Introduction
As a result of early diagnosis and improved
treatment strategies, the survival rates of colon cancer patients
have increased (1); however, the
five-year survival rate remains at <60% (2). For colon cancer in situ, surgery
is the primary curative method. However, in the later phases (e.g.
node-positive stage III), adjuvant chemotherapy is required
(1). As a first-line chemotherapeutic
agent for colon cancer, 5-fluorouracil (5-FU) is administered in
order to increase the likelihood of survival (3). The structure of 5-FU resembles the
pyrimidines of DNA and RNA (4);
therefore, it is able to disrupt nucleoside metabolism and be
incorporated into RNA and DNA molecules. This results in cell-cycle
arrest at G1 phase and at the G1/S boundary,
which prolongs DNA synthesis (5–7). Despite
its anticancer effects, the clinical use of 5-FU is limited by drug
resistance. 5-FU used alone, or in combination with other antitumor
drugs, has a relatively low response rate in the treatment of colon
cancer (8,9). Therefore, the mechanisms underlying 5-FU
resistance require further investigation.
Epithelial-mesenchymal transition (EMT) is a process
in which epithelial cells lose a number of endogenous
characteristics and acquire typical features of mesenchymal cells
(10). For instance, the cobblestone
appearance of epithelial cells changes to a spindle-like shape. In
addition, epithelial biomarkers (including E-cadherin) are lost,
while mesenchymal markers (such as N-cadherin, vimentin and Snail)
are acquired (11). EMT in cancer
cells has been identified to facilitate metastasis and
chemoresistance (12). Furthermore,
an EMT phenomenon has been identified in a number of cancer cells,
including cisplatin-resistant non-small-cell lung cancer (13), doxorubicin-resistant breast cancer
(14) and sorafenib-resistant
hepatocellular carcinoma cells (15).
Preventing EMT-associated signaling pathways plays an important
role in inhibiting cancer cell migration and invasion, as well as
reducing drug resistance (12). The
occurrence of the EMT is associated with complex signaling
pathways, including the Wnt, PI3K, Hedgehog, transforming growth
factor-β (TGF-β) and Notch pathways (16–18).
However, the details of these mechanisms in HCT-8 colon cancer
cells are yet to be elucidated.
In order to identify potential therapeutic targets
for the treatment of 5-FU-resistant colon cancer, the present study
investigated the pathway(s) involved in the induction of an EMT
phenotype and the acquisition of 5-FU resistance in HCT-8/5-FU
cells.
Materials and methods
Cells and reagents
The HCT-8/wild-type (WT) and HCT-8/5-FU cell lines
were obtained from KeyGen Biotech Co. Ltd. (Nanjing, China) and
cultured in RPMI-1640 containing 10% fetal bovine serum (FBS), 100
U/ml penicillin (Beyotime Institute of Biotechnology, Shanghai,
China) and 100 U/ml streptomycin (Beyotime Institute of
Biotechnology) at 37°C under an atmosphere of 5% CO2. In
order to maintain drug resistance, 15 µg/ml 5-FU was added to the
HCT-8/5-FU cells. The 5-FU and GDC0449 agents were purchased from
King York Co. Ltd. (Tianjin, China) and Selleck Chemicals (Houston,
TX, USA), respectively.
Immunofluorescent staining
The cells were fixed in 4% paraformaldehyde for 30
min, permeated with 0.1% Triton X-100 [Sangon Biotech (Shanghai)
Co., Ltd., Shanghai, China] for 10 min at room temperature and then
washed three times with 0.05% Tween-20. After blocking with 5%
bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for
30 min, primary monoclonal rabbit anti-human E-cadherin (dilution,
1:200; cat. no. 1702-1; Epitomics, Inc., Burlingame, CA, USA) and
monoclonal mouse anti-rabbit vimentin (dilution, 1:500; cat. no.
ab8069; Abcam, Cambridge, UK) antibodies were added and incubated
at 4°C overnight. Next, fluorescent donkey anti-rabbit Alexa Fluor
568-conjugated (dilution, 1:200; Invitrogen Life Technologies,
Carlsbad, CA, USA) or donkey anti-mouse Alexa Fluor 488-conjugated
(dilution, 1:200; Invitrogen Life Technologies) secondary
antibodies were added for 1 h at room temperature. DAPI (Beyotime
Institute of Biotechnology) was used to stain the nuclei of the
cells. Images were captured using a confocal laser scanning
microscope (TCS SP8; Leica Microsystems GmBH, Wetzlar,
Germany).
