Introduction
Prostate cancer is one of the most common
malignancies in male patients of advanced age and is the second
leading cause of cancer-associated mortality worldwide (1,2). The
incidence of prostate cancer has increased in China in recent years
(3), and the majority of cases is
diagnosed in the metastatic stages of the disease, and have thus
lost the opportunity for radical surgery (4,5). The use
of prostate cancer biomarkers, including the prostate-specific
antigen (PSA), and Gleason scores has revolutionized the screening,
detection and determination of prognosis of this disease (6). It would be informative to specifically
block immune biomarkers or molecules which could promote the
progression of PCa. However, reliable biomarkers associated with
the immune response of PCa are not established at present (7). Therefore, identification of immune
biomarkers of invasion and metastasis is required for the diagnosis
and prognosis of patients with prostate cancer.
Macrophages are the most abundant cancer stromal
cells involved in the host immune system (8). Previous studies have reported that
tumor-associated macrophages (TAMs) originate from circulating
monocytes and are a key component of the inflammatory
microenvironment (9–11). TAMs are recruited and maintained in
neoplastic tissues by various chemokines and cytokines. Recent
evidence also suggests that TAMs may be involved in cancer
progression as they release cytokines, extracellular matrix
proteins and growth factors [including transforming growth factor
(TGF)-β and vascular endothelial growth factor] that promote
proliferation, angiogenesis, and metastasis (9–11). In a
number of cancer types, including breast, colon and bladder cancer,
TAM infiltration has been found to be associated with poor outcome
(12–14).
Macrophages differentiate into functionally distinct
immunological populations depending on the cytokine environment.
Classically activated macrophages (CAMs) are considered to be
induced by interferon γ and lipopolysaccharides, which function
predominantly in inflammation, tissue damage and the killing of
intracellular microbes (15–17). However, alternatively activated
macrophages (AAMs) are induced under the influence of T-helper type
2 cytokines, particularly interleukin (IL)-4 and IL-13. This
phenotype features the induction of arginase 1 and upregulates the
expression of surface molecules, including CD206 (also known as
mannose receptor), and scavenger receptors (CD163) (14). Notably, this particular macrophage
subset is the predominant source of cytokines and chemokines,
including IL-10, TGF-β and chemokine C-C motif ligand 18 (CCL18).
Through such cytokine pathways, AAM may be involved in the
downregulation of inflammation, tissue remodeling and elimination
of tissue debris and apoptotic bodies, as well as tumor progression
(15–18).
Certain studies have demonstrated that the number
and density of AAMs in cancer tissues is highly increased when
compared with normal tissues, and is associated with the prognosis.
In addition, two recent studies have indicated that TAMs and AAMs
participated in the progression of prostate cancer (19–21).
However, the association between differentiation of macrophages and
metastasis of prostate adenocarcinoma (PCa) is not
well-established. Therefore, we hypothesized that AAMs may
contribute to the metastasis of PCa and are associated with
prognosis. In the present study, TAMs and AAMs were analyzed in
prostate tissues of PCa by immunofluorescence. In addition, the
association of TAMs and AAMs with the clinicopathological features
and outcome in these patients were investigated.
Patients and methods
Patients
Between January 2008 and March 2009, a total of 42
patients with PCa underwent surgery or needle aspiration biopsy
with diagnostic or curative intent in Guangdong General Hospital
(Guangzhou, China). The patients were followed up for 5 years in
order to obtain a complete set of clinical data. The inclusion
criteria for the study was: i) Patients who were diagnosed with PCa
with or without metastasis; ii) aged between 60 and 85 years old;
and iii) patients who fit the above criteria who agreed to sign
informed consents. Each patient was treated according to their
stage of disease. The patients had not received any form of therapy
before surgery. Radical resection (Ransurethral plasmakinetic
resection and testes resection) was used in T1-T2 PCa patients. All
the patients received androgen deprivation therapy using a
hormone-releasing hormone agonist analogue. Tumors were confirmed
histopathologically and were staged according to the AJCC/UICC TNM
System (22). The histological types
were determined by two independent clinical pathologists in a
double-blinded manner: all the samples were PCa. The samples were
collected were according to the status of metastasis, following
treatment. A total of 8 patients diagnosed with benign prostatic
hyperplasia were used as the control group. The study involving
human participants was approved by the Ethics Committee of
Guangdong General Hospital. Written informed consent was obtained
from the patients prior to enrollment.
