Introduction
Radiation-induced lung injury (RILI) is a common
dose-limiting complication of thoracic radiotherapy that usually
consists of radiation pneumonitis and radiation fibrosis (1–4). The
occurrence of RILI is a continuous and dynamic process of
damage-related signal transduction, amplification and feedback
(1,2,5).
However, the underlying molecular mechanisms and signaling pathways
remain insufficiently understood, compromising effective
intervention in RILI (3,5). Therefore, other potential mechanisms
should be explored to integrate a more comprehensive and detailed
strategy for the clinical prevention and treatment of RILI
(4).
Long non-coding RNAs (lncRNAs) are a novel class of
messenger RNA (mRNA)-like transcripts (6,7). In
contrast to small non-coding RNAs, including microRNA and
transcription initiation RNAs, lncRNAs are usually >200
nucleotides in length and lack an open reading frame (6–8). In
the last decade, increasing evidence has established that, apart
from microRNAs, lncRNAs may also be primary genetic regulators of
several important biological processes, including metabolism,
development and carcinogenesis (7,9).
Although lncRNAs have previously been reported to conduct a broad
spectrum of molecular and cellular roles by implementing different
modes of action, including chromatin modification and epigenetic
regulation, subcellular and structural organization of transcripts,
and regulation of the expression of neighboring genes either in
cis or trans form, a phenomenon known as
transvection, their physiological functions remain poorly
understood (6,7). Several studies have previously
reported a group of lncRNAs that may be involved in the modulation
of several cellular stress responses (10,11).
For example, the increased expression of the psoriasis
susceptibility-related RNA gene induced by stress (PRINS), a lncRNA
gene, is induced by stress signals, including ultraviolet-B
irradiation (IR), viral infection and translational inhibition
(12). Certain large intergenic
ncRNAs (lincRNAs) were also found to be regulated by the p53
pathway, which is involved in the DDR (13,14).
However, in terms of the ionizing IR-induced DDR, the deregulation
and biological functions of IR-responsive lncRNAs remain largely
unknown.
IR can also induce stress signals, leading to
systemic organ damage. Therefore, we hypothesized that lncRNAs may
be involved in the process of RILI and may play a key modulatory
role. In the present study, female C57BL/6 mice were used as an
RILI model (15,16) and several differentially-expressed
long intergenic radiation-responsive ncRNAs (LIRRs) were identified
through microarray screening and quantitative polymerase chain
reaction (qPCR) verification. Following bioinformatics analysis,
LIRR1 was chosen for further functional study. A recombinant
eukaryotic expression vector for LIRR1 was constructed and
transfected into the normal human epithelial BEAS-2B cell line.
Following RNA transcription-level confirmation by reverse
transcription (RT)-PCR, the radiosensitivity of the BEAS-2B cells
constantly overexpressing LIRR1 was assessed through clonogenic and
flow cytometry assays. The IR-induced DNA double-strand breaks
(DSBs) were visualized by immunofluorescence staining. The
associated mechanism was preliminarily revealed by western blot
analysis. The present results may provide novel mechanisms that
result in the occurrence and development of RILI in
vitro.
Materials and methods
Animals
C57BL/6 mice (8–9 weeks old; Slac Laboratory Animal
Co., Ltd., Shanghai, China) were used in the present study. All the
mice were housed and maintained in a mouse colony, with a maximum
of six mice per cage. All the animal procedures were conducted in
accordance with the regulations of the Animal Experimentation
Ethics Committee of Soochow University (Suzhou, Jiangsu,
China).
Radiation exposure
The mice were anesthetized with 50 mg/kg
pentobarbital sodium (Sigma-Aldrich, St. Louis, MO, USA) through
intraperitoneal injection prior to IR. An immobilization chamber
allowing simultaneous bilateral exposure was used for total body IR
(TBI). Using a 6-MeV electron beam accelerator (Siemens KD-2;
Siemens, Munich, Bavaria, Germany) at a dose rate of 200 cGy/min
and with a source to skin distance of 100 cm, 12 Gy was delivered
to the mid-plane. Thermoluminescence dosimeters were placed over
the selected mice to verify the administration of the correct dose
for quality assurance. For cultured cell exposure, an IR area of
20×20 cm and a source to skin distance of 100 cm were used, and
doses of 0, 0.5, 1, 2, 4 and 6 Gy were delivered separately to the
mid-plane.
Microarray and computational
analysis
The lungs of the mice in the sham-irradiated and 12
Gy TBI groups were homogenized in TRIzol (Invitrogen, Carlsbad, CA,
USA). Total RNA was isolated using a Qiagen RNase Mini kit (Qiagen,
Valencia, CA, USA). The RNA concentration and integrity were
measured using a NanoDrop ND-100 spectrophotometer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and denaturing agarose gel
electrophoresis, respectively.
The mouse lncRNA microarray analysis was performed
by KangChen Bio-tech (Shanghai, China). Briefly, mouse lung tissue
mRNA was purified using a mRNA-ONLY™ Eukaryotic mRNA Isolation kit
(Epicentre Biotechnologies, Madison, WI, USA) according to the
manufacturer’s instructions. An Agilent array platform (Agilent
Technologies, Inc., Santa Clara, CA, USA) was used for the
subsequent analysis. One-color fluorescence-labeled complementary
RNA, transcribed without 3′ bias, was prepared using a Quick Amp
labeling kit and hybridized with the Mouse lncRNA Microarray v2.0
(8×60 K; ArrayStar, Rockville, MA, USA), which contained 31,423
lncRNAs and 25,376 coding transcripts that were carefully collected
from the most authoritative databases, such as RefSeq (National
Center for Biotechnology Information, Bethesda, USA), UCSC
Knowngenes (University of California, Santa Cruz, CA, USA), and
Ensembl (Hinxton, Cambridgeshire, UK). Each treatment was performed
in triplicate, with three array sets being prepared for the sham
and irradiated lung tissues.