Flow cytometry
The cells were collected by centrifugation at 500 ×
g for 5 min, blocked with 5% BSA in PBS for 30 min (for vimentin,
this process was the same as for the immunofluorescent staining)
and incubated with the antibodies for 30 min at 4°C. Subsequent to
each treatment, the cells were washed with 2% BSA in PBS and
collected by centrifugation at 500 × g for 5 min. Fluorescence was
detected using a FACSCalibur flow cytometer (BD Biosciences,
Franklin, NJ, USA).
Wound-healing assay
Cellular motility was assessed using a wound repair
assay, as described previously (19–21).
Briefly, the cells were plated at a density of 1×104
cells per well in 24-well plates and incubated overnight in
serum-free medium. A straight line was then scratched through the
attached cells using a sterile tip. The suspended cells were
removed, and medium containing 2% FBS was added. Images were then
captured immediately (0 h) and at 48 h using a fluorescence
microscope (TS100, Nikon Corporation, Tokyo, Japan). The width of
the wound was measured using ImageJ software (National Institutes
of Health, Bethesda, MD, USA) in order to determine the extent of
migration.
Migration assay
Transwell chamber inserts were used in order to
investigate cellular migration in vitro. A 100 µl serum-free
suspension containing 5×104 cells was added to a chamber
with 8-µm pores (BD Biosciences). Next, the chamber was placed in a
24-well plate containing 10% FBS. Subsequent to a 24-h incubation,
a number of cells had migrated to the lower surface. These cells
were stained with crystal violet [Sangon Biotech (Shanghai) Co.,
Ltd.] and images were captured using a Leica CME microscope (Leica
Microsystems GmBH) and a Nikon Coolpix 54 camera (Nikon
Corporation). The migrated cells were counted in randomly-selected
fields.
5-FU chemosensitivity assay
The cells were plated into 96-well plates at a
density of 8×103 cells per well. Following attachment, a
series of 5-FU concentrations (2.5–20,000 µg/ml in two-fold serial
dilutions) were added. The plates were then incubated for 48 h at
37°C under 5% CO2. Next, 10 µl MTT (5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) was added, and the plates were
incubated for a further 4 h. Subsequently, 150 µl dimethyl
sulfoxide was added in order to dissolve the formazan crystals.
Finally, the absorbance was measured at 492 nm using a microplate
reader (Multiskan MK3; Thermo Fisher Scientific, Waltham, MA,
USA).
Cell proliferation assay
Cellular proliferation was analyzed at an absorbance
of 492 nm. The cells were seeded into 96-well plates at a density
of 3×103, 5×103 or 1×104 cells per
well. Subsequent to a 24 h incubation, the absorbance was measured
using an MTT assay. In addition, cells were plated at a density of
5×103 cells per well in 96-well plates, and the
absorbance was measured at 24, 48, 72 and 96 h by MTT assay.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted for the reverse
transcription reactions using Reverse Transcriptase M-MLV (Takara
Bio Inc., Otsu, Japan) and the Oligo dT18 primer (Takara
Bio Inc.). HCT-8/5-FU cells were exposed to 5 µM GDC0449 for 48 h.
Then the total RNA was extracted. Next, the cDNA was used in the
PCR analysis with 2X Taq Master Mix (25 µl), containing 0.2 mM dNTP
mixture and 2X PCR buffer (20 mM Tris-HCl, pH 8.3, 100 mM KCl, 3 mM
MgCl2; Takara Bio Inc.). The PCR conditions were as
follows: 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec, for
34 cycles followed by a subsequent elongation step at 72°C for 10
min. The PCR results were visualized on agarose gel. The images
were analyzed using ImageJ software. β-actin was used as an
endogenous control. The primer sequences were as follows: β-actin
forward, 5′-TGA AGT GTG ACG TGG ACATC-3′, and reverse, 5′-GGA GGA
GCA ATG ATC TTGAT-3′; N-cadherin forward, 5′-ACA GTG GCC ACC TAC
AAAGG-3′, and reverse, 5′-TGA TCC CTC AGG AAC TGTCC-3′.