Histology and immunofluorescence
Prostate tissues of PCa patients were collected by
surgery or needle aspiration biopsy and fixed in paraformaldehyde
for 12 h. Paraffin-embedded sections (8 µm) were cut and stained
with hematoxylin and eosin (Boster Biological Technology Ltd.,
Wuhan, China) for pathological diagnosis. For confocal imaging
analysis, paraffin-embedded sections (8 µm) were cut and dehydrated
in xylene and a graded alcohol series, followed by antigen
retrieval; Briefly, the sections were immersed in citrate buffer
with pH 6.0 in a specialized pressure cooker designed for antigen
retrieval (Head Biotechnology Ltd., Beijing, China). When the
cooker reached a temperature of 120°C and pressure of 117KPa, the
sections were boiled for 5 min, then cooled down in the cooker for
3–4 h. The sections were blocked with 1% bovine serum albumin
(Boster Biological Technology, Ltd., Wuhan, China) and normal goat
serum (Boster Biological Technology Company) for 30 min at 37°C,
and then incubated with a mouse monoclonal antibody against CD68
(ab955; Abcam, Cambridge, UK; dilution, 1:100) and a rabbit
polyclonal antibody against CD206 (ab64693; Abcam; dilution, 1:50)
overnight at 4°C. Subsequently, the sections were washed in
phosphate-buffered saline (PBS), and incubated with Alexa Fluor®
488 goat anti-rabbit IgG (A-24922; Invitrogen Life Technologies,
Carlsbad, CA, USA; dilution, 1:1,000) and Alexa Fluor® 633 goat
anti-mouse IgG (A-21052; Invitrogen Life Technologies; dilution,
1:1000) at 37°C for 30 min. Next, the sections were washed in PBS,
incubated with 4′,6-diamindino-2-phenylindole dihydrochloride
(Sigma-Aldrich, St. Louis, MO, USA; dilution, 1:100) for nuclear
staining, sealed with aqueous mounting medium (R&D Systems,
Inc., Minneapolis, MN, USA), and viewed under a Zeiss LSM 510
confocal imaging system (Carl Zeiss AG, Dresden, Germany). Five
fields of cancer cores from each section were randomly selected and
viewed; then, the number of CD68-positive cells and
CD68/CD206-double-positive cells were counted.
Statistical analysis
All the obtained numerical data are expressed as the
mean ± standard deviation. Comparisons between groups were
performed using an independent samples t-test and χ2
test. The association of TAMs and AAMs with clinical
characteristics was assessed using Pearson's correlation
coefficient. Overall survival was measured from the initiation of
diagnosis until the end of the observation period and analyzed
using the Kaplan-Meier method. Cox proportional hazards analysis
using a univariate or multivariate method was used to explore the
effect of variables on overall survival. All the statistical
calculations were processed using SPSS version 16.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features of PCa patients
A total of 42 patients (21 with metastasis and 21
without metastasis) diagnosed with prostate adenocarcinoma were
recruited. PCa with metastasis was defined as invasion of other
organs, including bone, visceral (such as bladder, testes, and
other organs) or lymph nodes. To assess if the PCa patients were
metastatic, bone scans, ultrasound, MRI scans (or CT or PET scans)
were conducted. Age and survival time did not differ significantly
between patients with and without metastasis. Patients with
metastasis possessed significantly higher PSA levels, Gleason
scores and tumor grades (Table
I).
 | Table I.Clinical features of prostate
adenocarcinoma patients with or without metastasis. |
Table I.
