Introduction
Hepatocellular carcinoma (HCC) is the sixth common
malignancy worldwide, and is the second cause of cancer-associated
mortality (1). At present, the
incidence of HCC is increasing, and the disease is expected to
become the third leading cause of cancer-associated mortality in
the USA by 2030 (2–4). Recently, the survival rates of HCC
patients have improved due to advances in early diagnosis and
surgical treatments, including hepatic resection and liver
transplantation (5). However,
patients with HCC exhibit high postoperative recurrence and
mortality rates, and thus, prognosis remains unsatisfactory
(6,7).
Molecular-targeted therapy has been used to treat HCC; however,
only limited improvements in patient survival rates have been
achieved (8,9). Therefore, the identification of novel
prognostic markers and therapeutic targets for HCC treatment is
required.
Urotensin II (UII) is an endogenous peptide that is
considered a ‘non-classical’ pro-angiogenic cytokine (10,11).
Previous studies have indicated that UII is involved in the
pathogenesis of a number of human malignancies, including breast,
bladder, colon and prostate cancer (11–14).
Additionally, following the identification of human UII as the
cognate ligand for the urotensin II receptor (UT), the function of
the UII/UT system in human diseases was extensively investigated
(15). Increased expression of UII
and UT has been identified in a number of tumor cell lines
(16,17), including HeLa cervical cancer cells,
BeWo choriocarcinoma cells, IMR-32 neuroblastoma cells, VMRC-RCW
human renal cell carcinoma cells, SW-13 adrenocortical carcinoma
cells, T98G glioblastoma cells, DLD-1 colorectal adenocarcinoma
cells and NB69 neuroblastoma cells. Furthermore, the proliferation
of certain tumor cells, including VMRC-RCW cells, SW-13 cells and
human pheochromocytoma cells, is stimulated by UII (16). A previous study demonstrated that the
motility and invasion of bladder cancer cells was significantly
decreased following UT knockdown using a specific small hairpin RNA
(12). Furthermore, our previous
study revealed that the UII/UT system is upregulated in
dithyinitrosamine-induced rat precancerous liver lesions (18), liver cirrhosis (19) and human liver cancer (20). Additionally, the UII/UT system has
been demonstrated to stimulate cell proliferation in a human
hepatoma cell line (BEL-7402 cells) via extracellular
signal-regulated kinase (ERK) 1/2, protein kinase C (PKC) and p38
mitogen-activated protein kinase (MAPK) signaling pathways
(20). These results indicate that
the UII/UT system may be involved in the development of HCC.
However, at present the clinical significance of UII expression in
HCC remains unclear.
The aim of the present study was to assess UII and
UT messenger RNA (mRNA) expression in surgical specimens obtained
from HCC patients, and to investigate the association between UII
mRNA expression and patient clinicopathological parameters and
overall survival rates.
Patients and methods
Patients and tissue collection
A total of 129 HCC patients that underwent surgical
resection between September 2007 and January 2014 at Xuanwu
Hospital, Capital Medical University (Beijing, China) were included
in the present study. None of the patients had received
chemotherapy or radiotherapy prior to surgery. HCC diagnosis was
based on the World Health Organization criteria (21). Tumor staging was determined according
to the sixth edition of the tumor-node-metastasis classification of
the International Union Against Cancer (22). The histological types were assigned
according to the grading system of Edmondson and Steiner (23). All patients received a single
intrahepatic arterial injection dose of 40 mg/m2
epirubicin 1 month after surgery. Preoperative clinical data,
including patient age, gender, pathological diagnosis, serum
α-fetoprotein (AFP) levels, time after surgery to last follow-up
and overall survival, were collected prospectively (Table I). The current study was approved by
the ethics committee of Xuanwu Hospital, Capital Medical University
(Beijing, China), and written informed consent was obtained from
all patients.
 | Table I.Associations between UII expression
and clinicopathological parameters in 129 hepatocellular carcinoma
patients. |
Table I.
Associations between UII expression
and clinicopathological parameters in 129 hepatocellular carcinoma
patients.
