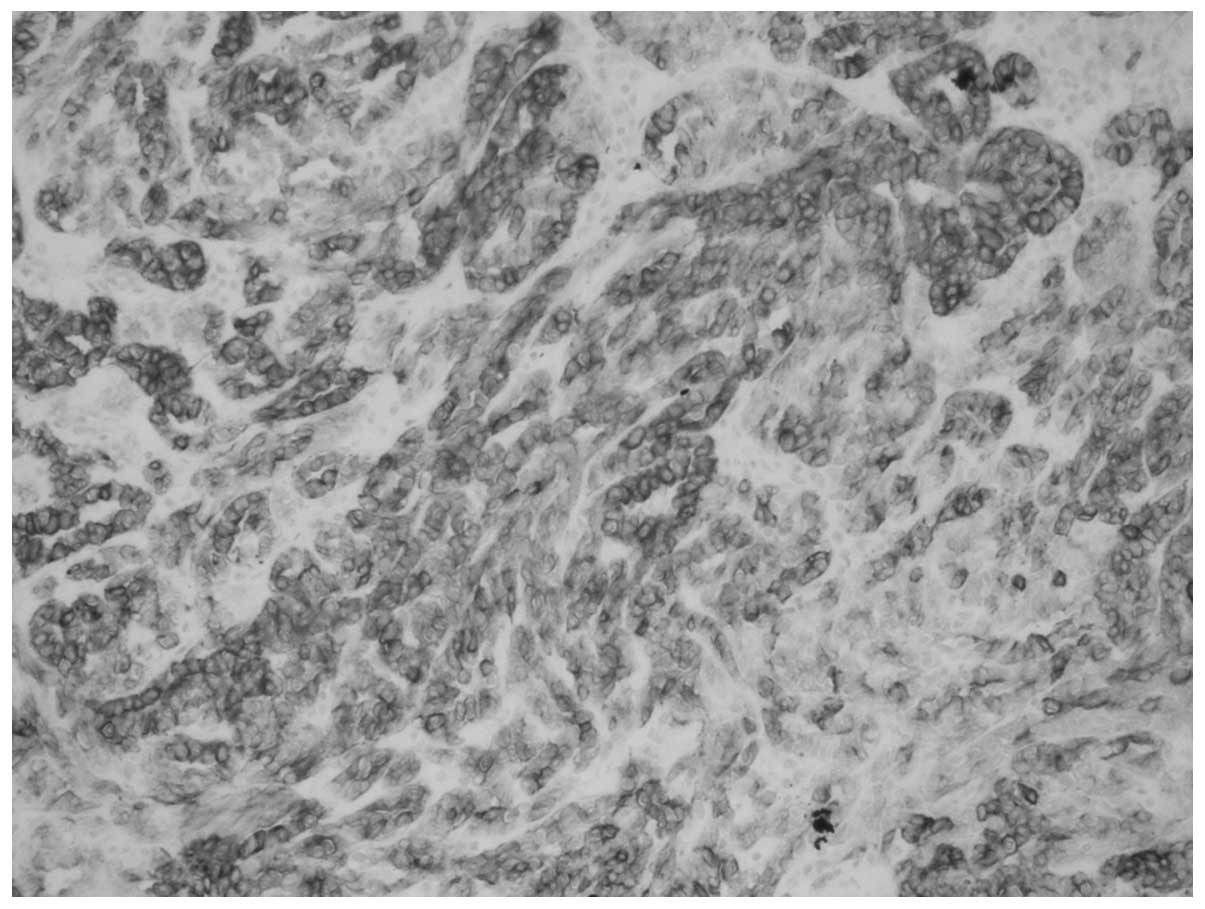
tubular spindle cell-like structures (Fig. 5). Tumor cells were smaller and
cubic-shaped or oval, with single small eosinophilic nucleoli and
low-grade nuclei (Fig. 6).
Occasionally, necrosis and foam cell infiltration were identified.
The myxoid stroma was stained by acidic mucus (Fig. 7).
