Introduction
Oral cancer, a type of head and neck cancer, refers
to cancerous tissues growing in the oral cavity and may arise as a
primary lesion originating in any of the mouth tissues (1). Oral cancer may also occur due to
metastasis from a distant site of origin or by extension from a
neighboring anatomical structure. Approximately 90% of oral cancer
cases are squamous cell carcinomas that originate in tissues lining
the mouth and lips (2). Squamous cell
carcinoma is managed by surgery, radiotherapy and chemotherapy,
alone or in combination; however, the five-year survival rate is
poor, at ~50% (2). Therefore, novel
diagnostic and therapeutic methods to detect squamous cell
carcinomas at an early stage and increase the survival rate are
urgently required.
Apoptosis, a strictly controlled mechanism of cell
death, is triggered by internal or external signals. This process
is crucial in the development and maintenance of multicellular
organisms through the elimination of superfluous or unwanted cells
(3). Cells that are undergoing
apoptosis exhibit characteristic morphological and functional
changes, including reduction of the cell and nucleus sizes, plasma
membrane blebbing, chromatin condensation and DNA fragmentation
(4). The induction of apoptosis in
malignant cells is a potential target for the development of novel
antitumor drugs (5,6). Tumor-induced angiogenesis, a process
leading to the development of new blood vessels from pre-existing
vessels, is critical in the development and dissemination of the
majority of malignant tumors (7).
Therefore, the development of antitumor drugs that possess
anti-angiogenic activity, to inhibit tumor angiogenesis, and
antitumor activity, to induce apoptosis in cancer cells, may be a
valuable strategy in cancer treatment.
Tumstatin, a 28-kDa C-terminal globular
noncollagenous domain of the α3 chain of type IV collagen, is an
antiangiogenic agent with distinct antitumor activity (8,9). Previous
studies revealed that tumstatin inhibits tumor growth in several
tumor models of malignant melanoma and glioma (10–12) and
has been suggested as a potential candidate to inhibit angiogenesis
and tumor growth in head and neck cancer models. In addition,
tumstatin has been demonstrated to delay primary tumor growth and
lymph node metastasis in an orthotopic oral squamous carcinoma
AT-84 animal model (13); however,
the apoptotic mechanism of tumstatin in oral squamous carcinoma
cells has not been fully investigated. In the current study, the
activity of tumstatin in mouse squamous cell carcinoma SCC-VII
cells and the underlying apoptotic mechanism were investigated. In
addition, the antitumor activity of tumstatin was evaluated in an
SCC-VII animal model.
Materials and methods
Cell culture
Mouse squamous cell carcinoma SCC-VII cells were
provided by Dr Han-Sin Chung (Samsung Medical Center, Seoul,
Korea). SCC-VII cells were grown at 37°C in an atmosphere of 5%
CO2 in RPMI-1640 medium (Thermo Fisher Scientific Inc.,
Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum
(FBS; Thermo Fisher Scientific Inc.) and 1% (v/v)
penicillin-streptomycin (Thermo Fisher Scientific Inc.). Stably
transfected Drosophila melanogaster Schneider 2 (S2) cells
expressing recombinant tumstatin (14) were grown at 27°C in HyClone SFX-insect
medium (Thermo Fisher Scientific Inc.) containing 300 µg/ml
hygromycin B (Duchefa Biochemie, Haarlem, Netherlands). The study
was approved by the Ethics Committee of Kyung Hee University
(Yongin, Korea).
Cytotoxicity assay
The cytotoxicity of recombinant tumstatin was
measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich, St. Louis, MO, USA) colorimetric assay. SCC-VII
cells were seeded into 6-well plates (Nunc A/S, Roskilde, Denmark)
at a density of 1×105 cells/well in 2 ml RPMI-1640
supplemented with 10% FBS, and incubated for 24 h. The cells were
then treated with RPMI-1640 containing 2% FBS and various
concentrations of recombinant tumstatin (1, 5, 10, 20 and 30
µg/ml). An equal volume of 30 mM HEPES (Polysciences, Inc.,
Warrington, PA, USA) was used as a negative control. After a 24-h
incubation period, 50 µl MTT [5 µg/ml in phosphate-buffered saline
(PBS)] was added to each well, and the cells were further incubated
at 37°C for 2 h. Subsequently, the medium was replaced with 100 µl
of dimethyl sulfoxide (Duchefa Biochemie) and the plate was
incubated for 5 min prior to measuring the optical density (OD) at
550 nm using an EL800 Universal Microplate Reader (BioTek
Instruments Inc., Winooski, VT, USA). Cell viability was calculated
as the percentage of viable cells in the recombinant
tumstatin-treated group relative to the control group, according to
the following equation: Cell viability (%) = [(ODRecombinant
tumstatin-ODBlank)/(ODControl-ODBlank)]
× 100.
Confocal microscopy
SCC-VII cells were seeded into 6-well plates (Nunc
A/S) at a density of 1×105 cells/well and incubated for
24 h. The cells were treated with RPMI-1640 containing 2% FBS and
various concentrations of recombinant tumstatin (1, 5, 10, 20 and
30 µg/ml) for 24 h. Next, the cells were harvested, washed with
ice-cold PBS buffer, resuspended in 20 µl PBS buffer and stained
with 0.2 µM YO-PRO-1 (Molecular Probes Inc., Eugene, OR, USA) for
20 min on ice in the dark. The fluorescence of the stained cells
was imaged under x40 objective magnification using a confocal laser
scanning microscope (LSM 510 Meta; Carl Zeiss, Oberkochen, Germany)
with a 488 nm argon laser, 543 nm HeNe laser and 633 nm HeNe
laser.
