Introduction
The morbidity of gastric cancer is particularly high
in China, where 23.9% of the total death toll resulting from tumors
was attributed to patients with gastric cancer in 2012 (1). Although favorable therapeutic outcomes
for the treatment of gastric cancer have been achieved by a
combination of surgery and chemotherapy, distant metastasis and
recurrence remain common in advanced stage patients (2,3).
Progression of the tumor cells may be induced by a variety of
factors, including multiple structural and functional genetic
alterations, cell-cycle regulators, adhesion molecules and growth
factors (2). Study of these factors
is of significant clinical value for further understanding the
underlying molecular mechanisms of gastric cancer progression, as
well as identifying novel therapeutic targets in gastric tissues
(4–6).
Autophagy is a common occurrence in cell metabolism,
and is involved in facilitating the cellular clearance of
aggregation-prone proteins, thus exerting a cytoprotective function
(7). Autophagy is a genetically
controlled process, including lysosomal degradation and recycling
of these proteins and cellular organelles. The process of autophagy
is activated under various conditions, including hypoxia,
inflammatory stimulation and lack of nutrients (8). Activation of autophagy has two potential
effects, depending on the environment; promoting non-apoptotic type
II programmed cell death or facilitating adaptive cell survival
(9–11). Cancer cells are able to survive under
hypoxic and hypo-nutrient microenvironments, and therefore
theoretically enhance autophagic ability to utilize recyclable
materials. However, there is evidence that autophagy may be capable
of promoting and suppressing cancer. Beclin-1, the first identified
mammalian autophagy effector, was reported to be deleted or
significantly decreased in ovarian, breast and prostate cancer,
suggesting that autophagy may be a tumor-suppressive mechanism
(12). Notably, previous studies have
indicated that low expression levels of Beclin-1 were predictive of
poor survival in B cell lymphoma-xL-overexpressing hepatocellular
carcinoma tissues (2,3). These observations indicate that
autophagy has a significant role in tumorigenesis and prognosis.
However, there have been limited studies regarding the roles of
autophagy in gastric cancer specifically (13).
In the present study, the expression of
autophagy-associated protein Beclin-1 and inflammatory cytokine
interferon-γ (IFN-γ) were evaluated in clinical gastric cancer
tissue samples and cell lines. The association between autophagy
and inflammation was also examined. The present study aimed to
elucidate the characteristics of autophagy in gastric cancer and
the effects of inflammation on this process.
Materials and methods
Patients and tissue samples
Between January 2010 and December 2014, clinical
gastric cancer and adjacent noncancerous gastric mucosa tissue
specimens were collected from 75 patients who had undergone
surgeries for radical resection of gastric cancer at The China
Japan Union Hospital of Jilin University (Changchun, China). The
study protocol was approved by the institutional review board for
human studies of The China Japan Union Hospital of Jilin
University, and informed consent was obtained from all patients.
Following collection, the tissues were divided into two parts, a
large part (50–100 mg) and a small one (10 mg). The 50–100 mg
samples were immediately frozen and stored in liquid nitrogen for
western blot analysis and quantitative polymerase chain reaction
(qPCR), while the 10 mg samples were washed with phosphate-buffered
saline (PBS; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) and stored in 4% paraformaldehyde (Wuhan Boster Biological
Technology, Ltd.) for further histological analysis. Details of the
patients are listed in Table I.
 | Table I.Details of patients included in the
present study (n=75). |
Table I.
Details of patients included in the
present study (n=75).
| Parameter | Patients, n |
|---|
| Gender |
|
| Male | 47 |
|
Female | 28 |
| Median age, years
(range) | 54 (38–65) |
| Median BMI,
kg/m2 (range) | 24.3 (17.6–28.4) |
| TNM stage |
|
| I | 16 |
| II | 40 |
| III | 19 |
Cell culture and stimulation
Human gastric cancer cell lines BGC-823 and MKN-28
(Cell Bank of the Chinese Academy of Sciences, Shanghai, China),
and human normal gastric mucosa epithelial cell line GES-1 (Cell
Bank of the Chinese Academy of Sciences, Shanghai, China), were
grown in Dulbecco's modified Eagle's medium supplemented with 10%
fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 100 U/ml penicillin and 100 g/ml streptomycin
(Sangon Biotech, Shanghai, China). Cells were tested for
authenticity by the Cell Bank of the Chinese Academy of Sciences
prior to purchase. Cells were maintained at 37°C in a humidified
atmosphere of 5% CO2. Cells were plated in 6-well plates
and experimental group cells were stimulated with 50 ng/ml IFN-γ
(BioLegend, Inc., San Diego, CA, USA) for 12, 24 and 48 h (14). The control group cells received the
same dose of PBS. Expression levels of Beclin-1 and LC3 were
analyzed by western blot analysis.