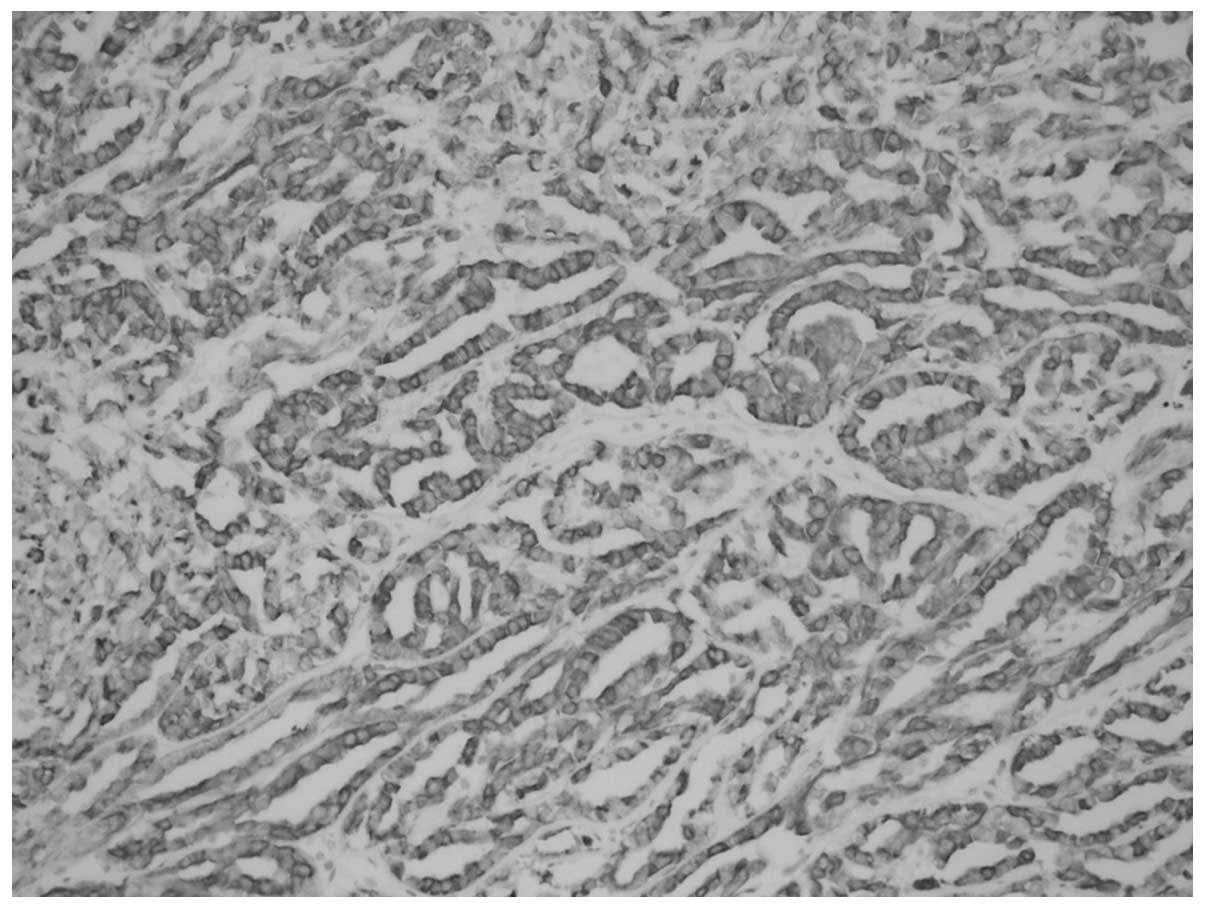
Immunohistochemistry
CK7 (Fig. 8), CK19
(Fig. 9), EMA (Fig. 10), Vimentin and P504S (AMACR)
showed positive expression in tumoral cells, but the tumoral cells
were CD10-negative.
Pathological results
The tumor was well-circumscribed, measuring
~7.0×6.5×6.5 cm. Invasion of the renal pelvis, renal sinus or
perirenal fat was not observed. Under the microscope (BX53,
Olympus, Tokyo, Japan), the tumor observed to be composed of small,
elongated cords or tubules. Myxoid stroma was demonstrated to be
interspersed among the tubular cells and appeared to exhibit
slender tubular spindle cell-like structures, and was stained by
acidic mucus. In terms of immunohistochemical staining, CK7, CK19
and AMACR had a positive expression in tumoral cells, while CD10
was negative in the tumorous cells. From these observations the
tumor was determined to be low grade T1bN0 MTSCC-K.