Cell cycle analysis
SCC-VII cells were seeded into 75 cm2
tissue culture flasks (T-75 flask; Nunc A/S) at a density of
1×106 cells/flask and incubated for 24 h in RPMI-1640
medium containing 2% FBS. Following treatment with various
concentrations of recombinant tumstatin (1, 5, 10, 20 and 30 µg/ml)
for 24 h, the cells were washed twice with ice-cold PBS buffer, and
cell pellets were fixed in 70% (v/v) cold ethanol overnight at
−20°C. The fixed cells were centrifuged at 700 × g for 5 min,
washed, resuspended in 100 µl PBS containing 1 mM RNase A (Qiagen,
Hilden, Germany) and then incubated for 30 min at 37°C. Next, the
cells were stained by incubation with 400 µl propidium iodide (PI;
50 µg/ml; Sigma-Aldrich) for 30 min in the dark and subsequently
filtered through a 40 µm nylon mesh (BD Biosciences, San Jose, CA,
USA). The DNA content of the stained cells was analyzed using a
FACSVantage SE flow cytometry system and CellQuest program (BD
Biosciences).
DNA fragmentation assay
SCC-VII cells were plated at a density of
1×106 cells/T-75 flask and cultured for 24 h. The cells
were treated with RPMI-1640 containing 2% FBS and 20 µg/ml
recombinant tumstatin for 6, 12, 24 and 48 h. Genomic DNA was
prepared using a QIAamp DNA Mini Kit (Qiagen) according to the
manufacturer's instructions. Next, the isolated genomic DNA samples
were subjected to electrophoresis on a 1.8% agarose gel at 50 V for
1.5 h. The gel was stained with ethidium bromide (EtBr;
Sigma-Aldrich) and visualized using an ultraviolet (UV)
transilluminator (Wealtech Corp., Reno, NV, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
SCC-VII cells were plated at a density of
1×106 cells/T-75 flask and cultured for 24 h. Next, the
cells were treated with RPMI-1640 medium containing 2% FBS and 20
µg/ml recombinant tumstatin for 12 h. Total RNA was isolated using
TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to
the manufacturer's instructions. Total RNA (2 mg) was then treated
with RNase-free DNase I (Life Technologies) and transcribed into
cDNA using an ImProm-II™ Reverse Transcription System (Promega
Corporation, Madison, WI, USA) in 20 ml reaction mixtures
containing oligo-dT primers according to the manufacturer's
protocols. PCR was performed with 2 ml cDNA and specific primers
using an LA Taq DNA Polymerase kit (Takara Bio Inc., Shiga, Japan).
The sense and antisense primers used were as follows: Fas
sense, 5′-TGCTCAGAAGGATTATATCAAGGAG-3′, and antisense,
5′-CGGGATGTATTTACTCAAGCTAAGA-3′; β-actin sense,
5′-AGAGAGGTATCCTGACCCTGAAGTA-3′, and antisense,
5′-AGTCTAGACGAACATAGCACAGCTT-3′. The PCR program consisted of an
initial denaturation at 94°C for 5 min, followed by 30 cycles of
94°C for 1 min, 55°C for 1 min and 72°C for 1 min, with a final
extension at 72°C for 10 min. Equal volumes of the PCR products
were separated on 1% agarose gels and visualized under UV light
after staining with EtBr. PCR products were quantified using the
Image J program (National Institutes of Health, Bethesda, MD,
USA).
Western blot analysis
SCC-VII cells were incubated in RPMI-1640 medium
containing 2% FBS and 20 µg/ml recombinant tumstatin for 6, 12, 24
and 48 h and then lysed with radioimmunoprecipitation assay buffer
(Thermo Fisher Scientific Inc.) supplemented with a protease
inhibitor cocktail (Roche, Basel, Switzerland). An RC/DC Bio-Rad
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used
to determine the protein concentration, according to the
manufacturer's instructions. Electrophoresis was then used to
separate the protein samples on a 10% polyacrylamide-sodium dodecyl
sulfate gel, and the samples were subsequently transferred onto a
polyvinylidene fluoride membrane (Pall Corporation, Port
Washington, NY, USA). The membranes were pre-incubated in blocking
solution [5% (w/v) skimmed milk in Tris-buffered saline containing
0.1% Tween-20] for 1 h, incubated with a rabbit polyclonal
anti-human Fas antibody (1:2,000 dilution in blocking solution) or
mouse monoclonal anti-avian β-actin (cat. no. sc-7886 and sc-47778,
respectively; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C. Subsequently, the samples were probed with
peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G
antibody (1:5,000 dilution in blocking solution; Santa Cruz
Biotechnology, Inc.). An enhanced chemiluminescence western
blotting detection reagent was applied to allow detection of the
protein bands (GE Healthcare Life Sciences, Stockholm, Sweden).
Tumor growth in an in vivo SCC-VII
animal model
A total of 18 five-week-old female C3H/HeJ mice with
an average weight of 20 g (purchased from Orient Bio Inc.,
Seongnam, Korea) were quarantined in a specific pathogen-free
environment, with access to water and food ad libitum and a
12 h light/12 h dark cycle. The mice were kept in an animal care
facility accredited by the Kyung Hee University Institutional
Animal Care and Use Committee. Animal care and experimental
procedures adhered to the Kyung Hee University guidelines for the
care and use of laboratory animals. To establish an in vivo
SCC-VII animal model, 1×106 SCC-VII cells in 100 µl PBS
were subcutaneously injected into the right flank of the C3H/HeJ
mice. After five days, when the tumors formed visible masses, the
animals were divided into three groups, each containing six mice.