Clinical features of prostate
adenocarcinoma patients with or without metastasis.
|
| Metastasis | Non-metastasis | P-value |
|---|
| Age, years | 70.05±10.05 | 73.00±7.76 | P>0.05 |
| PSA, ng/ml | 87.29±48.12 | 16.33±7.15 | P<0.01 |
| Gleason scores | 7.24±1.04 | 6.587±0.96 | P<0.05 |
| Tumor grade (T),
n |
|
| P<0.01 |
| 1–2 | 0 | 18 |
|
| 3–4 | 21 | 3 |
|
| Survival time, n |
|
| P>0.05 |
| <5
years | 8 | 4 |
|
| ≥5
years | 13 | 17 |
|
Identification of TAMs and AAMs in
prostate tissues of PCa patients with or without metastasis
Immunofluorescence analysis revealed that the mean
number of TAMs (CD68-positive cells) in the prostate tissues of PCa
patients with metastasis [45.29±7.25 cells/high power field (HPF)]
was significantly higher compared with that of PCa patients without
metastasis (33.57±5.25 cells/HPF; P<0.01). Co-localization
signals of CD68 and CD206 were identified as AAMs, and the numbers
of AAMs in PCa patients with and without metastasis were 29.43±5.68
cells/HPF and 9.14±5.29 cells/HPF, respectively (Fig. 1). The mean percentage of AAMs
(calculated as the number of AAMs / number of TAMs) in patients
with and without metastasis were 65.11±9.68 and 27.32±7.85%,
respectively. The differences in the number and percentage of AAMs
between these patient groups were statistically significant
(P<0.01).
Association of TAMs and AAMs with the
Gleason score and level of PSA
Pearson's correlation coefficient analysis revealed
that the numbers of AAMs and TAMs were significantly positively
correlated with the Gleason scores of the patients (P<0.01;
Table II). The number of TAMs and
the level of serum PSA were also found to be significantly
correlated (P<0.05). The percentage of AAMs was found to be
negatively correlated with Gleason scores and PSA levels. However,
the numbers of AAMs were not correlated with the level of serum
PSA.
 | Table II.Correlation between macrophages and
clinical features. |
Table II.
Correlation between macrophages and
clinical features.
| Parameters | Pearson correlation
coefficient | P-value |
|---|
| Number of TAMs vs.
Gleason scores |
0.723 | P<0.01 |
| Number of AAMs vs.
Gleason scores |
0.848 | P<0.01 |
| Percentage AAMs vs.
Gleason scores | −0.520 | P<0.05 |
| Number of TAMs vs.
level of PSA |
0.418 | P<0.05 |
| Number of AAMs vs.
level of PSA |
0.746 | P>0.05 |
| Percentage of AAMs
vs. level of PSA | −0.713 | P<0.01 |
Association of TAMs and AAMs with the
percentage of AAMs and survival
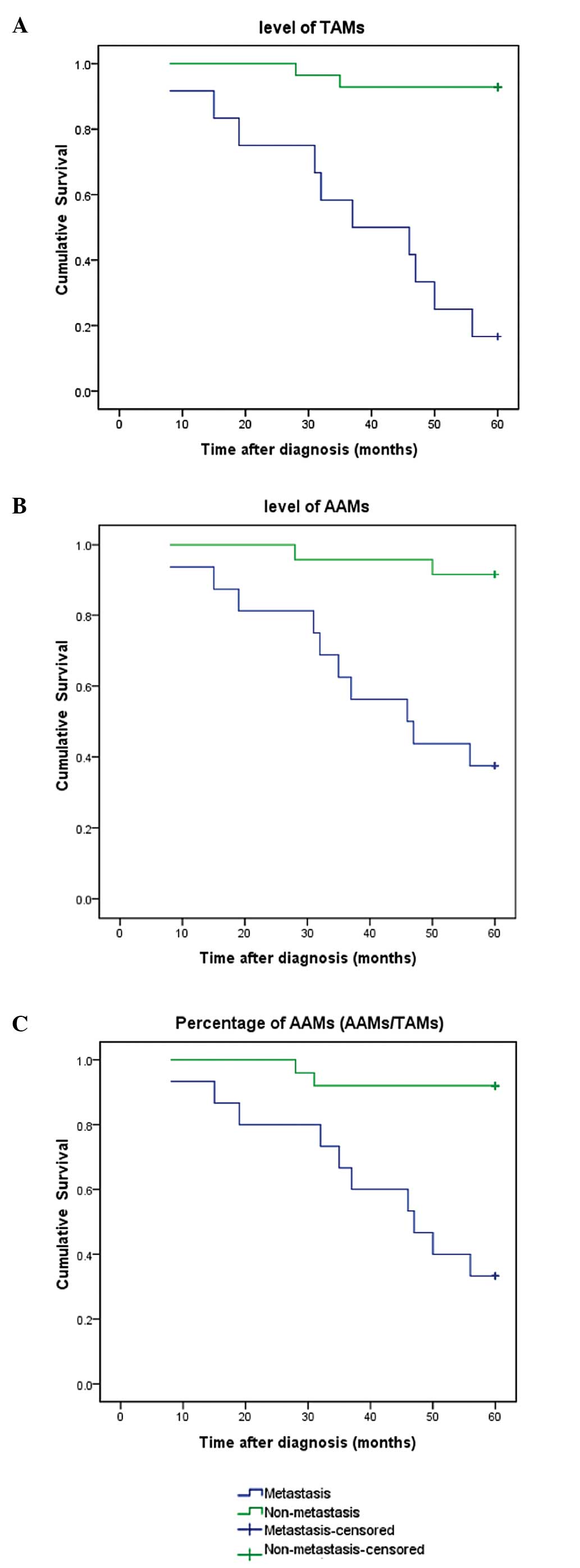
To assess the association between levels of TAMs,
AAMs and patients' overall survival time, patients were followed up