Discussion
To date, ~100 MTSCC-K cases have been reported
worldwide (6). MTSCC-K has a wide
age distribution, with an age range between 17 and 82 years and a
mean age of 53 years. The female incidence is approximately three-
or four-fold that of males (3,6–8).
Typically, the majority of patients present with asymptomatic
masses, often found incidentally by ultrasound (9,10). In
a few cases, the patient may present with flank pain or hematuria
(9).
One of the main features of MTSCC-K is the good
prognosis. However, the clinical symptoms and imaging
characteristics of the MTSCC-K are similar to the more common renal
cell carcinoma (RCC). Therefore, a clear preoperative diagnosis
becomes more difficult. Previously, it has been reported (11) that on renal magnetic resonance
imaging scans for the tumor, the T2 signal intensity ratio is 0.96
(intermediate between papillary, 0.67 and clear cell carcinoma,
1.41). Additionally, T1 images show a signal similar to that of a
normal renal cortex and postcontrast T1 VIBE images demonstrate
poor early phase enhancement. The current case underwent abdominal
CT scan prior to surgery and no clear enhancement was identified in
the arterial phase. However, marginal uneven enhancement was
identified in the venous phase. This change is consistent with the
previous literature and may provide references for the preoperative
clinical diagnosis of MTSCC-K. In addition, it has been reported
that fine needle aspiration biopsy may be diagnostic of MTSCC-K
(12), which may aid to improve
preoperative diagnosis rates.
Previously, Fine et al (3) described the histological types of
MTSCC-K and divided them into the following two categories:
Classic, abundance of extracellular mucin stroma; and mucin-poor,
little to no extracellular mucin. In addition, the unusual
organizational characteristics associated with MTSCC-K are as
follows: Papillary clear cell structure, necrosis, ectopic bone or
grit-like calcospherites and neuroendocrine differentiation has
also been reported (13).
In cytogenetic studies, genetic abnormalities found
in MTSCC-K cells are monosomies in chromosomes 1, 4, 6, 8 and 13,
and total or partial trisomies of chromosomes 7, 11, 16 and 17
(3). Simultaneously, the
immunophenotype of MTSCC-K tumor cells demonstrates a complex mode,
including epithelial markers (CK19, CK7 and AE1/AE3) and distal
convoluted tubule markers (EMACK19 and E-cadherin).
Since the incidence ratio of papillary RCC is second
to clear RCC and is also a relatively common pathological type of
RCC, the tubular architecture showing focal papillae are clear
clues pointing to MTSCC-K. Therefore, it is important to
differentially diagnose papillary RCC and MTSCC-K. Associated
studies (13) have previously
reported on a few of the immunohistochemical markers between
MTSCC-K cells and papillary RCC cells. By comparison, the
immunoreactivity in MTSCC-K was as follows: AMACR, 93%; CK7, 81%;
EMA, 95%; RCC Ma, 7%; CD10, 15%; HMWK, 15%; and c-kit, 5%. While in
papillary RCC, the immunoreactivity was as follows: AMACR, 95%;
CK7, 65%; EMA, 88%; RCC Ma, 25%; CD10, 80%; HWMK, 15%; and c-kit,
18%. AMACR was once the specific immunohistochemical marker of
papillary RCC, but high expression of AMACR has also been confirmed
in MTSCC-K cells. By immunohistochemistry, in addition to the rate
of CD10 expression differences existing in the two tumor cells,
other markers have shown similarities. Therefore, identification of
the two tumors relies mainly on pathological and karyotype
analysis. Papillary RCC does not have an extracellular matrix;
however, chromosomal gains, particularly in chromosomes 7 and 17,
and loss of chromosome Y characterize papillary RCC (4).