Each group was treated at three-day intervals with a peritumoral
injection of recombinant tumstatin (2.5 or 10 mg/kg in PBS) or PBS
(control) over a period of nine days. All the mice were euthanized
using CO2, 14 days after tumor inoculation and the
tumors were excised and weighed. A caliper was used to determine
the length and width of the tumors, and the tumor volume was
calculated using the standard formula: length × width2 ×
0.5 (15).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Student's t-test was used to compare different data
groups and all the data were analyzed using Microsoft Excel 2010
software (Microsoft Corporation, Redmond, WA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Recombinant tumstatin decreases the
viability of SCC-VII cells
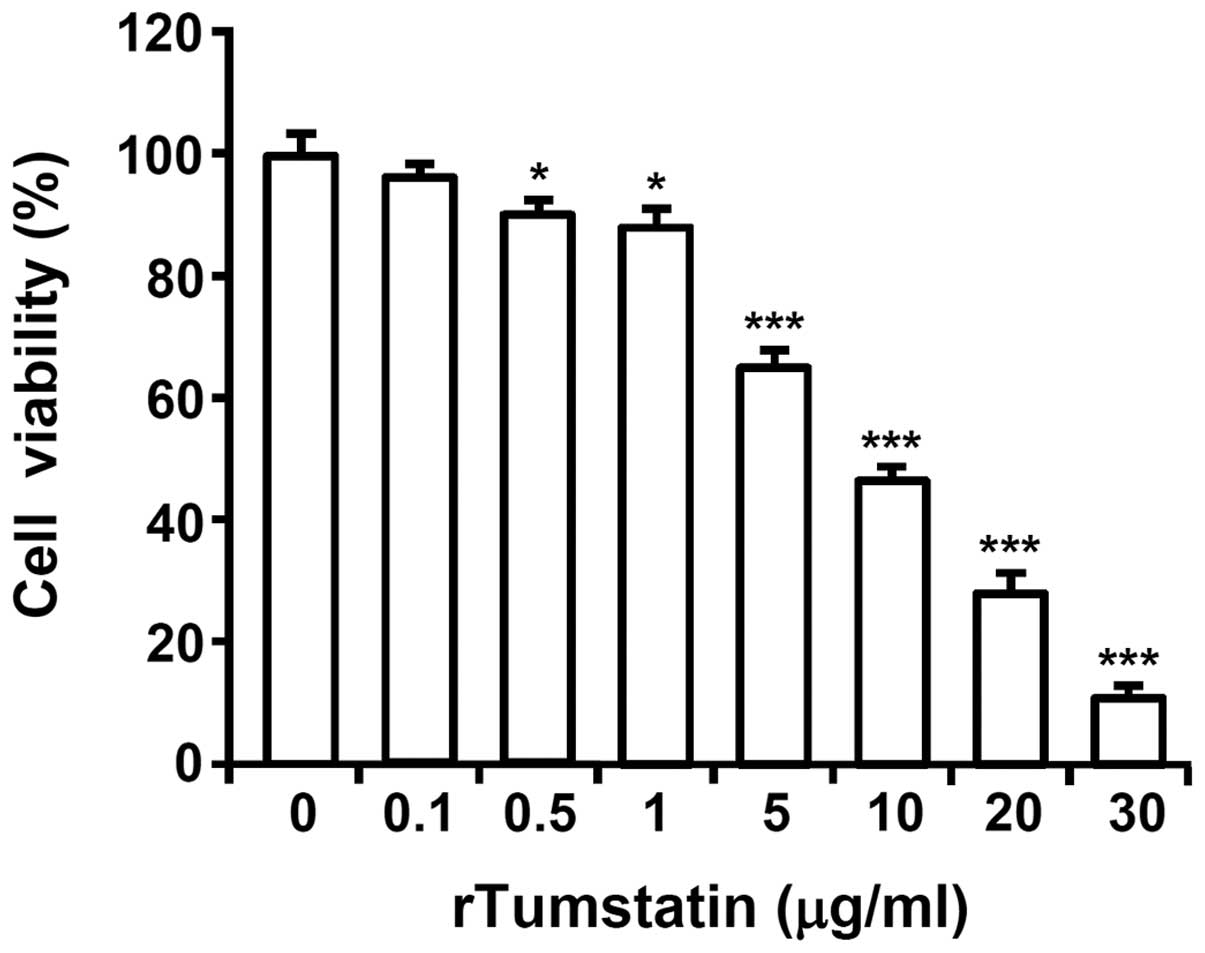
To evaluate the cytotoxic effect of recombinant
tumstatin, SCC-VII cells were treated with various concentrations
(0, 0.1, 0.5, 1, 5, 10, 20 and 30 µg/ml) of recombinant tumstatin
for 24 h, and cell viabilities were evaluated by an MTT assay.
Recombinant tumstatin decreased the viability of SCC-VII cells in a
dose-dependent manner. Following treatment with 30 µg/ml
recombinant tumstatin for 24 h, the viability of SCC-VII cells
decreased to 10% of that for untreated control cells (P= 0.000026).
The 50% inhibitory concentration value of recombinant tumstatin was
~9 µg/ml (Fig. 1).