Animal experiments
Female BALB/c nude mice (18–20 g, 6 weeks of age)
were purchased from Huafukang Co. Ltd. (Beijing, China). In total,
50 mice were used for the experiment, of which 10 were used for the
tumor bearing assay and 40 were used for the xenograft assay, with
20 mice in the experimental group and 20 mice in the control group.
The mice were bred at the animal center of Jilin University and
were maintained according to the Jilin University animal management
program. Mice were housed under specific pathogen-free conditions
at a temperature of 23±1°C and relative humidity of 30–70%. Mice
were maintained in a 12-h light-dark cycle, with free access to
water and ad libitum feeding with sterilized food.
Exponentially growing gastric cancer cells (BGC-823 and MKN-28
cells; 5×106 cells in 200 µl PBS) were subcutaneously
inoculated into the left hind leg of 10 mice. Tumor-bearing mice
were sacrificed 10 days later by CO2 inhalation, and the
tumors were dissected and divided into isometrical tissue blocks (2
mm in diameter). The tissue blocks were implanted into the back of
40 novel nude mice. These xenograft tumors were allowed to grow for
a further 14 days. Then the mice were randomly divided into two
groups (20 mice per group). The control group received a
subcutaneous injection of saline (50 µl; Qingdao Hope
Bio-Technology Co., Ltd., Qingdao, China) every 2 days for 14 days,
while the treatment group received a subcutaneous injection of
IFN-γ (0.5 ng in 50 µl) every 2 days for 14 days. Tumor volume
[(major axis × minor axis)2 × 0.5] (15) and status of the mice were measured
every day for 14 days from the commencement of treatment.
Immunohistochemistry
A total of 53 clinical gastric cancer and adjacent
noncancerous gastric mucosa tissue specimenscarcinomas tissues
previously fixed in paraformaldehyde were used for
immunohistochemistry study here. These samples were
triformol-fixed, dehydrated in a graded alcohol series, embedded in
paraffin and cut into sections. The aforementioned chemical
reagents were purchased from Sinopharm Chemical Reagent Co., Ltd.
(Shanghai, China). The sections were subsequently deparaffinized,
blocked with 3% hydrogen peroxide for 10 min, washed with deionized
water three times and sealed with serum (FBS; HyClone; GE
Healthcare Life Sciences) for 30 min at room temperature. The
sections were washed with deionized water and incubated for 30 min
at 37°C with primary polyclonal rabbit anti-human Beclin-1
antibodies (1:500 in 1% BSA in PBS containing 0.1% Tween; cat no.
ab62472; Abcam, Cambridge, MA, USA). Next, the sections were washed
three times with PBS and incubated with a biotinylated goat
anti-rabbit IgG secondary antibody (1:300 in 1% BSA in PBS
containing 0.1% Tween; cat. no. ab64257; Abcam) for 20 min at room
temperature. The sections were washed three times, followed by
detection with the HRP Substrate Kit and ABC kit (SK-4100 and
PK-6100, protocols provided in the kit. Vector Blue; Vector
Laboratories Inc., Burlingame, CA, USA). and nuclei were
counterstained with hematoxylin using the ULTRA Staining system
(Ventana Medical Systems, Tucson, AZ, USA). Negative controls were
obtained by substituting PBS for the primary antibody. The results
were evaluated based on the proportion of stained cells and the
staining intensity.
Tissues were interpreted as positive when a minimum
of weak-to-moderate cytoplasmic staining was identified in >30%
of the neoplastic cells. The rates of Beclin-1-positive cells were
graded as ++ when ≥61% of the cells were positive, + when 30–60%
were positive, and - when 0–29% were positive. Results were
independently reviewed by two pathologists.
qPCR
RNA in the clinical tissue samples was isolated
using mechanical homogenization (LabGEN 700 Homogenizer;
Cole-Parmer, Vernon Hills, IL, USA) and TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Complementary
DNA was synthesized from the total RNA using SuperScriptIII RNase
H-Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.). The expression levels of the genes were quantified using
TaqMan® Gene Expression assays (Applied Biosystems Life
Technologies, Foster City, CA, USA). The results were expressed as
relative expression, standardized against the expression of the
gene encoding β-actin. β-actin was used as the cDNA loading
control, and all qPCR read-outs were adjusted according to the
β-actin results. The primers used were as follows: Beclin-1
forward, 5′-CAAGATCCTGGACCGTGTCA-3′ and reverse,
5′-TGGCACTTTCTGTGGACATCA-3′; β-actin forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (16).