for >5 years. Higher levels of TAMs and AAMs, and higher
percentages of AAMs were associated with shorter overall survival
time, as demonstrated by the Kaplan-Meier analysis (Fig. 2). Univariate and multivariate analyses
were also performed separately to analyze several variables as
predictors of PCa. Univariate analysis results indicated that the
PSA levels, Gleason scores, metastatic status, T grade, number of
TAMs, number of AAMs and percentage of AAMs are predictive factors
for the overall survival of patients. Multivariate analyses
indicated that the Gleason scores, PSA levels and number of TAMs
are predictive factors for overall survival (Table III). Thus, high TAM and AAM levels
may be used as new markers to predict poor prognosis among patients
with late-stage PCa.
 | Table III.Univariate and multivariate Cox
proportional HRs for overall survival. |
Table III.
Univariate and multivariate Cox
proportional HRs for overall survival.
| Characteristic | HR (95% CI) | P-value |
|---|
| Univariate
analysis |
|
|
| Age,
years | 1.017
(0.952–1.086) | P>0.05 |
| PSA
level | 1.022
(1.011–1.033) | P<0.01 |
| Gleason
score | 4.014
(2.040–7.899) | P<0.01 |
| Tumor
grade | 2.538
(1.178–5.467) | P<0.05 |
|
Treatmenta | 0.722
(0.229–2.278) | P>0.05 |
|
Metastatic status | 12.888
(1.659–100.096) | P<0.05 |
| Number
of TAMs | 1.089
(1.030–1.151) | P<0.01 |
| Number
of AAMs | 1.182
(1.091–1.281) | P<0.01 |
|
Percentage of AAMs
(AAMs/TAMs) | 0.053
(0.070–0.411) | P<0.01 |
| Multivariate
analysis |
|
|
| PSA
level | 1.025
(1.002–1.051) | P<0.05 |
| Gleason
scores | 2.579
(0.481–13.836) | P<0.05 |
| Tumor
grade | 0.983
(0.732–1.011) | P>0.05 |
|
Metastatic status | 1.230
(1.030–1.533) | P>0.05 |
| Number
of TAMs | 1.524
(1.155–2.012) | P<0.01 |
| Number
of AAMs | 0.838
(0.677–1.039) | P>0.05 |
|
Percentage of AAMs
(AAMs/TAMs) | 1.531
(0.134–17.545) | P>0.05 |
Discussion
The current study demonstrated that the number of
TAMs and AAMs were significantly increased in PCa tissues of
patients with metastasis compared with that of patients without
metastasis. Furthermore, the number of AAMs and TAMs were
positively correlated Gleason scores, and the number of TAMs were
correlated with PSA levels. Additionally, higher levels of TAMs,
levels of AAMs and percentages of AAMs were associated with shorter
overall survival. In addition, univariate analysis indicated that
the level of PSA, Gleason scores, metastasis status, T grade,
number of TAMs, number of AAMs and percentage of AAMs are
predictors for overall survival, whilst multivariate analyses
demonstrated that Gleason scores, PSA level and number of TAMs are
predictors for overall survival.