An Agilent Technologies G2505B Scanner was used for
array scanning, and Agilent Feature Extraction software v11.0.1.1
was used to acquire the array images required for the data analysis
(both Agilent Technologies, Inc.). Quantile normalization and
subsequent data processing were conducted using the GeneSpring GX
v11.5.1 software package (Agilent Technologies, Inc.).
qPCR
Total RNA was extracted with TRIzol and
reverse-transcribed into complementary DNA (cDNA) using the
oligo(dT)18 primer and HiscriptTM First-strand cDNA
Synthesis kit (Vazyme Biotech (Nanjing) Co., Ltd, Nanjing, Jiangsu,
China). The transcription levels of LIRR1 and β-actin, the control
gene, were determined using the SYBR green method (Applied
Biosystems 7500FAST RT-PCR system; Applied Biosystems, Foster City,
CA, USA). The primers were designed using Primer3 software
(available from bioinfo.ut.ee/primer3; Table I). Each PCR used 0.2 μl platinum Taq
polymerase, 0.5 μl of each PCR primer (10 mM), 1 μl SYBR (20x), 1
μl cDNA, 2 μl 10X PCR buffer, 2 μl Mg2+ (25 mM), 0.5 μl
deoxynucleotide triphosphates (25 mM) and 20 μl double-distilled
water. For the RT-PCR, the denaturation step was established at
95°C for 2 min and then continued at 95°C for 10 sec, 60°C for 30
sec and 70°C for 30 sec for 40 cycles, with a final extension step
at 70°C for 8 min. All the RNA samples were analyzed in triplicate
for each tested transcript and the levels were normalized to
β-actin. Expression fold changes were calculated using the
2−ΔΔCt method (10).
 | Table IPrimers for quantitative polymerase
chain reaction. |
Table I
Primers for quantitative polymerase
chain reaction.
| Gene name | Forward | Reverse | Product length,
bp |
|---|
| β-actin |
CACTATTGGCAACGAGCGGT |
CAACGTCACACTTCATGATGGA | 119 |
| LIRR 1 |
CATGGTGGCTCACAATTATCTGTAA |
CATATATGTCAGTACACCATCACTGTCTTC | 85 |
| LIRR 2 |
CAACTTTTCCCTCGGGATTATAAC |
AGTACCCAAGGTTGAAGCCATAAA | 100 |
| LIRR 3 |
ACTCCAGTGACCTGCATGCA |
AGTGTGAAAGGCTCTGTGGGTTA | 76 |
| LIRR 4 |
TGGTCAGAACCCTTGCTATTTTATT |
AGAGGGCACTTCAGCCAAGTT | 75 |
| LIRR 5 |
CCGGGAGACATTGGCACTT |
AAGTTGACTTTGAACTGTGCCATATG | 82 |
| LIRR 6 |
AAAGCGGCAGCAGATTGC |
TGCCAGTAGGGTAAAGCAGTTCT | 81 |
| LIRR 7 |
TCTTCATTTCAGAGGGACAGCTT |
CTGTTGCAGAGCCATGAAGGT | 88 |
| LIRR 8 |
GGCAAGCTCTATGGCAACGA |
CATGTTCTTGAAGCTCAGGTTGA | 77 |
| LIRR 9 |
AGTTTTCAGCTATTGATAAACTTGGACA |
AAAAGTTTCCATTTTTTGGTGAGTAGA | 97 |
Cell culture and transfection
The human bronchial epithelial BEAS-2B cells were
originally obtained from the Type Culture Collection (Chinese
Academy of Sciences, Shanghai, China) and maintained at Jiangsu
Provincial Key Laboratory of Radiation Medicine and Protection
(Suzhou, China). The cells were cultured with Dulbecco’s modified
Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine
serum (Invitrogen, Carlsbad, CA, USA) at 37°C in an atmosphere of
5% CO2. The medium was changed every two days, and the
cells were sub-cultured every three days.
The recombinant plasmid pcDNA3.1/LIRR1 was
constructed by Jiangsu Provincial Key Laboratory of Radiation
Medicine and Protection as previously described (data not shown)
(17). The plasmid transfection was
conducted using lipofectamine (Invitrogen), as previously described
(18). G418 (500 μg/ml; Invitrogen,
Carlsbad, CA, USA) was used to acquire resistant clones with high
LIRR1 transcription. The LIRR1 transcription was determined using
an RT-PCR assay. the denaturation step was established at 95°C for
2 min and then continued at 95°C for 10 sec, 60°C for 30 sec and
70°C for 30 sec for 40 cycles, with a final extension step at 70°C
for 8 min.
Clonogenic assay
The exponential cells were plated at various cell
densities and irradiated with 0, 0.5, 1, 2, 4 and 6 Gy of X-ray
radiation. Following 12–14 days of incubation at 37°C, the cells
were fixed in methanol, which was followed by Giemsa staining. The
number of colonies per dish was counted and the surviving fractions
were calculated as the ratio of plating efficiencies for irradiated
and non-irradiated cells. Plating efficiency was defined as the
colony number divided by the number of cells plated for the
non-irradiated controls. The experiments were conducted in
triplicate and the data are presented as the mean ± standard
deviation from three independent experiments. All the survival
fractions were plotted onto a linear quadratic model using GraphPad
Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Flow cytometry
The cells were removed with trypsin and collected in
centrifuge tubes together with the culture medium. The detailed
method for flow cytometry has been previously described (17). The cell suspensions were collected
and centrifuged at 1,800 × g for 5 min. The supernatant was
discarded, and the cell pellets were washed with 1X
phosphate-buffered saline (PBS, Hyclone, Logan, UT, USA) and
centrifuged at 1,800 × g for 5 min. Finally, the cells were fixed
in 5 ml chilled 70% ethanol at 4°C for 4 h. Following
centrifugation and washing with 1× PBS three times, the cell
pellets were resuspended in 500 μl propidium iodine (10 μg/ml)
containing 300 μg/ml RNase (Sigma-Aldrich). The cells were then
incubated on ice for 30 min and filtered with a 53 μm nylon mesh.
The cell cycle distribution was calculated from 10,000 cells using
ModFit LT software (Becton Dickinson, San Jose, CA, USA) and
FACSCalibur (Becton Dickinson).