Western blot analysis
Cytoplasmic proteins were extracted from gastric
cancer cell lines and clinical samples using NE-PER Nuclear and
Cytoplasmic Extractions reagents (Beyotime Institute of
Biotechnology, Haimen, China). The lysates were resolved by 4–20%
SDS-PAGE gradient gels (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Blots were blocked and incubated
with rabbit anti-human polyclonal primary antibodies against
Beclin-1 (cat no. ab62472), LC3 (cat no. ab128025) and GADPH (cat
no. ab37168) at dilutions of 1:1,000 at 4°C overnight (Abcam).
Blots were then incubated with a secondary goat anti-rabbit
antibody (cat. no. sc-2004; 1:5,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h.
Blots were visualized using enhanced chemiluminescence detection
reagents and then exposed to X-ray film (Thermo Fisher Scientific,
Inc.) (17,18).
Fluorescence-activated cell sorting
(FACS) analysis for intracellular IFN-γ
FACS requires a small number of high quality tissue
samples and is expensive to run, therefore, only 5 of the 75 fresh
clinical samples were selected for analysis. The samples were
divided into sections and ground on a screen cloth. PBS was used to
wash the ground tissues, filtering immune cells through the cloth
by the wash buffer (PBS) and separating them from the cell debris.
These cells were collected by centrifugation of the washing liquid
and stained with phycoerythrin-CD3, allophycocyanin-CD4 and
fluorescein isothiocyanate-IFN-γ monoclonal antibodies (BioLegend,
Inc.). Data were analyzed using the BD FACS CantoII (BD
Biosciences, Franklin Lakes, NJ, USA) (19).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical significance was determined by the Student's
t-test, Mann-Whiney U test or Fisher's exact test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of Beclin-1 in gastric
cancer tissues and cell lines
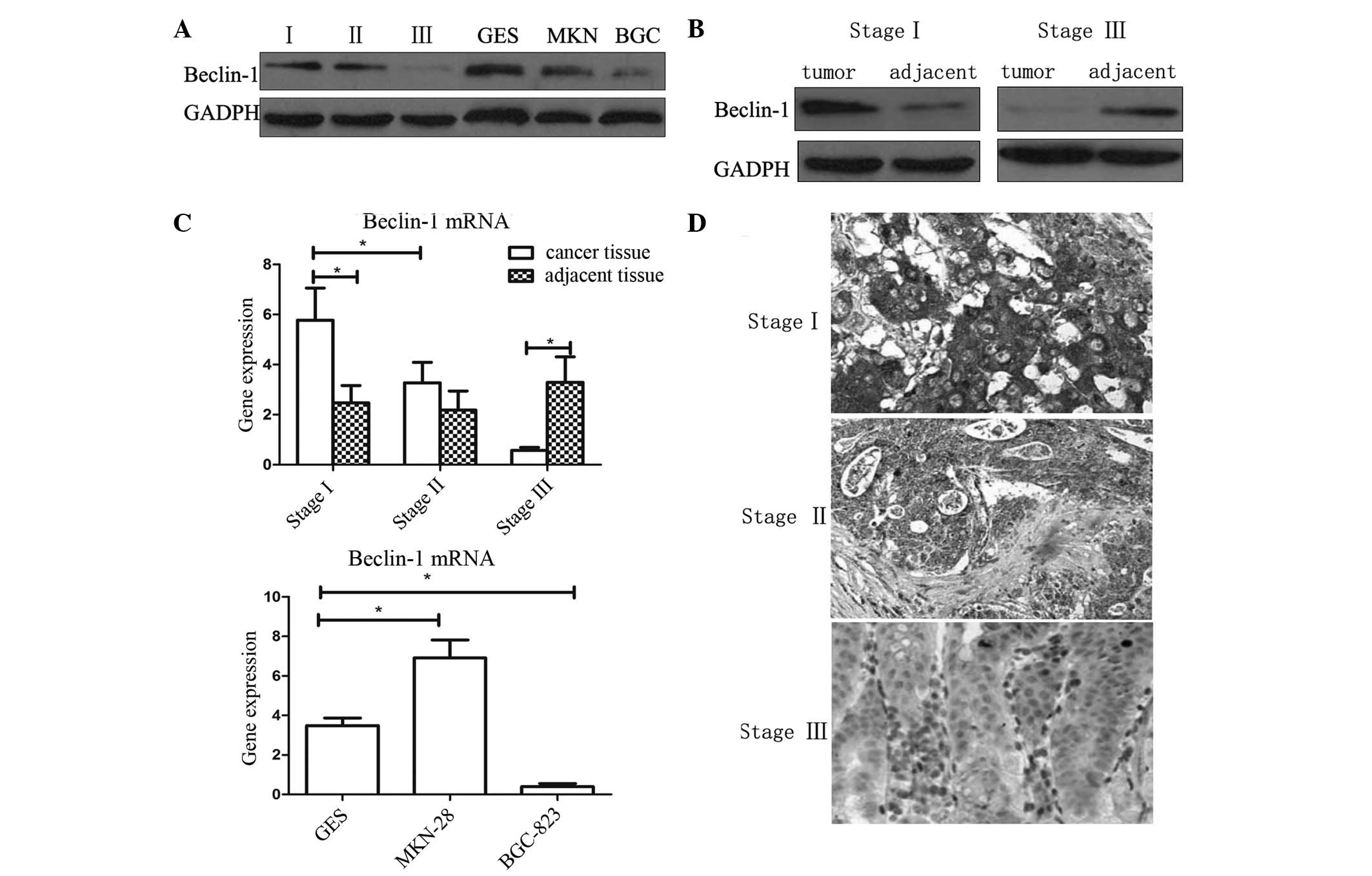
Immunohistochemistry and western blotting were used
to analyze the expression levels of Beclin-1 in gastric cancer
tissues and cell lines. As shown in Fig.
1, in tissues of tumor/node/metastasis (TNM) stage I and II,
the expression of Beclin-1 was higher than that in the adjacent
normal tissues (P<0.05). However, in tissues of TNM stage III,
expression of Beclin-1 was reduced compared with that of the
adjacent tissue (P<0.05). The results of immunohistochemistry
and western blot analysis were concurrent.