Statistical analysis
The results are presented as the mean ± standard
deviation. Statistical differences were determined using Student's
t-test. All statistical analyses were performed using GraphPad
Prism version 5.01 software (GraphPad Software, Inc., La Jolla, CA,
USA). A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
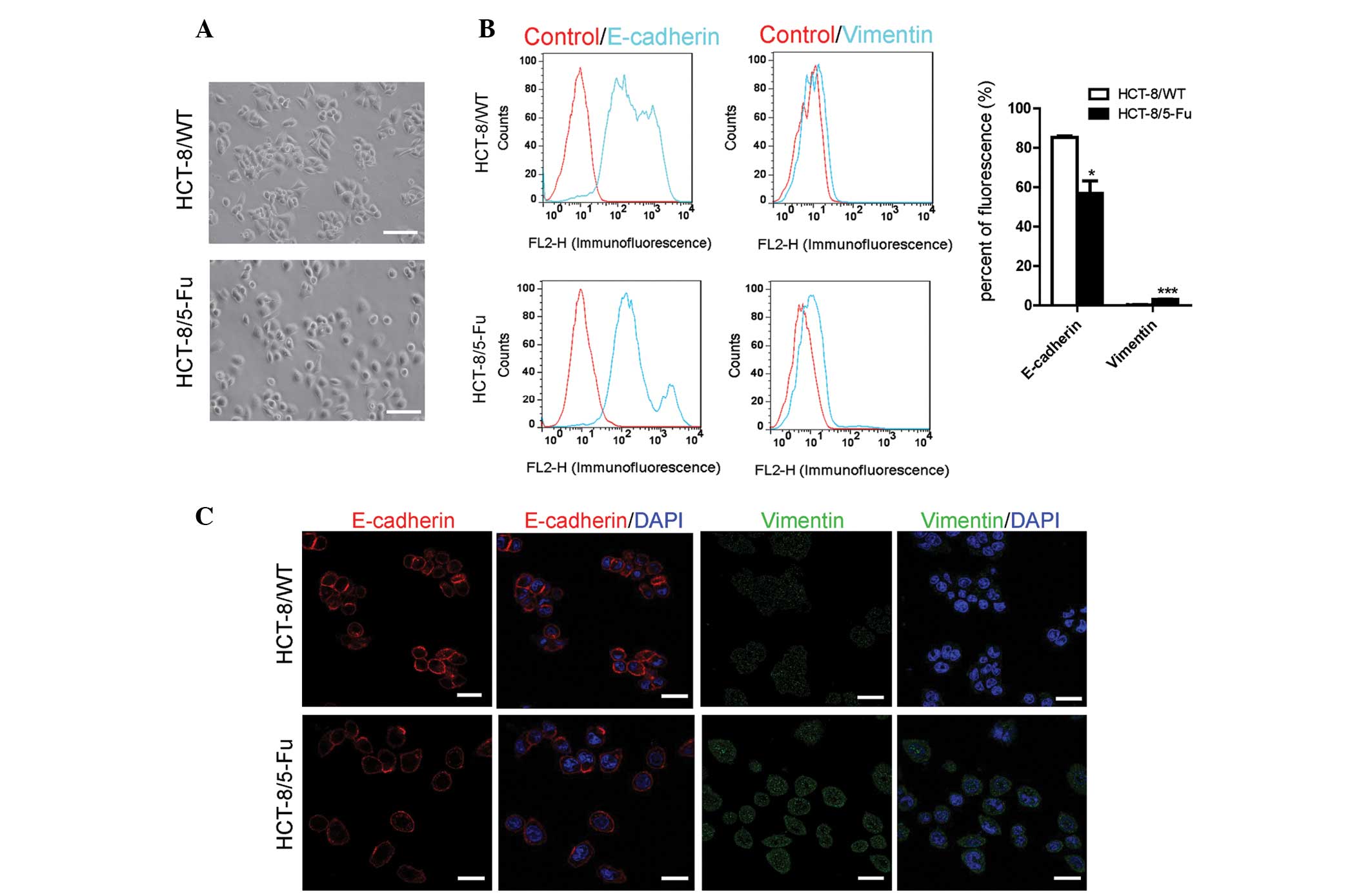
HCT-8/5-FU cells develop morphological
and molecular changes consistent with EMT signaling
The HCT-8/WT cells grew in clusters in vitro,
which is a typical feature of the epithelial phenotype. By
contrast, the HCT-8/5-FU cells were scattered and had few
connections as a result of the presence of 15 µg/ml 5-FU (Fig. 1A).