Recent studies have demonstrated that TAMs play a
critical biological role in prostate cancer initiation and
progression (10–21). Gollapudi et al (20) demonstrated that TAM levels were higher
in prostatic intraepithelial neoplasia compared with the levels in
benign tissue, while patients with higher Gleason scores contained
higher TAM numbers than those with lower Gleason scores. Similarly,
Herroon et al (23) presented
evidence that bone marrow macrophages, supplying the cysteine
protease cathepsin K, may be involved in CCL2- and
cyclooxygenase-2-driven pathways that contributed to tumor
progression in the bone. The present study also demonstrated that
the number of TAM (CD68-positive) cells in prostate tissues of PCa
patients with metastasis was significantly higher compared with the
number in PCa patients without metastasis. This indicates that TAMs
play an important role in the progression of metastasis in PCa
patients.
Macrophages are activated towards the alternatively
activated phenotype by stimulation with IL-4 and IL-13 (24,25).
Several studies have suggested that AAMs promote tumor growth,
angiogenesis and invasion (26–28).
However, the role of AAMs in metastasis of PCa was previously
undefined. Lanciotti et al (19) collected clinical and pathological data
from 93 patients treated with radical prostatectomy and analyzed
the association between CAMs and AAMs occurrence. They concluded
that a higher density of macrophages was associated with poor
prognosis and that AAM was significantly associated with tumor
extension. Furthermore, Comito et al (21) demonstrated that PCa cells participate
in the differentiation process through secretion of monocyte
chemotactic protein-1, facilitating monocyte recruitment,
macrophage differentiation and M2 polarization. This complex
interplay among cancer cells and AAMs contributes to increasing
tumor cell motility, ultimately facilitating the escape of cancer
cells from the primary tumor and metastatic spread; therefore,
Comito et al (21) concluded
that AAMs interact with cancer-associated fibroblasts during
prostate carcinoma progression. The present study investigated the
function of AAMs on PCa in a cellular or molecular level. The
current results demonstrated that the number of AAMs in PCa
patients with metastasis was significantly higher compared with
that in patients without metastasis. In addition, the percentage of
AAMs (number of AAMs/number of TAMs) was significantly higher in
PCa patients with metastasis. The collective findings of the
aforementioned studies and the present study suggest that AAMs may
promote the metastasis of PCa.
The present findings also revealed that numbers of
AAMs and TAMs are significantly positively correlated with Gleason
scores. The analysis of the TAM number and serum PSA level also
revealed a significant correlation. Numerous studies have
demonstrated that Gleason scores and PSA levels are associated with
the severity of clinical outcome. Huang et al (29) reported that the initial PSA level, a
PSA nadir of ≥2 ng/ml and shorter time to PSA nadir were associated
with disease progression. Furthermore, Chen et al (30) showed that initial level of PSA was a
potential predictive factor for biochemical progression. Petrylak
et al (31) demonstrated that
greater PSA declines were associated with survival in the Southwest
Oncology Group (SWOG) trial. Another study demonstrated that a PSA
decline of ≥30% within 3 months of chemotherapy initiation had the
highest degree of surrogacy for overall survival, confirming the
results of the SWOG trial (32).
These clinical trials indicated that the initial PSA level and PSA
declines were associated with prognosis. The biopsy Gleason scores,
defined as the sum of the most predominant Gleason grades, is a
prognostic factor significantly associated with the risk of
prostate cancer-specific mortality (PCSM) following conservative or
curative management (33,34). Studies have established an association
between the commonly used Gleason score levels (8–10 vs. 7 vs. ≤6)
and an increased risk of PCSM after adjusting for known prognostic
factors (35,36). These data may suggest that AAM and TAM
levels were consistent with the clinical features such as the
levels of PSA and the Gleason scores. However, the number of AAMs
was not correlated with the level of serum PSA. This phenomenon may
be due to the wide range of PSA variation.