The expression of Beclin-1 in BGC-823, MKN-28 and
normal gastric mucosa epithelial cell line GES-1 were analyzed by
western blotting. BGC-823 is a poorly-differentiated gastric
adenocarcinoma cell line, while MKN-28 is a well-differentiated
adenocarcinoma cell line. It was observed that the expression of
Beclin-1 in the poorly-differentiated BGC-823 cell line was
significantly lower compared with that of the other cells
(P<0.05). This result was in accordance with the results of the
analysis of clinical tissue samples. However, significant
differences in Beclin-1 expression were not observed between the
well-differentiated MKN-28 and the normal GES-1 cell lines.
Beclin-1 expression is correlated with
differentiation and depth of invasion
Immunopositivity for Beclin-1 was observed in 53/75
gastric adenocarcinoma patient samples analyzed in the present
study. Expression of Beclin-1 was observed in 20.00% of the
poorly-differentiated samples, 81.10% of the
moderately-differentiated samples and 86.96% of the
well-differentiated samples. There were significant differences
between the poorly-differentiated group and the well- and
moderately-differentiated groups (P<0.01; two-tailed Fisher's
exact test). There were no significant differences in Beclin-1
expression with respect to the existence of lymph node metastasis.
However, the expression of Beclin-1 was associated with the depth
of invasion. Samples of early gastric cancer expressed higher
levels of Beclin-1 than those of advanced gastric cancer. Details
are presented in Table II.
 | Table II.Beclin-1 expression in various types
of gastric carcinoma. |
Table II.
Beclin-1 expression in various types
of gastric carcinoma.
|
| Beclin-1 expression,
n (%) |
|---|
|
|
|
|---|
| Gastric carcinoma
characteristic | − | + | ++ | Total positive |
|---|
| Histological
grade |
|
|
|
|
|
Well | 3 | 9 | 11 | 20 (86.96) |
|
Moderate | 7 | 12 | 18 | 30 (81.10) |
|
Poor | 12 | 2 | 1 | 3
(20.00)a |
| Lymph node
metastasis |
|
|
|
|
| No | 6 | 6 | 8 | 14 (65.00) |
|
Yes | 15 | 17 | 22 | 39 (70.90) |
| Depth of
invasion |
|
|
|
|
|
EGC | 3 | 4 | 15 | 19
(83.63)b |
|
AGC | 19 | 19 | 15 | 34 (64.15) |
Infiltration of IFN-γ in cancer
tissues
FACS analysis was used to evaluate the percentage of
immune cells secreting IFN-γ (Fig. 2A and
B). Five fresh samples of a suitable size were selected for
FACS analysis. Western blotting was also used to directly study the
protein expression of IFN-γ (Fig.