Recombinant tumstatin induces
apoptosis in SCC-VII cells
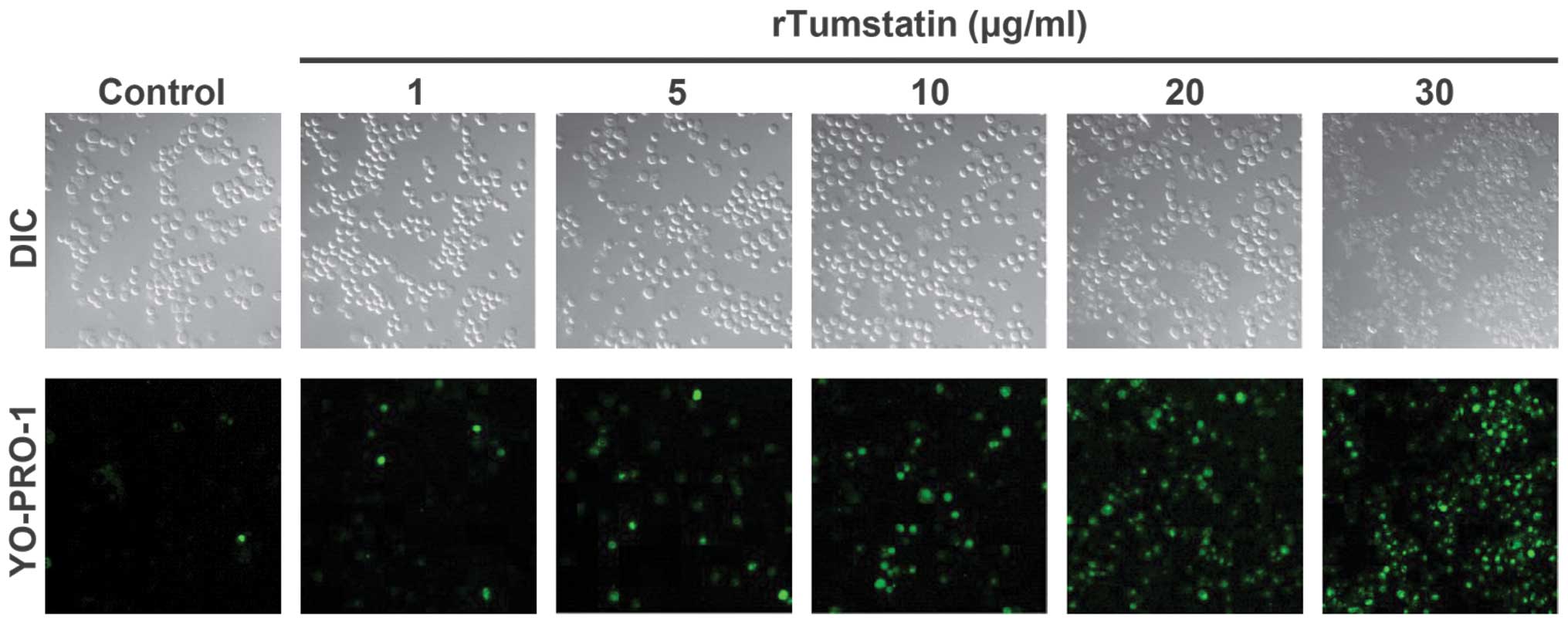
To determine whether the cytotoxic effect of
recombinant tumstatin was caused by apoptosis, recombinant
tumstatin-treated SCC-VII cells were stained with the cell
permeable green fluorescence dye, YO-PRO-1, as a marker of
apoptosis and monitored under a confocal microscope (Fig. 2). Recombinant tumstatin increased the
number of YO-PRO-1-stained cells in a dose-dependent manner. Cell
cycle analysis was performed to determine the sub-G1 apoptotic
population of recombinant tumstatin-treated SCC-VII cells. The
cells were treated with various concentrations (1, 5, 10, 20, and
30 µg/ml) of recombinant tumstatin for 24 h and their DNA contents
were analyzed by flow cytometry following PI staining. As shown in
Fig. 3A, the number of cells in the
sub-G1 population increased in a dose-dependent manner following
treatment with recombinant tumstatin. After 24 h of incubation with
recombinant tumstatin at a concentration of 1, 5, 10, 20 or 30
µg/ml, the number of cells in the sub-G1 population increased to
0.37, 0.51, 3.13, 15.65 and 46.81% of total cells, respectively
(Fig. 3B). The induction of apoptosis
by recombinant tumstatin was further characterized by a DNA
fragmentation assay. Genomic DNA was purified from SCC-VII cells
following treatment with 20 µg/ml recombinant tumstatin for the
indicated times (6, 12, 24 and 48 h) and subjected to agarose gel
electrophoresis to assess DNA fragmentation (Fig. 3C). DNA fragmentation was observed in
recombinant tumstatin-treated cells and increased in a
time-dependent manner. These results indicate that recombinant
tumstatin induces apoptosis in SCC-VII cells.