Kaplan-Meier analysis revealed that higher TAM and
AAM numbers, and higher percentages of AAMs were associated with
shorter overall survival. This indicates that TAMs and AAMs may be
closely connected with the prognosis of PCa. The combination of TAM
and AMM numbers, and percentage of AAMs is, therefore, a sensitive
marker for poor prognosis. In addition, the level of PSA, Gleason
scores, metastatic status and clinical stage of disease were found
to be significantly predictive of overall survival by univariate
and multivariate analyses. The classical prognostic factors of
clinical stage, such as level of PSA and Gleason scores, have been
used for over a decade to categorize patients at the time of
diagnosis into broad risk groups that help to determine appropriate
management strategies (33–36). The present results are consistent with
these factors. Finally, univariate analysis demonstrated that the
number of TAMs, number of AAMs and percentage of AAMs are
predictors for overall survival, whilst multivariate analyses
indicated that the number of TAMs is a predictor for overall
survival. Thus, these data indicated that high levels of TAMs and
AAMs may be used as a pathological and immune marker to predict
poor prognosis among patients with late-stage PCa in addition to
Gleason scores. However, the number of AAMs was not found to be a
predictor of the overall survival based on multivariate analysis.
This may be due to the particular samples used in the current
study, and more cases should be recruited in further studies.
In conclusion, the results demonstrated that AAMs
may be important in the metastasis of PCa and may be used as
potential immune biomarkers of poor prognosis in patients with
late-stage PCa. However, the underlying mechanism of AAMs in the
metastasis of PCa and how this may be blocked remain to be
established and require further investigation.
Acknowledgements
This study was supported by a grant from the Medical
Scientific Research Foundation of Guangdong Province, China (no.
B007).
References
|
1
|
Collin SM, Martin RM, Metcalfe C, et al:
Prostate-cancer mortality in the USA and UK in 1975–2004: An
ecological study. Lancet Oncol. 9:445–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han SJ, Zhang SW, Chen WQ and Li CL:
Analysis of status and trends of prostate cancer incidence in
China. Lin chuang Zhong Liu Xue Za Zhi. 18:330–335. 2013.
|
|
4
|
Tammela T: Endocrine treatment of prostate
cancer. J Steroid Biochem Mol Biol. 92:287–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loblaw DA, Virgo KS, Nam R, et al:
American Society of Clinical Oncology : Initial hormonal management
of androgen-sensitive metastatic, recurrent, or progressive
prostate cancer: 2006 update of an American Society of Clinical
Oncology practice guideline. J Clin Oncol. 25:1596–1605. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phillips JG, Aizer AA, Chen MH, Zhang D,
Hirsch MS, Richie JP, Tempany CM, Williams S, Hegde JV, Loffredo MJ
and D'Amico AV: The effect of differing Gleason scores at biopsy on
the odds of upgrading and the risk of death from prostate cancer.
Clin Genitourin Cancer. 12:e181–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohammed AA: Biomarkers in prostate
cancer: New era and prospective. Med Oncol. 31:1402014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiarugi P, Paoli P and Cirri P: Tumor
microenvironment and metabolism in prostate cancer. Semin Oncol.
41:267–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gollapudi K, Galet C, Grogan T, et al:
Association between tumor-associated macrophage infiltration, high
grade prostate cancer and biochemical recurrence after radical
prostatectomy. Am J Cancer Res. 3:523–529. 2013.PubMed/NCBI
|
|
10
|
Schmieder A, Michel J, Schönhaar K, Goerdt
S and Schledzewski K: Differentiation and gene expression profile
of tumor-associated macrophages. Semin Cancer Biol. 22:289–297.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solinas G, Schiarea S, Liguori M, et al:
Tumor-conditioned macrophages secrete migration-stimulating factor:
A new marker for M2-polarization, influencing tumor cell motility.
J Immunol. 185:642–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su S, Liu Q, Chen J, et al: A positive
feedback loop between mesenchymal-like cancer cells and macrophages
is essential to breast cancer metastasis. Cancer Cell. 25:605–620.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takayama H, Nishimura K, Tsujimura A, et
al: Increased infiltration of tumor associated macrophages is
associated with poor prognosis of bladder carcinoma in situ after
intravesical bacillus Calmette-Guerin instillation. J Urol.