2C). In the present study, it was demonstrated that the
majority of immune cells which were IFN-γ positive, were
CD3(+)CD4(+), indicating that they were Th1 cells. However, the
percentage of IFN-γ-positive cells in stage I/II cases was
significantly higher than that of cases of stage III (Fig. 2).
IFN-γ induces autophagy in gastric
cancer cells
IFN-γ was subsequently used to induce autophagy in
gastric cancer cells. Beclin-1 was originally highly expressed in
MKN-28 cells, while rarely expressed in BGC-823 cells. The
poorly-differentiated BGC-823 cell line was therefore selected for
this in vitro experiment. In order to investigate whether
autophagy was induced in the BGC-823 cells, the expression levels
of autophagy-associated proteins LC3 and Beclin-1 were analyzed. As
shown in Figs. 1 and 3, in the control cells, low levels of LC3
and Beclin-1 were detected, however, following treatment with IFN-γ
for 48 h, the expression levels of these two proteins increased
significantly (Fig. 3A). Furthermore,
the ratio of LC3-II/LC3-I (represented by the two bands) increased
significantly compared with that of the control groups, indicating
the activation of autophagy in these cells (Fig. 3).
Activation of autophagy inhibits tumor
growth in xenograft nude mice
A rodent xenograft model was designed to detect
whether autophagy could inhibit xenograft growth in vivo.
The results revealed that tumor tissues from IFN-γ-treated mice
exhibited significantly increased expression levels of LC3 and
Beclin 1 (Fig. 3). In addition, it
was noted that following implantation of the tumor tissues, the
mice which received IFN-γ injection had significant smaller
xenograft tissues. In addition, 4 weeks later the survival rate of
those mice which received IFN-γ injection was higher than that of
the control group (which only received saline). All these results
indicated an anti-cancer effect of autophagy in gastric cancer
cells.
Discussion
Autophagy is the process of collecting and degrading
intracellular proteins and organelles in lysosomes to preserve
protein and organelle quality, as well as recycling certain
materials to sustain metabolism and survival during starvation
(12). Autophagy is therefore
important for the survival of cells and tissues, and the loss of
autophagy is typically destructive (16). However, in cancer, the role of
autophagy is more complex (19).
Various preclinical and clinical studies have thus been undertaken,
aiming to develop therapeutic agents targeting autophagy (14–20).
However, compared with apoptosis, data indicating an association
between cancer pathogenesis and autophagic cell death are limited
(21,22).
Beclin-1 is a mammalian ortholog of yeast Atg6,
which has a critical role in the vesicle nucleation phase of
autophagy (1). The Beclin-1 gene is
located on chromosome 17q21, which is frequently deleted in breast,
ovarian and prostate cancer (23).
Furthermore, Beclin-1+/− mutant mice demonstrate a
particularly high incidence of tumors, indicating that Beclin-1 may
potentially be a haploinsufficient tumor suppressor (24–26). Thus,
in the present study, the expression of Beclin-1 was evaluated in
human gastric cancer specimens and cell lines. Unexpectedly, it was
revealed that early-stage gastric cancer tissues expressed high
levels of Beclin-1. The mRNA and protein expression levels of
Beclin-1 were higher in these early-stage tissues compared with
those of the adjacent normal mucosa. This result differed from the
situation previously identified in breast cancer (20), indicating a distinct role for Beclin-1
in gastric cancer compared with other types of cancer. The
overexpression of Beclin-1 observed in the early stages of gastric
cancer in the present study may be interpreted in several ways.