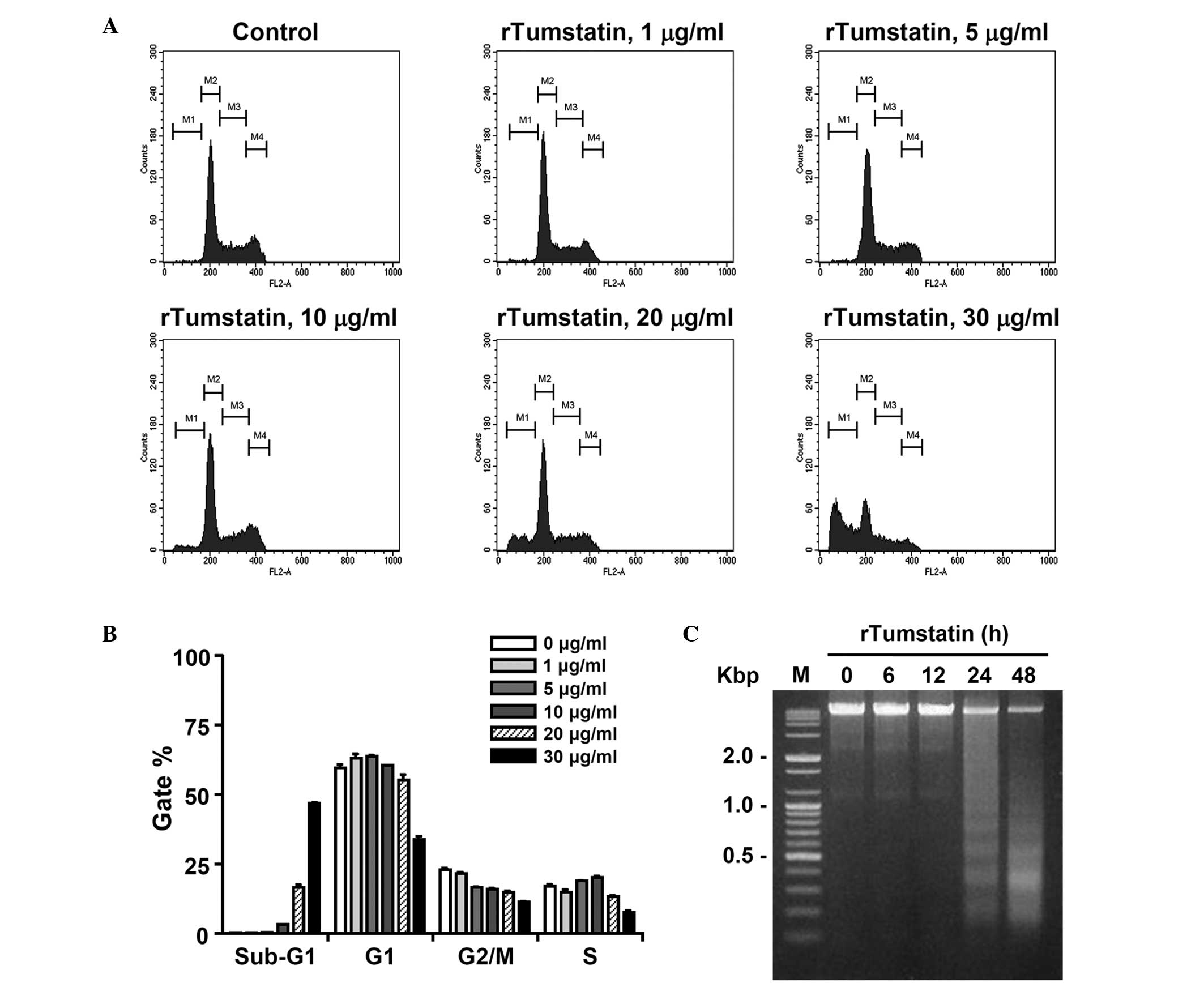 | Figure 3.Flow cytometric analysis of sub-G1
cell populations and DNA fragmentation of rTumstatin-treated
SCC-VII cells. (A) DNA content of SCCVII cells treated with
different concentrations (1, 5, 10, 20, and 30 µg/ml) of rTumstatin
for 24 h, measured using a flow cytometer subsequent to staining
with propidium iodide. (B) Cell cycle distribution data from three
independent experiments, performed as described in (A). (C) DNA
fragmentation of SCC-VII cells that were treated with 20 µg/ml
recombinant tumstatin for the indicated times (6, 12, 24 and 48 h)
and analyzed by agarose gel electrophoresis. rTumstatin,
recombinant tumstatin; M, 100 bp DNA ladder size marker. |
The induction of apoptosis in SCC-VII
cells by tumstatin is mediated by Fas
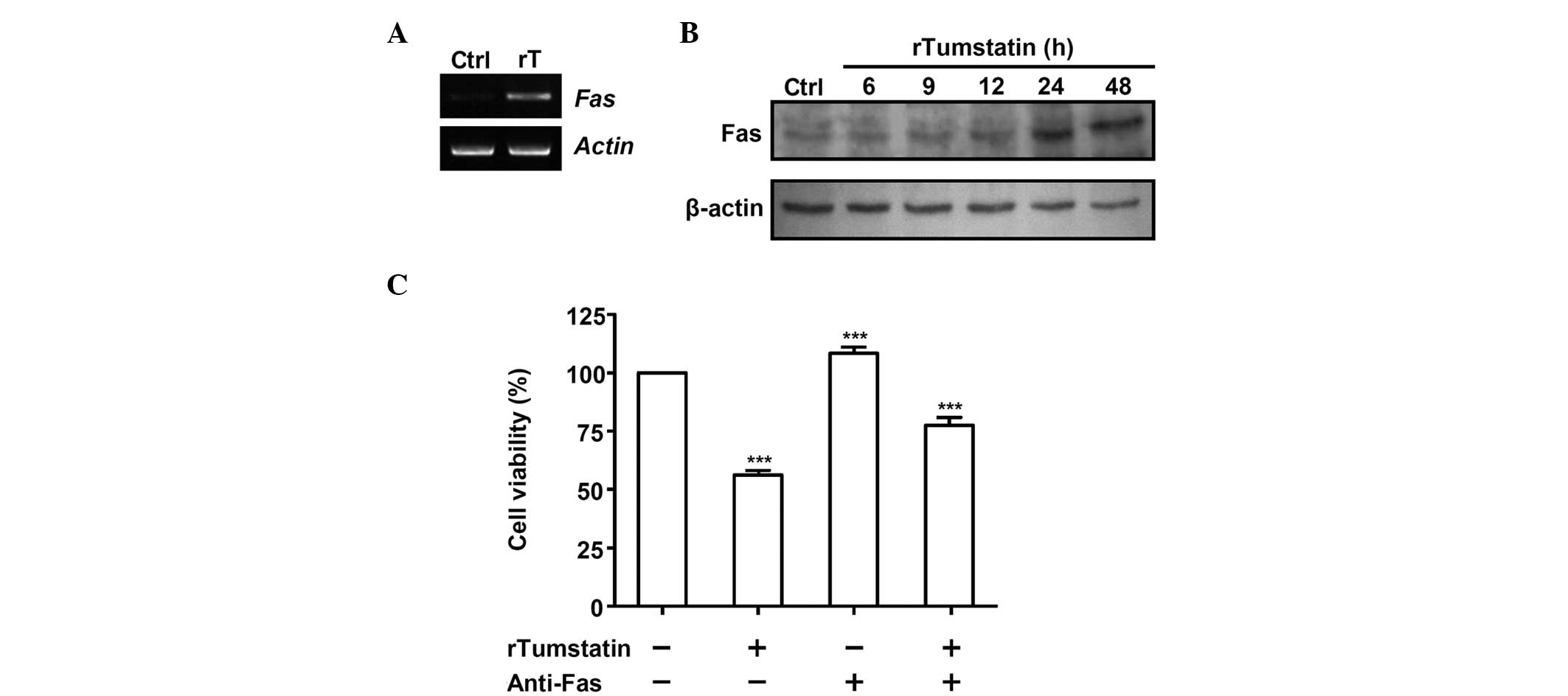
Expression of the Fas gene in recombinant
tumstatin-treated SCC-VII cells was determined by RT-PCR (Fig. 4A). Total RNA samples were prepared
from the SCC-VII cells following treatment with 20 µg/ml
recombinant tumstatin for 12 h. The level of Fas transcript
was markedly increased in the SCC-VII cells treated with
recombinant tumstatin compared with that of untreated control
cells, based on β-actin expression that was used as an internal
control. The induction of Fas expression by recombinant
tumstatin was further confirmed by western blot analysis, which
revealed that treatment with recombinant tumstatin increased the
protein level expression of Fas (Fig.
4B); following treatment of SCC-VII cells with 20 µg/ml
recombinant tumstatin for 24 or 48 h, the expression of Fas protein
increased by 2.8- and 3-fold, respectively, relative to that of