Immunofluorescence assay
The cells were seeded on a sterile glass chamber
(Becton Dickinson, Franklin Lakes, NJ, USA) at the appropriate
density and incubated overnight at 37°C in an atmosphere of 5%
CO2. Following exposure to 4 Gy X-ray IR for 1 h, the
cells were fixed using 4% formaldehyde for 30 min and then
permeabilized using 0.25% Triton X-100. The 1% bovine serum albumin
was used for blocking for 1 h at room temperature. The primary
rabbit anti-human monoclonal anti-phosphorylation γ-H2AX antibody
(1:1,000; Epitomics, Burlingame, CA, USA) was used for a 2-h
incubation at room temperature. A specific monoclonal goat
anti-rabbit secondary antibody conjugated to CY3 (1:2,000; Beyotime
Institute of Biotechnology, Haimen, China) was incubated in the
dark for 1 h at room temperature, followed by 5 μg/ml Hoechst 33342
nuclear staining (Sigma-Aldrich). Fluorescence was observed using
laser scanning confocal microscopy at a wavelength of 650 nm within
30 min. The number of foci in 50 cells in each sample was counted
randomly.
Western blot assay
Western blot analysis was performed as previously
described (17). Briefly, the
following primary antibodies were used for immunoblotting: RAD50
(catalog number 3427), KU70 (clone, D10A7), KU80 (clone, C48E7),
p-p53 (Ser15; clone, D4S1H), p21 (clone, 12D1) (all 1:1,000
dilution; Cell Signaling Technology, Danvers, MA, USA),
cyclin-dependent kinase 2 (CDK2; clone, M2; dilution, 1:1,000;
Santa Cruz Biotech, CA, USA), mouse double minute 2 homolog (MDM2;
clone, S1357; dilution, 1:1,000; Epitomics Inc., Hangzhou,
Zhejiang, China) and β-actin (clone, C4; dilution, 1:1,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The appropriate
secondary antibodies (1:5,000; Bioworld Technology Inc., St. Louis
Park, MN, USA), enhanced chemiluminescence system (Union Bioscience
Corporation, Hangzhou, Zhejiang, China), and X-film (Carestream
Health, Shanghai, China) were used for developing the immunoblot
using pre-stained markers (catalog number 26616; Thermo Fisher
Scientific, Inc.) as the molecular size standard.
Statistical analysis
The data are presented as the mean ± standard
deviation. The statistical comparisons of the experimental results
between the treated and control groups were performed using the
two-tailed Student’s t-test. All statistical tests were measured
using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Screening, validation and analysis of
differentially-expressed lincRNA LIRR1 from the RILI mouse
model
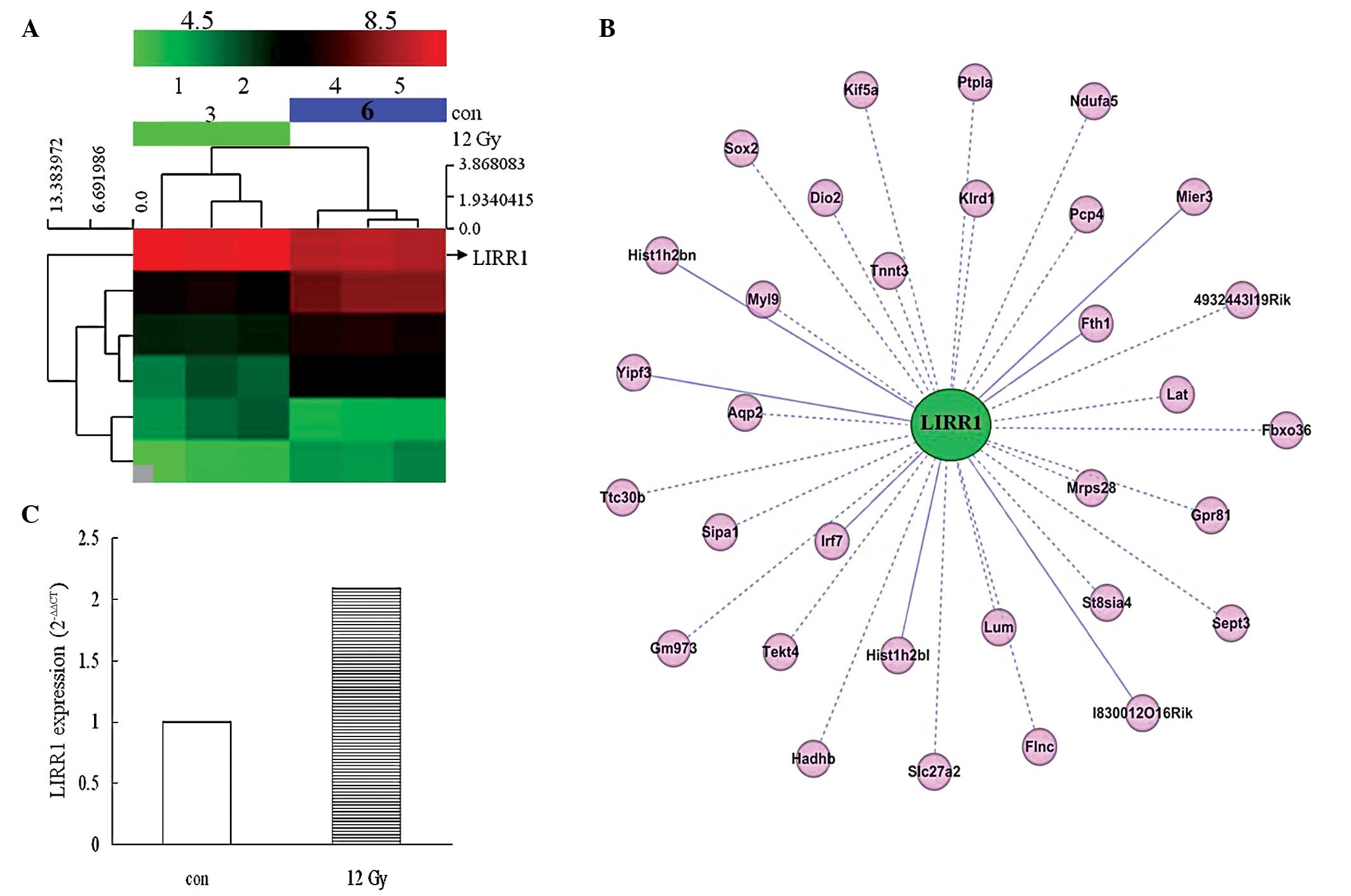
Six differentially-expressed lincRNAs that were near
coding genes (distance, <300 kb) and whose expression was
significantly different between the two groups (TBI vs. normal, log
fold change≥2.0; P≤0.05) were identified. As shown in the
hierarchical clustering dendrogram, all six lung tissue samples
were arranged into two groups, TBI vs. normal control, and the
results revealed a distinguishable lincRNA expression profile
(Fig. 1A). The six lncRNAs were
divided into two groups based on their response to IR. Group I
included LIRR1 and 5, which were induced following 24 h of X-ray
exposure, and group II included LIRR2, 3, 4 and 6, which decreased
following exposure.