EMT-associated biomarkers were assessed in order to
determine whether the acquisition of 5-FU resistance induced EMT
changes on the molecular level. Compared with the HCT-8/WT cells,
HCT-8/5-FU cells demonstrated a significant reduction in the level
of E-cadherin and an upregulation in the expression of vimentin
(Fig. 1B–D).
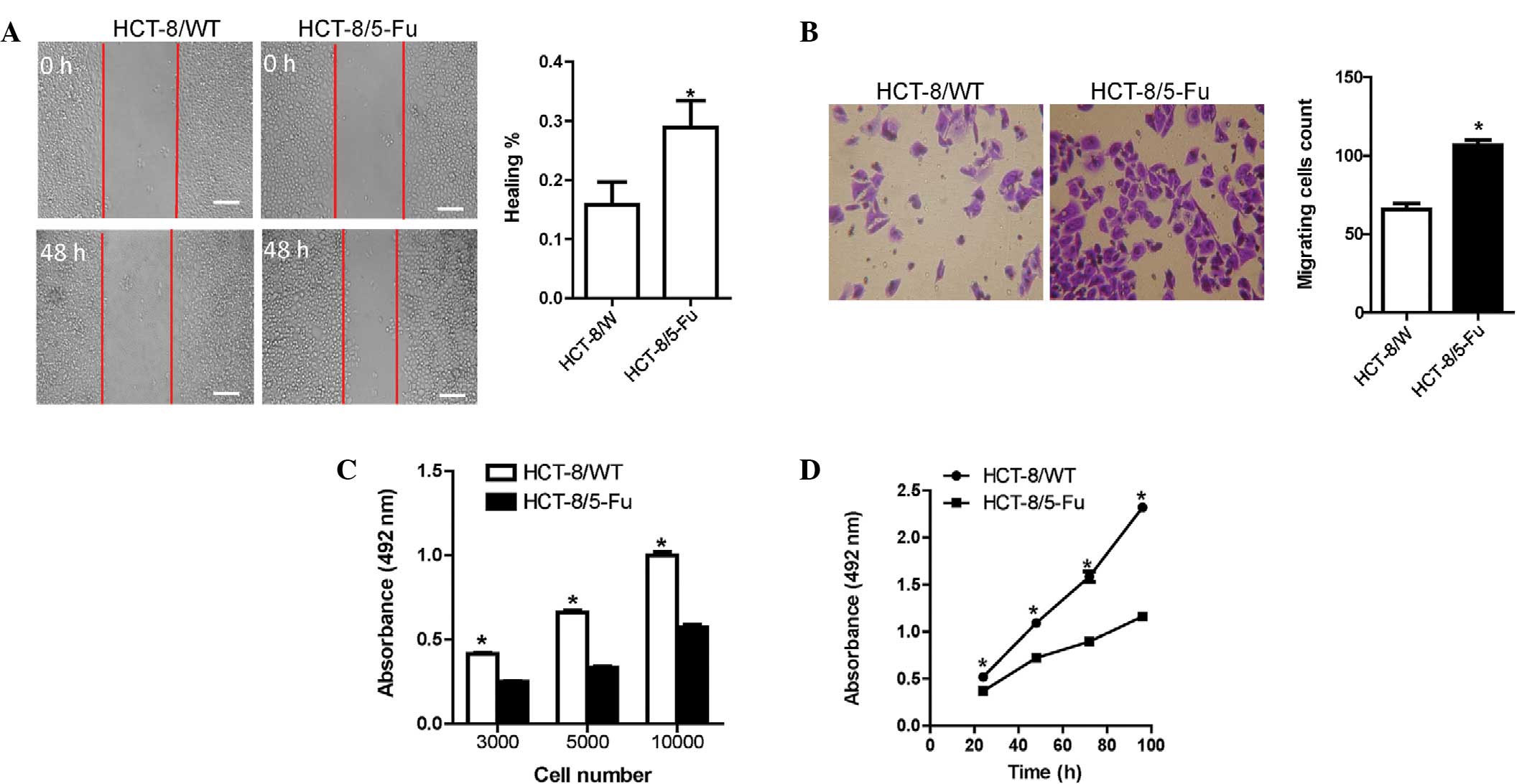
HCT-8/5-FU cells exhibit increased
migration but reduced proliferation
The HCT-8/5-FU cells exhibited a higher capacity for
wound-healing compared with the HCT-8/WT cells (Fig. 2A). In addition, the number of migrated
HCT-8/5-FU cells was significantly higher compared with the
HCT-8/WT cells (Fig. 2B).