control cells. These data indicate that recombinant tumstatin
increases the expression of Fas in SCC-VII cells.
Cell viability was determined in SCC-VII cells
following treatment with recombinant tumstatin in the presence or
absence of anti-Fas antibody (Fig.
4C). The presence of anti-Fas marginally increased the
viability of recombinant tumstatin-treated SCC-VII cells: When
SCC-VII cells were treated for 12 h with 20 µg/ml recombinant
tumstatin in the absence of anti-Fas, the cell viability decreased
to 56.3% of that for untreated control cells. Following treatment
with 20 µg/ml recombinant tumstatin in the presence of anti-Fas,
the cell viability decreased to 77.6% of that for untreated control
cells. Therefore, in anti-Fas-treated SCC-VII cells, the decrease
in cell viability due to recombinant tumstatin was diminished by
49% (P=0.0003), indicating that anti-Fas attenuated the apoptotic
effects of recombinant tumstatin in the SCC-VII cells.
Recombinant tumstatin inhibits tumor
growth in an SCC-VII animal model
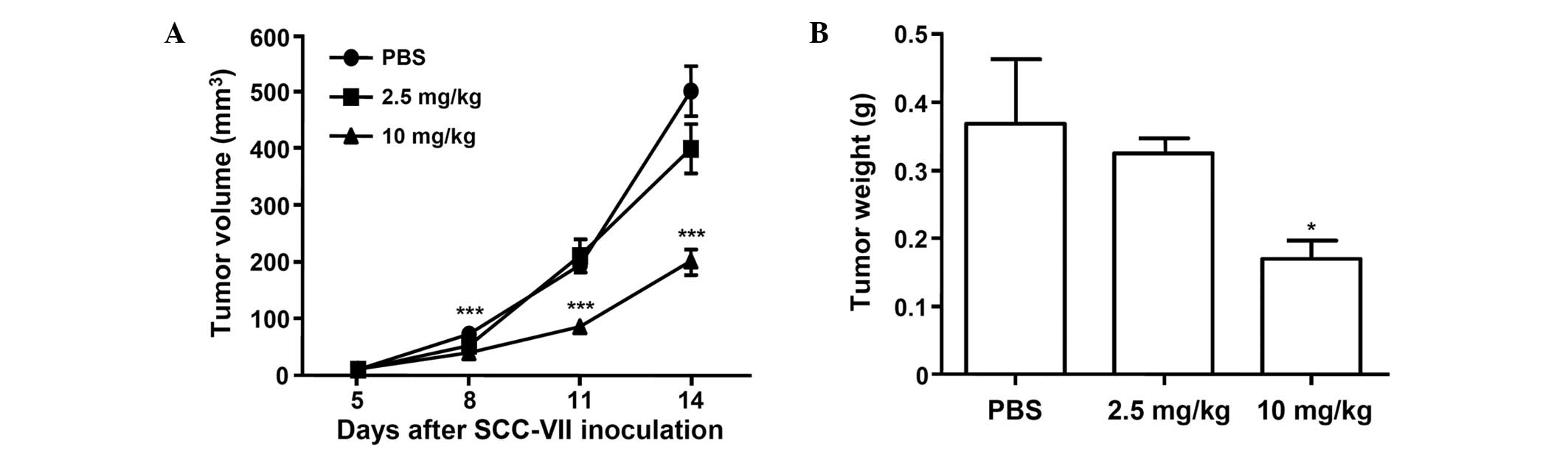
The antitumor activity of recombinant tumstatin was
examined in an SCC-VII animal model using C3H/HeJ mice. At five
days after subcutaneous implantation of SCC-VII cells, the mean
tumor volume was ~10 mm3. C3H/HeJ mice were divided into
groups and injected with recombinant tumstatin for nine days. No
acute side effects, such as body weight loss, hair loss, lethargy
or mortality, were observed (data not shown). In the control
(PBS-treated) group, the tumors grew rapidly and reached an average
volume of 501±43.9 mm3 (mean ± SD) on day 14 after
inoculation with SCC-VII cells (Fig.