The 273-bp X-ray inducible lincRNA LIRR1, which was
located on the positive strand of chromosome 1 and was adjacent to
the Stat4 gene (distance, <300 bp), was classified as a sense
overlap lncRNA. This type of lncRNA is able to exert biological
effects through gene transcription and expression regulation
(19). As shown in Fig. 1B, further bioinformatics analysis
revealed the co-expression pattern of LIRR1 with 32 coding genes,
which are mainly associated with cell cycle regulation (Sept3,
Sipal and Sox2), chromatin structure (Hist1h2b1, Hist1h2b1n and
Mier3), immune response (Klrd1, Lat and Irf7), mitochondrial
structure (Ndufa5, Hadhb, and Mrps28), collagen fibril organization
and circumferential growth, corneal transparency and epithelial
cell migration and tissue repair (Lum) (Fig. 1B). The induced transcription of
LIRR1 in TBI mouse lung tissues was also validated by qPCR
(Fig. 1C). Thus, LIRR1 was selected
for further study. The sequence of LIRR1 was acquired from the UCSC
Genome Browser database (http://genome.ucsc.edu/) according to the microarray
screening results.
LIRR1 regulates the radiosensitivity of
human bronchial epithelial BEAS-2B cells to X-ray IR
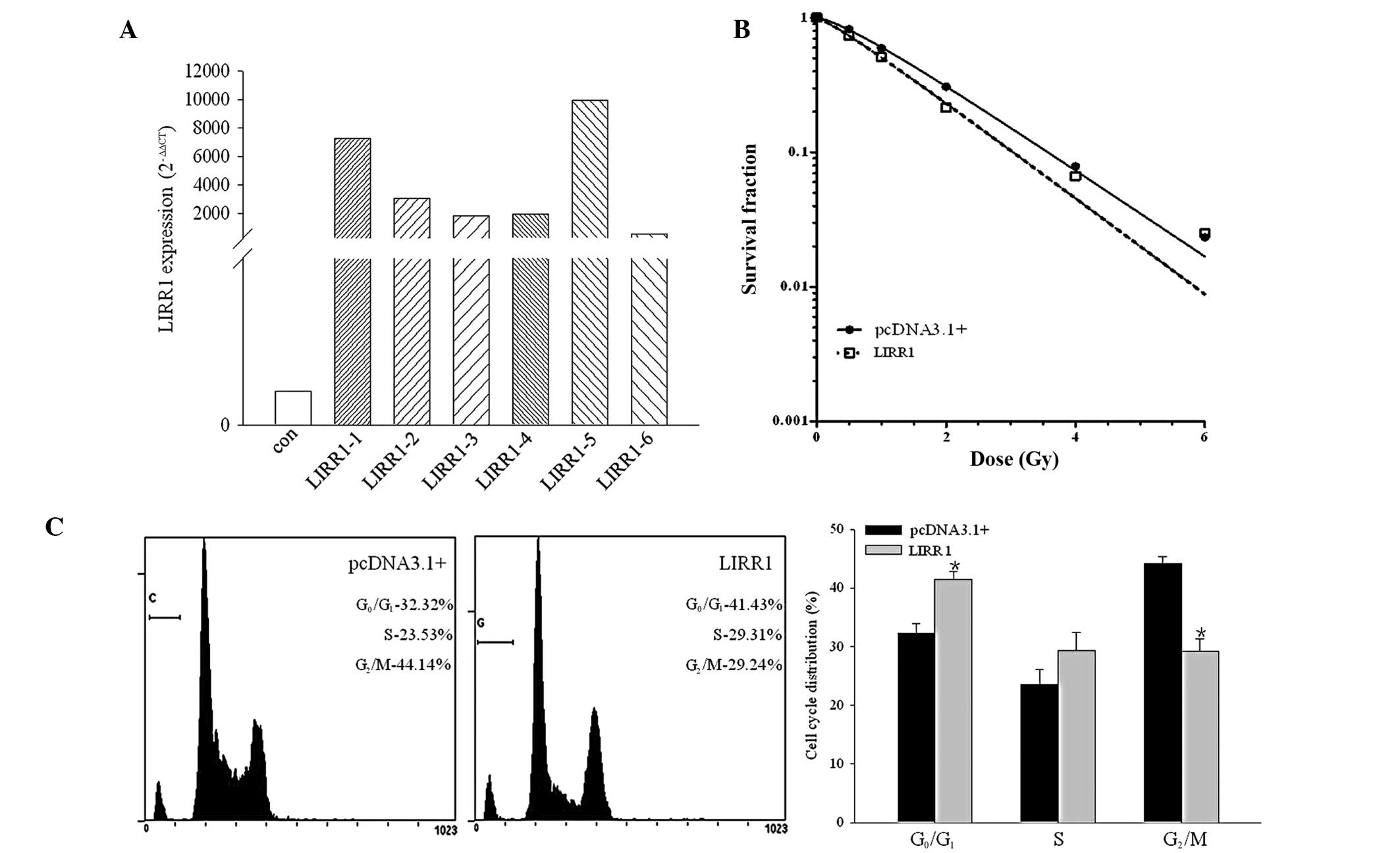
In the present study, the transcription level of
LIRR1 was measured by RT-PCR, as shown in Fig. 2A. The transcription of the six
selected resistant clones, which were stably transfected with the
pcDNA3.1/LIRR1 plasmid, was increased significantly compared with
that of the parental BEAS-2B cells. Clone 5 was identified for the
following studies due to the highest transcription level of LIRR1.