During the progression of cancer, cancer cells gradually acquire
the capacity to evade cell death. Autophagy is an important type of
cell death in tumors (23–26) and Beclin-1 may contribute to its
induction. However, although in the early stages, Beclin-1 was
strongly expressed in gastric cancer tissues, such changes were not
observed in late-stage gastric cancer specimens (TNM stage III). By
contrast, the expression levels of Beclin-1 were reduced in stage
III tumors, compared with those of the adjacent mucosa. This
observation indicated that Beclin-1 expression occurred at a
relatively early stage. It appeared that with the progression of
the tumor, gastric cancer tissues tended to lose their autophagic
capacity. Notably, in the cell-based studies, similar results were
observed: Expression levels of autophagy-associated proteins,
Beclin-1 and LC3, were low in the poorly-differentiated BGC823 cell
line but significantly higher in the well-differentiated MKN28 cell
line. Further clinical analysis revealed that the depth of invasion
was a significant factor affecting the expression of Beclin-1,
while lymph node metastasis was not. Considering the fact that for
gastric cancer, depth of invasion is more relevant in clinical
diagnosis, the findings of the present study revealed that
autophagy may have a significant role in this process.
Previous studies have revealed that autophagy is
frequently activated in cancer cells following drug treatment
(13–16,20,24,26).
A number of allosteric and catalytic inhibitors of mammalian target
of rapamycin (mTOR), phosphoinositide-3-kinase-AKT, tyrosine kinase
signaling and activators of energy sensing pathways may induce
autophagy in cells (27). Therefore,
it was hypothesized that autophagic cell death may be involved in
the mechanism of action of certain anticancer drugs. The Akt-mTOR
signaling pathway is the major negative signaling pathway against
autophagy (28). Despite the fact
that these chemotactic cytokines and pathways have been extensively
studied, studies regarding the role of inflammation in autophagy
are limited. It was previously reported that gastric carginogenesis
was able to be inhibited by IFN-γ, likely through the induction of
autophagy and apoptosis (29). Thus
the expression and roles of IFN-γ in gastric carcinogenesis and
autophagy were evaluated in the present study. The results
indicated that, notably, in the early stages of gastric cancer, the
infiltration and secretion of IFN-γ was relatively high. Activated
IFN-γ-secreting cells infiltrated the cancer tissues and protein
expression levels were significantly increased. However, in stage
III cancer tissues, limited IFN-γ expression was detected. It has
previously been widely recognized that IFN-γ is a significant
anti-tumor cytokine (7,23–25). This
change in IFN-γ expression may occur as a result of immune evasion
by the cancer cells. Since the expression of autophagy-associated
protein Beclin-1 also fluctuated during cancer progression, it was
hypothesized that there may be an association between these two
factors.
In the present study, IFN-γ was used to directly
stimulate BGC-823 cells, which originally expressed low levels of
Beclin-1. It was demonstrated that following IFN-γ stimulation, the
expression levels of LC3 and Beclin-1 were increased. As previous
studies have confirmed that increasing expression of LC3 and
Beclin-1 lead to increased numbers of autophagosomes (30–32), the
increase in expression of these two proteins observed in the
present study indicate that autophagy is activated in gastric
cancer cells following in vitro stimulation with IFN-γ.
Therefore, for stage III cancer cells, the lack of IFN-γ may be a
significant factor in the loss of autophagy.
The animal experiments utilized in the present study
were designed to confirm the results of the in vitro
experiments. To further evaluate the therapeutic effects of IFN-γ,
a xenograft rodent model was generated in nude mice. The mice with
xenografts typically succumbed within 2 weeks. However, following
injection of IFN-γ, the survival rate of the xenograft mice
significantly increased, indicating that the application of IFN-γ
may aid the limitation of tumor growth and extend the survival
time. IFN-γ has a significant role in the immune system for
restricting the progression and survival of tumor cells. However,
Th1 cells, the major cell which secretes IFN-γ, is typically
decreased in the local area of cancer tissues or lose this ability
to secrete IFN-γ. Therefore application of recombinant IFN-γ may
represent a novel therapeutic strategy for the treatment of gastric
cancer. Furthermore, this effect may be mediated via the activation
of autophagy.
In conclusion, the results of the current study
revealed that Beclin-1, an autophagy-associated protein, exhibited
a complex expression pattern in gastric cancer tissues at various
clinical stages. In contrast to other tumors, including breast or
prostate cancer, the expression of Beclin-1 in well-differentiated
gastric cancer tissues and cells was higher compared with that in
adjacent tissues or normal cell lines. However, in
poorly-differentiated tissues and cells the opposite effect was
observed. The results also revealed that there was a decrease in
the infiltration and secretion of IFN-γ associated with the
progression of gastric cancer. In vitro and in vivo
studies indicated that there were associations between IFN-γ and
autophagy. Additionally, IFN-γ not only activated autophagy in
gastric cancer cells, but also enhanced the survival rate in a
rodent xenograft model. Thus IFN-γ may represent a novel target for
the inhibition of gastric cancer via the mechanism of
autophagy.