5A). The mean size of the primary tumor in animals treated with
2.5 and 10 mg/kg recombinant tumstatin was determined to be to 79.6
and 40.3% (399±43.6 and 202±20.2 mm3, respectively) of
that in the control group at 14 days. Similarly, the mean tumor
weight for the animals treated with 2.5 and 10 mg/kg recombinant
tumstatin was 88.2 and 46.2% of that in the control group,
respectively (Fig. 5B). Together,
these results indicate that recombinant tumstatin inhibited tumor
growth in the SCC-VII animal model.
Discussion
The present study examined the effect of tumstatin
on the viability of mouse squamous cell carcinoma SCC-VII cells and
investigated the mechanism involved in tumstatin-induced apoptosis.
Recombinant tumstatin, expressed and purified from stably
transfected D. melanogaster S2 cells (14), was found to inhibit the viability of
SCC-VII cells in a dose-dependent manner (Fig. 1) and induce apoptosis, as
characterized by confocal microscopic analysis of YO-PRO-1-stained
cells (Fig. 2), an increase in the
sub-G1 apoptotic cell population (Fig. 3A
and B) and DNA fragmentation (Fig.
3C).
Tumstatin combines with an
αvβ3 integrin and suppresses the activity of
focal adhesion kinase, phosphatidylinositol 3-kinase and protein
kinase B, resulting in the inhibition of integrin-induced
cap-dependent protein synthesis and suppression of endothelial cell
proliferation (16,17). The T7 peptide of tumstatin, a 25-amino
acid region encompassing amino acids 74–98, has been reported to be
responsible for the anti-angiogenic activity associated with
tumstatin, eventually causing the suppression of tumor growth
(18). Tumstatin also exerts direct
antitumor activity by suppressing tumor cell proliferation and
inducing tumor cell apoptosis. Peptide 19, encompassing amino acids
185–203, is responsible for the antitumor activity of tumstatin
(8). In addition, peptide 19 has been
demonstrated to inhibit the activation of polymorphonuclear
leukocytes (19) and inhibit the
proliferation of human gastric cancer SGC-7901 cells and hepatoma
HepG2 cells to induce apoptosis (20,21).
Furthermore, previous studies have reported that peptide 19
increased the expression of Fas, Fas ligand, and caspase-3 in
orthotopic SGC-7901-induced tumor tissues (20) and upregulated the expression of
caspase-9, Fas, p53, Bax and Bid in human hepatoma cells (21).
In our preliminary experiments that led to the
present study, the expression of apoptosis-associated genes was
assessed by microarray analysis using an Agilent mouse cDNA chip
and total RNA prepared from tumstatin-treated SCC-VII cells.
Expression of the Fas gene increased by 1.73-fold in the
SCC-VII cells following treatment with 20 µg/ml recombinant
tumstatin for 12 h (data not shown). Semi-quantitative RT-PCR and
western blot analysis confirmed that treatment with recombinant
tumstatin increased the levels of Fas transcript and protein
(Fig. 4A and B). In addition,
treatment with anti-Fas increased the viability of SCC-VII cells
treated with recombinant tumstatin compared with that of cells
treated only with recombinant tumstatin (Fig. 4C). Fas is a death domain-containing
member of the tumor necrosis factor receptor superfamily that plays
a central role in the physiological regulation of apoptosis
(22). The Fas-mediated apoptosis
pathway involves the activation of procaspases. In particular, the
activation of procaspase-8 induces the cleavage of the proapoptotic
Bcl-2 family member, Bid (23). This
triggers the oligomerization of the apoptotic Bcl-2 family members,
Bak or Bax, and the release of mitochondrial cytochrome c.
The released cytochrome c forms an apoptosome with Apaf-1,
resulting in the sequential activation of procaspase-9 and -3. In
our preliminary study, the microarray analysis results revealed
that Fas signaling pathway-associated genes, including Fas,
caspase-3, caspase-8, caspase-9 and Bid, were
upregulated by recombinant tumstatin; by contrast, the
antiapoptotic Bcl-2 gene was downregulated (data not shown).
Therefore, these results indicate that tumstatin induces apoptosis
in SCC-VII cells through the Fas signaling pathway.
In the present study, the antitumor activity of
recombinant tumstatin was also investigated in a SCC-VII animal
model using C3H/HeJ mice. Tumstatin has been reported to suppress
the tumor growth of human renal cell carcinoma (786-O cells) and
prostate carcinoma (PC-3 cells) in mouse xenograft models (8,24).
Tumstatin has also been demonstrated to inhibit tumor growth in
malignant melanoma, glioma and subcutaneous glioblastoma animal
models (10–12,25). In an
orthotopic oral squamous carcinoma AT-84 animal model, treatment
with medium containing tumstatin delayed primary tumor growth and
lymph node metastasis; however, the effects of tumstatin on
suppressing the growth of AT-84-induced tumors were weak compared
with tumstatin-free media (13). By
contrast, in the present study, recombinant tumstatin effectively
reduced the tumor size and weight in the SCC-VII animal model
(Fig. 5), indicating that tumstatin
exhibits an antitumor activity that suppresses the tumor growth of
squamous cell carcinoma cells.
In conclusion, the results of the current study
demonstrated that tumstatin induced apoptosis and inhibited
proliferation of mouse oral squamous cell carcinoma SCC-VII cells
in vitro, and inhibited tumor growth in vivo.