At the same time, six clones that were stably transfected with the
pcDNA3.1 vector were also assessed using RT-PCR assay to determine
their LIRR1 transcription level (data not shown). One of the six
resistant clones exhibited a similar transcription pattern to the
parental BEAS-2B cells and was therefore also selected for the
following study.
LncRNA LIRR1 markedly affected the radiosensitivity
of the BEAS-2B cells. As shown in Fig.
2B, the mock transfectant, BEAS-2B/pc3.1, revealed a typical
clonogenic survival curve with a shoulder, indicating the potential
of DNA damage repair. Exogenous LIRR1 significantly radiosensitized
the BEAS-2B cells to X-ray IR.
Flow cytometry was performed 24 h after the cells
were exposed to 4 Gy of X-ray IR. As shown in Fig. 2C, the LIRR1 transfectant cells
exhibited a significantly increased proportion of cells in the
G1 phase (P<0.05) compared with the mock
BEAS-2B/pc3.1 transfectant cells following IR.
LIRR1 regulates γ-H2AX focus formation in
BEAS-2B cells following IR
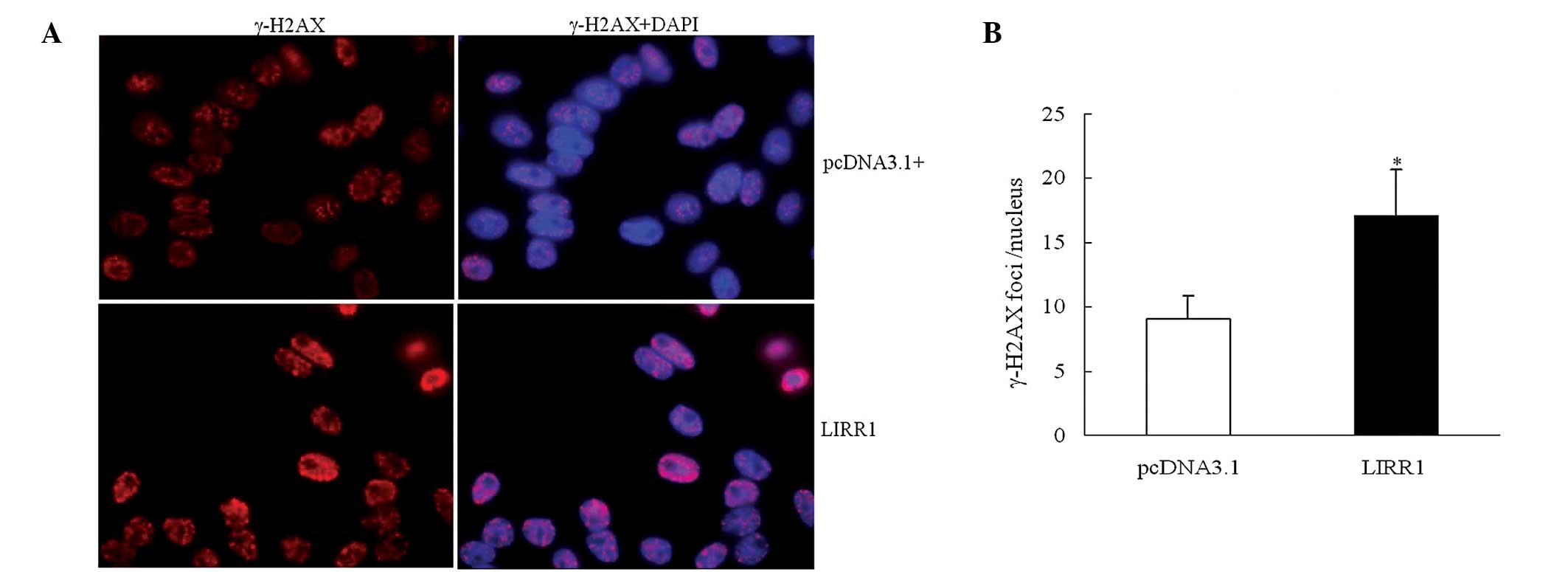
The IR-induced phospho-γ-H2AX foci formation was
regarded as the initial DDR, activating different cascades and
leading to different outcomes (20,21).
In the present study, immunofluorescence staining of phospho-γ-H2AX
was visualized 1 h after X-ray IR to investigate the potential
functions of LIRR1 on the IR-induced DDR. As shown in Fig. 3, there were approximately nine
IR-induced γ-H2AX foci per nucleus in the pcDNA3.1 transfectants.
Significantly increased numbers of γ-H2AX foci were observed in the
BEAS-2B cells exhibiting a high LIRR1 transcription level, with ~17
per nucleus (t=−14.46; P<0.01). The present results suggest that
lincRNA LIRR1 is able to inhibit IR-induced DNA damage repair.
LIRR1 is involved in DSB damage signaling
pathway regulation following IR in vitro
Western blot analysis was performed to verify the
mechanisms of LIRR1 modulating radiosensitivity. As shown in
Fig. 4A, when exposed to X-ray
radiation, the high transcription of LIRR1 led to the decreased
expression of DSB sensors KU70 and KU80, DNA damage repair protein
RAD50, cell cycle G1 phase checkpoint CDK2 and p53
partner MDM2 compared with the control group. The increased
expression of the key regulator of DDR-phosphorylated p53 and p21
was observed due to the high transcription level of LIRR1. The
present results are not only in accordance with the data drawn from
the clonogenic assay, flow cytometry and immunofluorescence
staining, but are also consistent with the bioinformatics analysis
of the co-expression pattern of LIRR1 (Fig. 1B).