References
|
1
|
Zhou WH, Tang F, Xu J, Wu X, Yang SB, Feng
ZY, Ding YG, Wan XB, Guan Z, Li HG, et al: Low expression of Beclin
1, associated with high Bcl-xL, predicts a malignant phenotype and
poor prognosis of gastric cancer. Autophagy. 8:389–400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vigen RA, Kodama Y, Viset T, Fossmark R,
Waldum H, Kidd M, Wang TC, Modlin IM, Chen D and Zhao CM:
Immunohistochemical evidence for an impairment of autophagy in
tumorigenesis of gastric carcinoids and adenocarcinomas in rodent
models and patients. Histol Histopathol. 28:531–542.
2013.PubMed/NCBI
|
|
3
|
Xia P, Wang JJ, Zhao BB and Song CL: The
role of beclin-1 expression in patients with gastric cancer: A
meta-analysis. Tumour Biol. 34:3303–3307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tu SP, Quante M, Bhagat G, Takaishi S, Cui
G, Yang XD, Muthuplani S, Shibata W, Fox JG, Pritchard DM and Wang
TC: IFN-γ inhibits gastric carcinogenesis by inducing epithelial
cell autophagy and T-cell apoptosis. Cancer Res. 71:4247–4259.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vegesna AK, Miller LS, Barbe MF, Braverman
AS, Khan F and Ruggieri MR: Su1162 nicotinic receptor stimulation
causes enhanced relaxation of gastric clasp, gastric sling and
lower esophageal circular muscle fibers from patients with
Barrett's esophagus - a possible pathophysiologic mechanism for
GERD. Gastroenterology. 142:S440–S441. 2012. View Article : Google Scholar
|
|
6
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR-and
hypoxia-induced factor 1α-mediated signaling. Autophagy. 7:966–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun PH, Zhu LM, Qiao MM, Zhang YP, Jiang
SH, Wu YL and Tu SP: The XAF1 tumor suppressor induces autophagic
cell death via upregulation of Beclin-1 and inhibition of Akt
pathway. Cancer Lett. 310:170–180. 2011.PubMed/NCBI
|
|
8
|
Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang
J, Qu X and Liu Y: β-Elemene-induced autophagy protects human
gastric cancer cells from undergoing apoptosis. BMC Cancer.
11:1832011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HS, Lee SH, Do SI, Lim SJ, Park YK and
Kim YW: Clinicopathologic correlation of beclin-1 expression in
pancreatic ductal adenocarcinoma. Pathol Res Pract. 207:247–252.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
11
|
Pan WR, Chen PW, Chen YL, Hsu HC, Lin CC
and Chen WJ: Bovine lactoferricin B induces apoptosis of human
gastric cancer cell line AGS by inhibition of autophagy at a late
stage. J Dairy Sci. 96:7511–7520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JM, Huang S, Wu TT and Sinicrope FA:
555 Association of autophagy regulator beclin 1 with response to
neoadjuvant chemoradiation in rectal carcinoma. Gastroenterology.
142(Suppl 1): S111–S112. 2012. View Article : Google Scholar
|
|
13
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji D, Zhang Z, Cheng L, Chang J, Wang S,
Zheng B, Zheng R, Sun Z, Wang C, Zhang Z, et al: The combination of
RAD001 and MK-2206 exerts synergistic cytotoxic effects against
PTEN mutant gastric cancer cells: Involvement of MAPK-dependent
autophagic, but not apoptotic cell death pathway. PLoS One.
9:e851162014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hossain MA, Kim DH, Jang YJ, Yoon JH, Moon
JO, Chung HY, Kim GY, Choi YH, Copple BL and Kim ND: Aspirin
induces apoptosis in vitro and inhibits tumor growth of human
hepatocellular carcinoma cells in a nude mouse xenograft model. Int
J Oncol. 40:1298–1304. 2012.PubMed/NCBI
|
|
16
|
Roesly HB, Khan MR, Chen HD, Hill KA,
Narendran N, Watts GS, Chen X and Dvorak K: The decreased
expression of Beclin-1 correlates with progression to esophageal
adenocarcinoma: the role of deoxycholic acid. Am J Physiol
Gastrointest Liver Physiol. 302:G864–G872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Claerhout S, Lim JY, Choi W, Park YY, Kim
K, Kim SB, Lee JS, Mills GB and Cho JY: Gene expression signature
analysis identifies vorinostat as a candidate therapy for gastric
cancer. PLoS One. 6:e246622011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vigen RA, Chen D and Zhao CM: Pathobiology
of gastric carcinoids and adenocarcinomas in rodent models and
patients: Studies of gastrocystoplasty, gender-related factors and
autophagy. Cell/Tissue Injury and Cytoprotection/Organoprotection
in the Gastrointestinal Tract: Mechanisms, Prevention and
Treatment. Filaretova LP and Takeuchi K: 30:(Basel). S. Karger AG.