The multiplication capacity of the two HCT-8 cell
lines was investigated using a modified MTT assay. Different
numbers of HCT-8/WT cells demonstrated increased proliferation
following a 48-h plating period compared with the HCT-8/5-FU cells
(Fig. 2C). Similar results were
observed for the proliferation assay at various times (Fig. 2D).
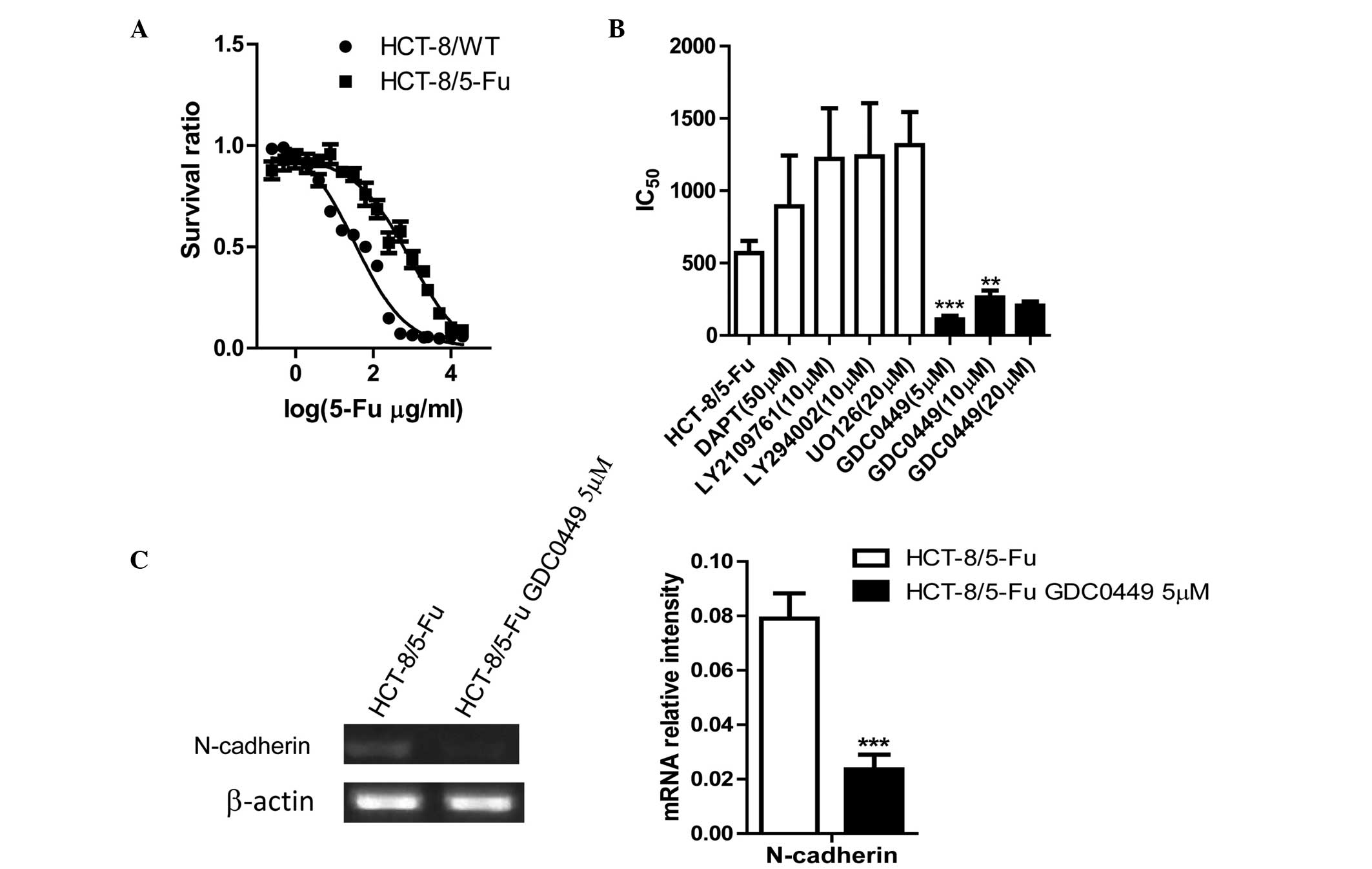
GDC0449, an inhibitor of the Hedgehog
signaling pathway, reverses drug resistance and EMT in HCT-8/FU
cells
MTT assays were performed in order to analyze 5-FU
resistance in the cell lines. The half maximal inhibitory
concentration (IC50) of the HCT-8/5-FU cells (899.2
µg/ml) was ~26-fold higher compared with that of HCT-8/WT cells
(33.53 µg/ml) (Fig. 3A).