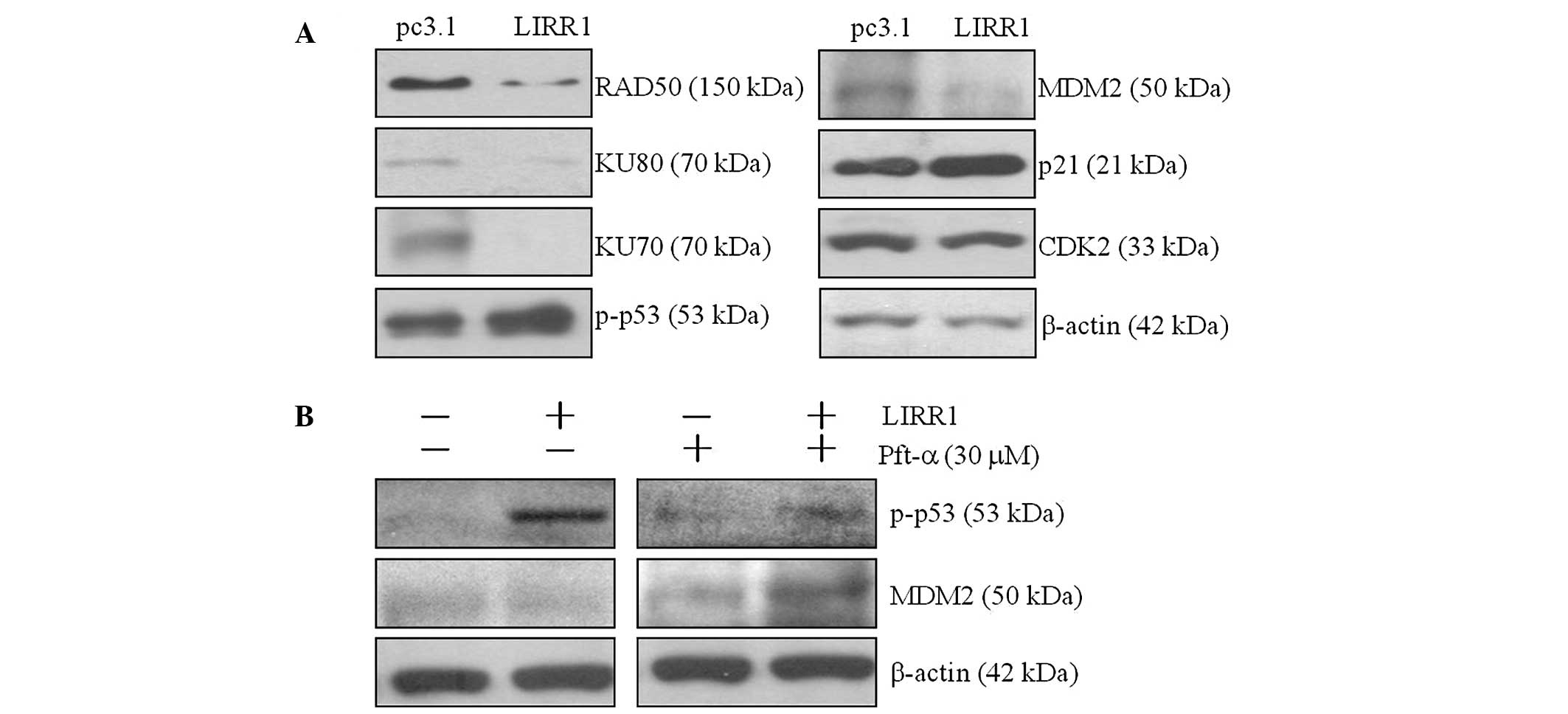 | Figure 4LIRR1 was involved in the regulation
of the DDR signaling pathway after IR. (A) Western blot analyses
were performed to determine the expression of DDR sensors and the
downstream cascades in LIRR1-overexpressing BEAS-2B cells. The
cells were exposed to 4 Gy of irradiation, and 50 μg of protein was
assessed using SDS-PAGE. Immunoblotting with the indicated specific
antibodies of the DDR signaling pathway was subsequently conducted.
The bands were analyzed through densitometry, with β-actin as the
loading control. (B) Following IR, the cells were cultured in the
presence or absence of the p53 inhibitor Pft-α. The expression
levels of p-p53 and MDM2 were determined by western blot analysis.
A representative result was gained following three independent
experiments. LIRR, long intergenic radiation-responsive non-coding
RNA; DDR, DNA damage response; IR, irradiation; p-p53, phospho-p53;
MDM2, mouse double minute 2 homolog; Pft-α, Pifithrin-α; CDK2,
cyclin-dependent kinase 2. |
Phosphorylation and the subsequent activation of p53
and its transcriptional induction are the key initial responses to
cell cycle distribution and DNA damage (21–24).
In the present study, it was identified that the high transcription
level of LIRR1 was coordinated with IR-induced p53 phosphorylation.
Following the use of Pifithrin-α, a specific inhibitor of p53
activation, the decreased MDM2 expression in LIRR1-overexpressing
cells following IR was restored, suggesting that LIRR1 could
mediate the DDR signaling in a p53-dependent manner (Fig. 4B).
Discussion
Guttman et al (8) demonstrated that lincRNAs are regulated
during their development and in response to diverse signaling cues,
including DNA damage, which may affect various human diseases
(19). In total, >3,000 human
lincRNA cases have been identified, of which, <1% have been
characterized (14). The
differentially-expressed lincRNAs associated with RILI have not
been previously reported. In the present study, the
differentially-expressed lincRNA was identified in C57BL/6 mouse
lung tissue 24 h after TBI using microarray screening and RT-PCR
validation. The expression of the six differential lincRNAs either
increased, LIRR1 and 5, or decreased, LIRR 2 3, 4 and 6, in the
irradiated mice compared with their sham-irradiated
counterparts.
LIRR1 was chosen for further functional study due to
the following reasons (1): LIRR1
was induced by X-ray IR, which may induce a variety of coding genes
involved in the intracellular metabolic pathways and play an
important role in cell cycle regulation (involving the GADD family,
MDM2, WAF1 and CDK) apoptosis (involving the Bcl-2 family death
receptors and caspase family), DNA damage repair (involving ATM,
p53, Ras, PKC and ErbB) and cell growth regulation (involving EGFR
and IGF-1) (20,21,24,25–29).