Overall, MTSCC-K exhibits a lower malignant degree
and an improved prognosis compared with other types of RCC
(14). Generally, radical
nephrectomy is the best treatment and no additional treatment is
required following surgery. The tumor is typically of low
pathological stage at the time of excision and it is extremely rare
for cases to present with metastases to lymph nodes and other
organs at the time of diagnosis (15). To date, an extremely small number of
reported cases have presented with distant metastasis and no
tumor-related mortality has been reported (6). However, MTSCC-K, as a type of renal
cancer, requires close follow-up after surgery.
References
|
1
|
Jung SJ, Yoon HK, Chung JI, et al:
Mucinous tubular and spindle cell carcinoma of the kidney with
neuroendocrine differentiation: report of two cases. Am J Clin
Pathol. 125:99–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
MacLennan GT, Farrow GM and Bostwick DG:
Low-grade collecting duct carcinoma of the kidney: report of 13
cases of low-grade mucinous tubulocystic renal carcinoma of
possible collecting duct origin. Urology. 50:679–684. 1997.
View Article : Google Scholar
|
|
3
|
Fine SW, Argani P, DeMarzo AM, et al:
Expanding the histologic spectrum of mucinous tubular and spindle
cell carcinoma of kidney. Am J Surg Pathol. 30:1554–1560. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Srigley JR, Eble JN, Grignon DJ and
Hartwick RWJ: Unusual renal cell carcinoma (RCC) with prominent
spindle cell change possibly related to the loop of Henle.
Laboratory Investigation. 79(1)Lippincott Williams & Wilkins;
Philadelphia, PA: pp. 107A1999
|
|
5
|
Lopez-Belron AL, Scrpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar
|
|
6
|
MacLennan GT and Cheng L: Neoplasms of the
kidney. Urologic Surgical Pathology. Bostwick DG and Cheng L: 2nd
edition. Mosby-Elsevier; Philadelphia, PA: pp. 104–106. 2008
|
|
7
|
Grigore A, Toma L, Stoicea M, et al: Rare
renal tumor - mucinous tubular and spindle cell carcinoma. Rom J
Morphol. 53:167–171. 2012.PubMed/NCBI
|
|
8
|
Srigley JR: Tumors of the kidney: Mucinous
tubular and indle cell carcinoma. Pathology and Genetics of Tumours
of the Urinary System and Male Genital Organs. Eble JN, Sauter G,
Epstein JI and Sesterhenn IA: IARC Press; Lyon: pp. 402004
|
|
9
|
Eble JN: Mucinous tubular and spindle cell
carcinoma and post-neuroblastoma carcinoma: newly recognised
entities in the renal cell carcinoma family. Pathology. 35:499–504.
2003. View Article : Google Scholar
|
|
10
|
Eble JN and Young RH: Tumors of the
urinary tract. Diagnostic Histopathology of Tumors. Fletcher CDM:
3rd edition. Churchill Livingstone; Edinburgh: pp. 5372007
|
|
11
|
Oliva MR, Glickman JN, Zou KH, et al:
Renal cell carcinoma: T1 and T2 signal intensity characteristics of
papillary and clear cell types correlated with pathology. AJR Am J
Roentgenol. 192:1524–1530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marks-Jones DA, Zynger DL, Parwani AV and
Cai G: Fine needle aspiration biopsy of renal mucinous tubular and
spindle cell carcinoma: report of two cases. Diagn Cytopatho.
38:51–55. 2010.
|
|
13
|
Paner GP, Srigley JR, Radhakfishnan A, et
al: Immunohistochemical analysis of mucinous tubular and spindle
cell carcinoma and papillary renal cell carcinoma of the kidney:
significant immunophenotypic overlap warrants diagnostic caution.
Am J Surg Pathol. 30:13–19. 2006. View Article : Google Scholar
|
|
14
|
Kumari N, Chhabra P, Dewan U and Jain M:
Renal mucinous tubular and spindle cell carcinoma. Indian J Pathol
Microbiol. 52:400–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ursani NA, Robertson AR, Schieman SM, et
al: Mucinous tubular and spindle cell carcinoma of kidney without
sarcomatoid change showing metastases to liver and retroperitoneal
lymph node. Hum Pathol. 42:444–448. 2011. View Article : Google Scholar
|