Next, drug sensitivity was assessed following
exposure of HCT-8/5-FU cells to inhibitors of EMT-associated
signaling pathways. The Hedgehog pathway inhibitor, GDC0449, was
found to reverse drug resistance (Fig.
3B). The optimum concentration of GDC0449 was 5 µM, which
decreased the IC50 by ~8-fold compared with the
untreated HCT-8/5-FU cells (Fig.
3B).
RT-PCR was performed in order to confirm whether the
inhibitor, GDC0449, reversed the EMT signals. The EMT-associated
biomarker, N-cadherin, was downregulated following treatment with
GDC0449 (Fig. 3C).
Discussion
Chemoresistance limits the effectiveness of colon
cancer treatment. Therefore, it is important to identify the
mechanisms that are involved in drug resistance. The present study
used wild-type (HCT-8/WT) and 5-FU-resistant (HCT-8/5-FU)
colorectal cancer cells to investigate the molecular mechanisms and
cellular behaviors involved in 5-FU resistance.
The present study verified that HCT-8/5-FU cells
undergo EMT processes based on the following results: i) The
cellular morphology changed from adherent to scattered; ii) the
level of EMT-associated biomarkers were altered (E-cadherin
declined and vimentin increased); and iii) the migration potential
increased. These results are in accordance with those of previous
studies, which demonstrated that EMT markers change at the
molecular level in colon cancer cells (22,23).
Therefore, the present study provided evidence that 5-FU resistance
is associated with the EMT.
The EMT is a complex process that involves several
signaling pathways. A number of studies have reported an
overexpression of EMT-associated signaling pathways, including the
Notch, Wnt and nuclear factor-κB in human colon cancer cells
(24,25). In order to determine which pathway(s)
are involved in the process of drug resistance in HCT-8/5-FU cells,
the present study used inhibitors of the Notch (DAPT), TGF-β
(LY2109761), PI3K (LY294002), extracellular-signal-regulated kinase
(UO126) and Hedgehog (GDC0449) signaling pathways. The results
identified that the inhibitor of the Hedgehog signaling pathway,
GDC0449, was most effective at reversing drug resistance, with 5 µM
determined to be the optimal concentration of GDC0449. In addition,
EMT biomarkers were analyzed using RT-PCR. The results demonstrated
that the EMT-associated biomarker, N-cadherin, was
downregulated.
In conclusion, the present study revealed that 5-FU
induces an EMT phenotype in HCT-8/5-FU colon cancer cells and that
this change is associated with the Hedgehog signaling pathway. The
Hedgehog inhibitor, GDC0449, increased drug sensitivity. These
findings present a novel clinical therapy for the treatment of
colon cancer with acquired resistance to 5-FU.
Acknowledgements
This study was supported by grants from the Program
for New Century Excellent Talents in University of The Ministry of
Education of China (no. NCET-12-0880), the Fundamental Research
Funds for the Central Universities (no. JUSRP51311A), the China
National Natural Science Foundation grants (nos. 81100185 and
81273437) and an NSFC-RGC joint grant (no. 81361168001).
References
|
1
|
Cunningham D, Atkin W, Lenz HJ, et al:
Colorectal cancer. Lancet. 375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verdecchia A, Francisci S, Brenner H, et
al: EUROCARE-4 Working Group: Recent cancer survival in Europe: a
2000–02 period analysis of EUROCARE-4 data. Lancet Oncol.