However, the biological function and associated mechanisms of
IR-induced lincRNAs remain largely unknown (2). The 273-nt lincRNA locus on chromosome
1 was co-expressed with 27 coding genes (Fig. 1B), which are mainly associated with
cell cycle regulation (Sept3, Sipal and Sox2), chromatin structure
(Hist1h2b1, Hist1h2b1n and Mier3), immune response (Klrd1, Lat and
Irf7), mitochondrial structure (Ndufa5, Hadhb and Mrps28), collagen
fibril organization and circumferential growth, corneal
transparency, and epithelial cell migration and tissue repair
(Lum).
The IR-induced DDR consists of several highly
complex and coordinated signaling pathways, including programmed
cell death, cell cycle regulation and DNA repair pathways, with
each exerting different effects on cells (21,23).
In the present study, a gain-of-function strategy was used, and the
effect of LIRR1 on cell survival following IR was investigated
through a clonogenic assay. LIRR1 overexpression significantly
reduced clonogenic formation following IR and therefore, LIRR1
facilitated cell death following IR.
Another important pathway of DDR is the activation
of cell-cycle checkpoints (23,24).
Treatment of mammalian cells with IR causes delays in the movement
of cells through the G1, S and G2 phases. In
the present study, LIRR1-overexpressing BEAS-2B cells exhibited a
significant increase in the G1 phase distribution of the
cell cycle following IR.
As one of the earliest occurring events, the
phosphorylation of H2AX, also known as γ-H2AX, is necessary for the
recruitment of other proteins involved in DDR (30,31).
Cells lacking H2AX exhibit a substantial increase in
radiosensitivity (20). BEAS-2B
cells with a high LIRR1 transcription level produce a higher amount
of γ-H2AX foci following IR than the ‘mock’ constantly transfected
control cells. Along with the results of the clonogenic assay, the
phenotype of increased radiosensitivity may reflect the potential
regulatory effects exerted by LIRR1 on the associated regulatory
factors of DDR.
Previous studies have revealed that hundreds of
proteins can be phosphorylated by two key kinases, ataxia
telangiectasia mutated (ATM) and ataxia telangiectasia and
Rad3-related (ATR), in response to DNA damage (22,28).
Therefore, these kinases serve as the signals to activate several
downstream effectors of the DDR, including DNA repair, cell cycle
checkpoints and apoptosis. One of the most important proteins
activated by ATM and ATR is p53 (24,29).
Once activated by DSB-induced ATM phosphorylation, the
stabilization of the p53-MDM2 complex is disturbed (29). Consequently, the p53 protein is no
longer degraded. A series of genes is induced by activated p53,
which affects the checkpoints or promotes apoptosis. The induction
of the p53 downstream effector, p21, inhibits the function of
cyclin-CDKs complex and interrupts cell entry into the S phase
(29).
The present study reports the evident decrease of
DNA repair protein KU70, KU80 and RAD 50 in LIRR1-overexpressing
BEAS-2B cells following IR and the marked activation of p53, the
decrease in MDM2, the substantial inducement of p21 and the
suppression of CDK2. These findings may explain the resulting block
of the cell cycle checkpoint and DNA damage repair. In accordance
with this finding, the control cells clearly did not demonstrate
the same expression pattern as the aforementioned proteins.
Furthermore, Pifithrin-α was used following IR. The ability of this
p53 inhibitor to affect MDM2 expression indicates the direct role
of p53 in modulating the radiation response in LIRR1-overexpressing
BEAS-2B cells. The present findings suggest a potential biological
role of lincRNAs in RILI, which is considered the most common
clinical complication of thoracic radiotherapy. However, the
molecular basis of such a role remains unclear.
In conclusion, according to the present preliminary
study, varying lincRNAs expression may be involved in the
regulation of the RILI process and can be profiled to aid the
diagnosis, prognosis and even therapy of RILI. LIRR1 may be a novel
molecular target of IR, as it was able to modulate the
radiosensitivity of BEAS-2B cells through the DDR signaling
pathways in a p53-dependent manner. However, several questions
remain unanswered (1). It should be
determined whether the variable lincRNAs identified in the present
study respond only to IR or not, as a group of long
stress-responsive non-coding transcripts, including Xist and
HOTAIR, has already been identified (2). Although the biological functions of
certain long IR-induced non-coding RNAs were initially explored,
the precise roles and mechanisms of LIRR-ncRNAs in RILI development
mentioned in the present study necessitate further in vivo
and in vitro experimental investigation.
Acknowledgements
This study was supported by National Science
Foundation of China (grant no. 81020108028, 81172706, 81170468 and
81302382) and the Priority Academic Program Development of Jiangsu
Higher Education Institutions.
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
lincRNA
|
long intergenic non-coding RNA
|
|
RILI
|
radiation-induced lung injury
|
|
TBI
|
total body irradiation
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
qPCR
|
quantitative polymerase chain
reaction
|
References
|
1
|
Puthawala K, Hadjiangelis N, Jacoby SC,
Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH,
Lukashev ME, et al: Inhibition of integrin alpha(v)beta6, an
activator of latent transforming growth factor-beta, prevents
radiation-induced lung fibrosis. Am J Respir Crit Care Med.
177:82–90. 2008. View Article : Google Scholar
|
|
2
|
Christofidou-Solomidou M, Tyagi S, Tan KS,
Hagan S, Pietrofesa R, Dukes F, Arguiri E, Heitjan DF, Solomides CC
and Cengel KA: Dietary flaxseed administered post thoracic
radiation treatment improves survival and mitigates
radiation-induced pneumonopathy in mice. BMC Cancer. 11:2692011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathew B, Jacobson JR, Berdyshev E, Huang
Y, Sun X, Zhao Y, Gerhold LM, Siegler J, Evenoski C, Wang T, et al:
Role of sphingolipids in murine radiation-induced lung injury:
protection by sphingosine 1-phosphate analogs. FASEB J.
25:3388–3400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh JH, Craft JM, Townsend R, Deasy JO,
Bradley JD and El Naqa I: A bioinformatics approach for biomarker
identification in radiation-induced lung inflammation from limited
proteomics data. J Proteome Res. 10:1406–1415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Walton W, Cook DN, Hua X, Tilley
S, Haskell CA, Horuk R, Blackstock AW and Kirby SL: The chemokine,
CCL3, and its receptor, CCR1, mediate thoracic radiation-induced
pulmonary fibrosis. Am J Respir Cell Mol Biol. 45:127–135. 2011.