181:1894–1900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Q, Peng RQ, Wu XJ, et al: The density
of macrophages in the invasive front is inversely correlated to
liver metastasis in colon cancer. J Transl Med. 8:132010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:453–461. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Badylak SF, Valentin JE, Ravindra AK, et
al: Macrophage phenotype as a determinant of biologic scaffold
remodeling. Tissue Eng Part A. 14:1835–1842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Troidl C, Möllmann H, Nef H, et al:
Classically and alternatively activated macrophages contribute to
tissue remodelling after myocardial infarction. J Cell Mol Med.
13:3485–3496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lanciotti M, Masieri L, Raspollini MR, et
al: The role of M1 and M2 macrophages in prostate cancer in
relation to extracapsular tumor extension and biochemical
recurrence after radical prostatectomy. Biomed Res Int.
2014:4867982014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gollapudi K, Galet C, Grogan T, et al:
Association between tumor-associated macrophage infiltration, high
grade prostate cancer and biochemical recurrence after radical
prostatectomy. Am J Cancer Res. 3:523–529. 2013.PubMed/NCBI
|
|
21
|
Comito G, Giannoni E, Segura CP, et al:
Cancer-associated fibroblasts and M2-polarized macrophages
synergize during prostate carcinoma progression. Oncogene.
33:2423–2431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng L, Montironi R, Bostwick DG,
Lopez-Beltran A and Berney DM: Staging of prostate cancer.
Histopathology. 60:87–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herroon MK, Rajagurubandara E, Rudy DL, et
al: Macrophage cathepsin K promotes prostate tumor progression in
bone. Oncogene. 32:1580–1593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy BS, Bush HM, Sundareshan V, et al:
Characterization of macrophage activation states in patients with
cystic fibrosis. J Cyst Fibros. 9:314–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heymann F, Trautwein C and Tacke F:
Monocytes and macrophages as cellular targets in liver fibrosis.
Inflamm Allergy Drug Targets. 8:307–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Desguerre I, Mayer M, Leturcq F, et al:
Endomysial fibrosis in Duchenne muscular dystrophy: a marker of
poor outcome associated with macrophage alternative activation. J
Neuropathol Exp Neurol. 68:762–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mathai SK, Gulati M, Peng X, et al:
Circulating monocytes from systemic sclerosis patients with
interstitial lung disease show an enhanced profibrotic phenotype.
Lab Invest. 90:812–823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higashi-Kuwata N, Jinnin M, Makino T, et
al: Characterization of monocyte/macrophage subsets in the skin and
peripheral blood derived from patients with systemic sclerosis.
Arthritis Res Ther. 12:R1282010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang SP, Bao BY, Wu MT, et al: Impact of
prostate-specific antigen (PSA) nadir and time to PSA nadir on
disease progression in prostate cancer treated with
androgen-deprivation therapy. Prostate. 71:1189–1197. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen CH, Hsieh JT, Huang KH, Pu YS and
Chang HC: Predictive clinical indicators of biochemical progression
in advanced prostate cancer patients receiving Leuplin depot as
androgen deprivation therapy. PLoS One. 9:e1050912014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Petrylak DP, Ankerst DP, Jiang CS, et al:
Evaluation of prostate-specific antigen declines for surrogacy in
patients treated on SWOG 99-16. J Natl Cancer Inst. 98:516–521.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Armstrong AJ, Garrett-Mayer E, Ou Yang YC,
et al: Prostate-specific antigen and pain surrogacy analysis in
metastatic hormone-refractory prostate cancer. J Clin Oncol.
25:3965–3970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Partin AW, Kattan MW, Subong EN, et al:
Combination of prostate-specific antigen, clinical stage and
Gleason score to predict pathological stage of localized prostate
cancer. A multi-institutional update. JAMA. 277:1445–1451. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
D'Amico AV, Whittington R, Malkowicz SB,
et al: Biochemical outcome after radical prostatectomy, external
beam radiation therapy, or interstitial radiation therapy for
clinically localized prostate cancer. JAMA. 280:969–974. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crook J and Ots AF: Prognostic factors for
newly diagnosed prostate cancer and their role in treatment
selection. Semin Radiat Oncol. 23:165–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Phillips JG, Aizer AA, Chen MH, et al: The
effect of differing gleason scores at biopsy on the odds of
upgrading and the risk of death from prostate cancer. Clin
Genitourin Cancer. 12:e181–e187. 2014. View Article : Google Scholar : PubMed/NCBI
|