8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed. Efficacy of adjuvant
fluorouracil and folinic acid in colon cancer. International
Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT)
investigators. Lancet. 345:939–944. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rutman RJ, Cantarow A and Paschkis KE:
Studies in 2-acetylaminofluorene carcinogenesis. III. The
utilization of uracil-2-C14 by preneoplastic rat liver and rat
hepatoma. Cancer Res. 14:119–123. 1954.PubMed/NCBI
|
|
5
|
Thomas DM and Zalcberg JR: 5-fluorouracil:
a pharmacological paradigm in the use of cytotoxics. Clin Exp
Pharmacol Physiol. 25:887–895. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noordhuis P, Holwerda U, Van der Wilt CL,
et al: 5-Fluorouracil incorporation into RNA and DNA in relation to
thymidylate synthase inhibition of human colorectal cancers. Ann
Oncol. 15:1025–1032. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giacchetti S, Perpoint B, Zidani R, et al:
Phase III multicenter randomized trial of oxaliplatin added to
chronomodulated fluorouracil-leucovorin as first-line treatment of
metastatic colorectal cancer. J Clin Oncol. 18:136–147.
2000.PubMed/NCBI
|
|
9
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Wever O, Pauwels P, De Craene B, et al:
Molecular and pathological signatures of epithelial-mesenchymal
transitions at the cancer invasion front. Histochem Cell Biol.
130:481–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosanò L, Cianfrocca R, Spinella F, et al:
Acquisition of chemoresistance and EMT phenotype is linked with
activation of the endothelin A receptor pathway in ovarian
carcinoma cells. Clin Cancer Res. 17:2350–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurokawa M, Ise N, Omi K, et al: Cisplatin
influences acquisition of resistance to molecular-targeted agents
through epithelial-mesenchymal transition-like changes. Cancer Sci.
104:904–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Sun Y, Chen L, et al: miRNA-200c
increases the sensitivity of breast cancer cells to doxorubicin
through the suppression of E-cadherin-mediated PTEN/Akt signaling.
Mol Med Rep. 7:1579–1584. 2013.PubMed/NCBI
|
|
15
|
Huang XY, Ke AW, Shi GM, et al:
αB-crystallin complexes with 14-3-3ξ to induce
epithelial-mesenchymal transition and resistance to sorafenib in
hepatocellular carcinoma. Hepatology. 57:2235–2247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Zeng R, Xu C, et al: Erbin
inhibits TGF-β1-induced EMT in renal tubular epithelial cells
through an ERK-dependent pathway. J Mol Med (Berl). 90:563–574.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conti B, Minutolo A, Arciello M and
Balsano C: Are Hedgehog and Wnt/β-catenin pathways involved in
hepatitis C virus-mediated EMT? J Hepatol. 58:636–637. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Zhou Y, Xie C, et al: Genome-wide
screening reveals an EMT molecular network mediated by Sonic
hedgehog-Gli1 signaling in pancreatic cancer cells. PLoS One.
7:e431192012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shan D, Chen L, Njardarson JT, et al:
Synthetic analogues of migrastatin that inhibit mammary tumor
metastasis in mice. Proc Natl Acad Sci USA. 102:3772–3776. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shan D, Chen L, Wang D, Tan YC, Gu JL and
Huang XY: The G protein G alpha(13) is required for growth
factor-induced cell migration. Dev Cell. 10:707–718. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S and Huang XY: Ca2+ influx through
L-type Ca2+ channels controls the trailing tail contraction in
growth factor-induced fibroblast cell migration. J Biol Chem.
280:27130–27137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Todosi AM, Gavrilescu MM, Aniţei GM, et
al: Colon cancer at the molecular level - usefulness of
epithelial-mesenchymal transition analysis. Rev Med Chir Soc Med
Nat Iasi. 116:1106–1111. 2012.PubMed/NCBI
|
|
23
|
Bhangu A, Wood G, Mirnezami A, Darzi A,
Tekkis P and Goldin R: Epithelial mesenchymal transition in
colorectal cancer: Seminal role in promoting disease progression
and resistance to neoadjuvant therapy. Surg Oncol. 21:316–323.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin H, Gong W, Zhang C and Wang S:
Epigallocatechin gallate inhibits the proliferation of colorectal
cancer cells by regulating Notch signaling. Onco Targets Ther.
6:145–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang FX, Deng AJ, Li M, Wei JF, Qin HL and
Wang AP: (3S)-1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid from
Cichorium endivia. L induces apoptosis of human colorectal cancer
HCT-8 cells. Molecules. 18:418–429. 2012. View Article : Google Scholar : PubMed/NCBI
|