Furthermore, the tumstatin-induced apoptosis in SCC-VII cells was
found to be mediated by the Fas signaling pathway. These findings
implicate tumstatin as a candidate antitumor drug for the treatment
of squamous cell carcinomas.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education, Science and Technology (grant
no. NRF-2013RA1A2062398).
References
|
1
|
Koch WM, Stafford E and Bajaj G: Cancer of
the oral cavity. Part A: General principles and managementHead and
neck cancer: A multidisciplinary approach. Harrison LB, Sessions RB
and Hong WK: 3rd. Lippincott Williams and Wilkins; Philadelphia,
PA: pp. 250–265. 2009
|
|
2
|
Feller L and Lemmer J: Oral squamous cell
carcinoma: Epidemiology, clinical presentation and treatment. J
Cancer Ther. 3:263–268. 2012. View Article : Google Scholar
|
|
3
|
Raff MC, Barres BA, Burne JF, Coles HS,
Ishizaki Y and Jacobson MD: Programmed cell death and the control
of cell survival: Lessons from the nervous system. Science.
262:695–700. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980.PubMed/NCBI
|
|
5
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boedefeld WM 2nd, Bland KI and Heslin MJ:
Recent insights into angiogenesis, apoptosis, invasion and
metastasis in colorectal carcinoma. Ann Surg Oncol. 10:839–851.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeshima Y, Colorado PC, Torre A, Holthaus
KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE and
Kalluri R: Distinct antitumor properties of a type IV collagen
domain derived from basement membrane. J Biol Chem.
275:21340–21348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamano Y and Kalluri R: Tumstatin, the NC1
domain of α3 chain of type IV collagen, is an endogenous inhibitor
of pathological angiogenesis and suppresses tumor growth. Biochem
Biophys Res Commun. 333:292–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pasco S, Brassart B, Ramont L, Maquart FX
and Monboisse JC: Control of melanoma cell invasion by type IV
collagen. Cancer Detect Prev. 29:260–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pasco S, Ramont L, Venteo L, Pluot M,
Maquart FX and Monboisse JC: n vivo overexpression of
tumstatin domains by tumor cells inhibits their invasive properties
in a mouse melanoma model. Exp Cell Res. 301:251–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawaguchi T, Yamashita Y, Kanamori M,
Endersby R, Bankiewicz KS, Baker SJ, Bergers G and Pieper RO: The
PTEN/Akt pathway dictates the direct alphaVbeta3-dependent
growth-inhibitory action of an active fragment of tumstatin in
glioma cells n vitro and n vivo. Cancer Res.
66:11331–11340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung IS, Son YI, Ko YJ, Baek CH, Cho JK
and Jeong HS: Peritumor injections of purified tumstatin delay
tumor growth and lymphatic metastasis in an orthotopic oral
squamous cell carcinoma model. Oral Oncol. 44:1118–1126. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeon HK, Chang KH, Kim KI and Chung IS:
Functional expression of recombinant tumstatin in stably
transformed Drosophila melanogaster S2 cells. Biotechnol Lett.
25:185–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alessandri G, Filippeschi S, Sinibaldi P,
Mornet F, Passera P, Spreafico F, Cappa PM and Gullino PM:
Influence of gangliosides on primary and metastatic neoplastic
growth in human and murine cells. Cancer Res. 47:4243–4247.
1987.PubMed/NCBI
|
|
16
|
Maeshima Y, Colorado PC and Kalluri R: Two
RGD-independent alpha v beta 3 integrin binding sites on tumstatin
regulate distinct anti-tumor properties. J Biol Chem.
275:23745–23750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeshima Y, Sudhakar A, Lively JC, Ueki K,
Kharbanda S, Kahn CR, Sonenberg N, Hynes RO and Kalluri R:
Tumstatin, an endothelial cell-specific inhibitor of protein
synthesis. Science. 295:140–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maeshima Y, Yerramalla UL, Dhanabal M, et
al: Extracellular matrix-derived peptide binds to alpha(v)beta (3)
integrin and inhibits angiogenesis. J Biol Chem. 276:31959–31968.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monboisse JC, Garnotel R, Bellon G, Ohno
N, Perreau C, Borel JP and Kefalides NA: The alpha 3 chain of type
IV collagen prevents activation of human polymorphonuclear
leukocytes. J Biol Chem. 269:25475–25482. 1994.PubMed/NCBI
|
|
20
|
Li YJ, Sun LC, He Y, Liu XH, Liu M, Wang
QM and Jin XM: The anti-tumor properties of two tumstatin peptide
fragments in human gastric carcinoma. Acta Pharmacol Sin.
30:1307–1315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Li J, Xu H, Zhang Y, Liu Y and Liu
X: Mitochondria-mediated tumstatin peptide-induced HepG2 cell
apoptosis. Int J Mol Med. 24:653–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López-Hernández FJ, Ortiz MA and
Piedrafita FJ: The extrinsic and intrinsic apoptotic pathways are
differentially affected by temperature upstream of mitochondrial
damage. Apoptosis. 11:1339–1347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeshima Y, Manfredi M, Reimer C, Holthaus
KA, Hopfer H, Chandamuri BR, Kharbanda S and Kalluri R:
Identification of the anti-angiogenic site within vascular basement
membrane-derived tumstatin. J Biol Chem. 276:15240–15248. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oliveira-Ferrer L, Wellbrock J, Bartsch U,
Penas EM, Hauschild J, Klokow M, Bokemeyer C, Fiedler W and Schuch
G: Combination therapy targeting integrins reduces glioblastoma
tumor growth through antiangiogenic and direct antitumor activity
and leads to activation of the pro-proliferative prolactin pathway.
Mol Cancer. 12:1442013. View Article : Google Scholar : PubMed/NCBI
|