View Article : Google Scholar :
|
|
6
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cabili MN, Trapnell C, Goff L, Koziol M,
Tazon-Vega B, Regev A and Rinn JL: Integrative annotation of human
large intergenic noncoding RNAs reveals global properties and
specific subclasses. Genes Dev. 25:1915–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: new links in cancer progression. Cancer
Res. 71:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silva JM, Perez DS, Pritchett JR, Halling
ML, Tang H and Smith DI: Identification of long stress-induced
non-coding transcripts that have altered expression in cancer.
Genomics. 95:355–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizutani R, Wakamatsu A, Tanaka N, Yoshida
H, Tochigi N, Suzuki Y, Oonishi T, Tani H, Tano K, Ijiri K, et al:
Identification and characterization of novel genotoxic
stress-inducible nuclear long noncoding RNAs in mammalian cells.
PLoS One. 7:e349492012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sonkoly E, Bata-Csorgo Z, Pivarcsi A,
Polyanka H, Kenderessy-Szabo A, Molnar G, Szentpali K, Bari L,
Megyeri K, Mandi Y, et al: Identification and characterization of a
novel, psoriasis susceptibility-related noncoding RNA gene, PRINS.
J Biol Chem. 280:24159–24167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: new links in cancer progression. Cancer
Res. 71:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnston CJ, Manning C, Hernady E, Reed C,
Thurston SW, Finkelstein JN and Williams JP: Effect of total body
irradiation on late lung effects: hidden dangers. Int J Radiat
Biol. 87:902–913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zaidi A, Jelveh S, Mahmood J and Hill RP:
Effects of lipopolysaccharide on the response of C57BL/6J mice to
whole thorax irradiation. Radiother Oncol. 105:341–349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao Y, Ge CM, Meng QH, Cao JP, Tong J and
Fan SJ: Adenovirus-mediated expression of Tob1 sensitizes breast
cancer cells to ionizing radiation. Acta Pharmacol Sin.
28:1628–1636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiao Y, Sun KK, Zhao L, Xu JY, Wang LL and
Fan SJ: Suppression of human lung cancer cell proliferation and
metastasis in vitro by the transducer of ErbB-2.1 (TOB1). Acta
Pharmacol Sin. 33:250–260. 2012. View Article : Google Scholar
|
|
19
|
Khalil AM, Guttman M, Huarte M, Garber M,
Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van
Oudenaarden A, Regev A, Lander ES and Rinn JL: Many human large
intergenic noncoding RNAs associate with chromatin-modifying
complexes and affect gene expression. Proc Natl Acad Sci USA.
106:11667–11672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugrue T, Brown JA, Lowndes NF and Ceredig
R: Multiple facets of the DNA damage response contribute to the
radioresistance of mouse mesenchymal stromal cell lines. Stem
Cells. 31:137–145. 2013. View Article : Google Scholar
|
|
21
|
Foltánková V, Legartová S, Kozubek S,
Hofer M and Bártová E: DNA-damage response in chromatin of
ribosomal genes and the surrounding genome. Gene. 522:156–167.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiloh Y and Ziv Y: The ATM protein
kinase: regulating the cellular response to genotoxic stress, and
more. Nat Rev Mol Cell Biol. 14:197–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cipressa F and Cenci G: DNA damage
response, checkpoint activation and dysfunctional telomeres: face
to face between mammalian cells and Drosophila. Tsitologiia.
55:211–217. 2013.PubMed/NCBI
|
|
24
|
Senturk E and Manfredi JJ: p53 and cell
cycle effects after DNA damage. Methods Mol Biol. 962:49–61. 2013.
View Article : Google Scholar
|
|
25
|
Insinga A, Cicalese A, Faretta M, Gallo B,
Albano L, Ronzoni S, Furia L, Viale A and Pelicci PG: DNA damage in
stem cells activates p21, inhibits p53, and induces symmetric
self-renewing divisions. Proc Natl Acad Sci USA. 110:3931–3936.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roos WP and Kaina B: DNA damage-induced
cell death: from specific DNA lesions to the DNA damage response
and apoptosis. Cancer Lett. 332:237–248. 2013. View Article : Google Scholar
|
|
27
|
Jiang G, Plo I, Wang T, Rahman M, Cho JH,
Yang E, Lopez BS and Xia F: BRCA1-Ku80 protein interaction enhances
end-joining fidelity of chromosomal double-strand breaks in the G1
phase of the cell cycle. J Biol Chem. 288:8966–8976. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park J, Jo YH, Cho CH, Choe W, Kang I,
Baik HH and Yoon KS: ATM-deficient human fibroblast cells are
resistant to low levels of DNA double-strand break induced
apoptosis and subsequently undergo drug-induced premature
senescence. Biochem Biophys Res Commun. 430:429–435. 2013.
View Article : Google Scholar
|
|
29
|
Pant V, Xiong S, Jackson JG, Post SM,
Abbas HA, Quintás-Cardama A, Hamir AN and Lozano G: The p53-Mdm2
feedback loop protects against DNA damage by inhibiting p53
activity but is dispensable for p53 stability, development, and
longevity. Genes Dev. 27:1857–1867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong Z, Kase Y, Moritake T, Gerelchuluun
A, Sun L, Suzuki K, Terunuma T, Yasuoka K, Kumada H, Anzai K, et
al: Lineal energy-based evaluation of oxidative DNA damage induced
by proton beams and X-rays. Int J Radiat Biol. 89:36–43. 2013.
View Article : Google Scholar
|
|
31
|
Wu J, Clingen PH, Spanswick VJ,
Mellinas-Gomez M, Meyer T, Puzanov I, Jodrell D, Hochhauser D and
Hartley JA: γ-H2AX foci formation as a pharmacodynamic marker of
DNA damage produced by DNA cross-linking agents: results from 2
phase I clinical trials of SJG-136 (SG2000). Clin Cancer Res.
19:721–730. 2013. View Article : Google Scholar
|