202–211. 2012. View Article : Google Scholar
|
|
19
|
Zhou W, Yue C, Deng J, Hu R, Xu J, Feng L,
Lan Q, Zhang W, Ji D, Wu J, et al: Autophagic protein beclin 1
serves as an independent positive prognostic biomarker for
non-small cell lung cancer. PLoS One. 8:e803382013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Won KY, Kim GY, Lim SJ and Kim YW:
Decreased Beclin-1 expression is correlated with the growth of the
primary tumor in patients with squamous cell carcinoma and
adenocarcinoma of the lung. Hum Pathol. 43:62–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen
X, Chen X, Zhang CY, Zhang Q and Zen K: MicroRNA-30a sensitizes
tumor cells to cis-platinum via suppressing beclin 1-mediated
autophagy. J Biol Chem. 287:4148–4156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kapoor V, Paliwal D, Baskar Singh S,
Mohanti BK and Das SN: Deregulation of Beclin 1 in patients with
tobacco-related oral squamous cell carcinoma. Biochem Biophys Res
Commun. 422:764–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baspinar S, Bircan S, Orhan H, Kapucuoglu
N and Bozkurt KK: The relation of beclin 1 and bcl-2 expressions in
high grade prostatic intraepithelial neoplasia and prostate
adenocarcinoma: A tissue microarray study. Pathol Res Pract.
210:412–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong Y, You L, Liu H, Li L, Meng H, Qian Q
and Qian W: Potent antitumor activity of oncolytic adenovirus
expressing Beclin-1 via induction of autophagic cell death in
leukemia. Oncotarget. 4:860–874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu DM, Wang GL, Chen L, Xu YY, He S, Cao
XL, Qin J, Zhou JM, Zhang YX and E Q: The expression of beclin-1,
an autophagic gene, in hepatocellular carcinoma associated with
clinical pathological and prognostic significance. BMC Cancer.
14:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sivridis E, Koukourakis MI, Mendrinos SE,
Karpouzis A, Fiska A, Kouskoukis C and Giatromanolaki A: Beclin-1
and LC3A expression in cutaneous malignant melanomas: A biphasic
survival pattern for beclin-1. Melanoma Res. 21:188–195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang L, Wang S, Li SS and Yang XM:
Prognostic significance of Beclin-1 expression in laryngeal
squamous cell carcinoma. Pathol Oncol Res. 19:771–777. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhan Z, Li Q, Wu P, Ye Y, Tseng HY, Zhang
L and Zhang XD: Autophagy-mediated HMGB1 release antagonizes
apoptosis of gastric cancer cells induced by vincristine via
transcriptional regulation of Mcl-1. Autophagy. 8:109–121. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hasui K, Nagai T, Wang J, Jia X, Aozasa K,
Izumo S, Kawano Y, Kanekura TS, Eizuru Y and Matsuyama T:
Immunohistochemistry of programmed cell death in archival human
pathology specimens. Cells. 1:74–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marquez RT and Xu L: Bcl-2: Beclin 1
complex: Multiple, mechanisms regulating autophagy/apoptosis toggle
switch. Am J Cancer Res. 2:214–221. 2012.PubMed/NCBI
|
|
31
|
Wang J, Pan XL, Ding LJ, Liu DY, Da-Peng
Lei and Jin T: Aberrant expression of beclin-1 and LC3 correlates
with poor prognosis of human hypopharyngeal squamous cell
carcinoma. PLoS One. 8:e690382013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hasui K, Wang J, Jia X, Tanaka M, Nagai T,
Matsuyama T and Eizuru Y: Enhanced autophagy and reduced expression
of cathepsin D are related to autophagic cell death in Epstein-Barr
virus-associated nasal natural killer/T-cell lymphomas: An
immunohistochemical analysis of beclin-1, LC3, mitochondria (AE-1)
and cathepsin D in nasopharyngeal lymphomas. Acta Histochem
Cytochem. 44:119–131. 2011. View Article : Google Scholar : PubMed/NCBI
|