|
|
| UII mRNA
expression |
|
|---|
|
|
|
|
|
|---|
| Parameter | Patients,
(n=129) | Low (n=53) | High (n=76) | P-value |
|---|
| Age, years |
|
|
| 0.421 |
|
<60 | 59 | 22 | 37 |
|
| ≥60 | 70 | 31 | 39 |
|
| Gender |
|
|
| 0.398 |
| Male | 80 | 35 | 45 |
|
|
Female | 49 | 18 | 31 |
|
| HBsAg |
|
|
| 0.139 |
|
Positive | 78 | 28 | 50 |
|
|
Negative | 51 | 25 | 26 |
|
| AFP, ng/ml |
|
|
| 0.854 |
|
≤400 | 55 | 24 | 31 |
|
|
>400 | 74 | 29 | 45 |
|
| Liver
cirrhosis |
|
|
| 0.295 |
|
Present | 87 | 33 | 54 |
|
|
Absent | 42 | 20 | 22 |
|
| Esophageal
varices |
|
|
| 0.388 |
|
Present | 77 | 34 | 43 |
|
|
Absent | 52 | 19 | 33 |
|
| Histological
differentiation |
|
|
| <0.001 |
|
Well/moderate | 58 | 38 | 20 |
|
|
Poor | 71 | 15 | 56 |
|
| Tumor size, cm |
|
|
| <0.001 |
| ≤5 | 62 | 42 | 20 |
|
|
>5 | 67 | 11 | 56 |
|
| Pathological
stage |
|
|
| 0.026 |
|
I–II | 58 | 30 | 28 |
|
|
III–IV | 71 | 23 | 48 |
|
HCC tissues and adjacent healthy liver tissues were
resected, and the resected tissues obtained during surgery were
divided into two sections: The first section was immediately snap
frozen and stored in liquid nitrogen prior to RNA and protein
extraction, and the second section was fixed in 10% neutral
buffered formalin (OriGene Technologies, Inc., Beijing, China) for
24 h at room temperature and embedded in paraffin for subsequent
immunohistochemical analysis. Liver function was assessed using the
Child-Pugh scoring system (24). Only
those patients who were classified as Child-Pugh class A were
included in the study. Patients with portal vein thrombosis,
metastasis, systemic hypertension, chronic kidney disease
(creatinine >177 mmol/l and blood urea nitrogen >9 mmol/l),
diabetes mellitus or aortic valve diseases were excluded from the
present study.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA extraction and complementary DNA (cDNA)
synthesis were performed as previously described (19). Total RNA was extracted from
snap-frozen liver biopsy specimens using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The cDNA was reverse
transcribed using the High-Capacity cDNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. UII, UT and vascular endothelial
growth factor (VEGF) gene expression were quantified by qPCR. The
housekeeping gene GAPDH was used as the internal control for target
genes. Primer sequences (Invitrogen; Thermo Fisher Scientific,
Inc.) are presented in Table II.
mRNA expression was measured using SYBR® Green Real-Time
PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and a 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
PCR was performed in a 20-µl reaction mixture containing 2 µg cDNA,
1 µl of each primer and 10 µl SYBR Green PCR Master Mix. The
conditions for PCR amplification were as follows: 94°C
pre-denaturation for 5 min, 94°C denaturation for 30 sec, 60°C
annealing for 30 sec, and 72°C extension for 2 min, for a total of
40 cycles. qPCR analysis was performed as previously described
(19,25). Comparative quantification cycle (Cq)
calculations were all relative to the control group. GADPH Cq
values were subtracted from gene Cq values to give a final Cq
value. ΔΔCq values were achieved by subtracting the average control
ΔCq value, and the expression of UII and UT relative to the control
was derived by using the equation 2−ΔΔCq (26). All experiments were performed in
triplicate.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Primer
sequence | Product size
(bp) | NCBI accession
number |
|---|
| UII | (F)
5′-TCTCCTTGACTCCAGGGAAATA-3′ | 104 | NM-006786.2 |
|
| (R)
5′-GCAGTATCTGTAGAAGGGAAGC-3′ |
|
|
| UT | (F)
5′-CCCAACGCAACCCTCAA-3′ | 96 | NM-018949.1 |
|
| (R)
5′-CGACAGCAGAGTCCCAATG-3′ |
|
|
| VEGF | (F)
5′-CACTGAGGAGTCCAACATCAC-3′ | 97 | NC-000006.12 |
|
| (R)
5′-AGGAAGCTCATCTCTCCTATGT-3′ |
|
|
| GADPH | (F)
5′-AGCCACATCGCTCAGACAC-3′ | 67 | NM-002046.3 |
|
| (R)
5′-GCCCAATACGACCAAATCC-3′ |
|
|
Immunohistochemistry
Immunohistochemical analysis was performed as
previously described (19). The
paraffin-embedded samples were cut into 4-µm sections and subjected
to immunohistochemical staining using the EliVision™ Plus kit
(Maxim Biotechnology Development, Co., Ltd., Fuzhou, China),
according to the manufacturer's protocol. Tissue sections were
incubated with rabbit anti-human UT antibody (1:200; catalog no.
LS-A372; LifeSpan BioSciences, Inc., Seattle, WA, USA) for 18 h in
a humidified chamber at 4°C and washed three times with PBS. An
enhancer was added for 30 min, followed by three washes in PBS. The
sections were then incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit antibody (1:1,500; catalog no.
ZDR-5306; Beijing Zhongshan Biotechnology Co., Ltd., Beijing,
China) for 20 min at 37°C, followed by three washes with PBS.
Finally, immunoreactivity was visualized following incubation with
3,3′-diaminobenzidine (Maxim Biotechnology Development, Co., Ltd.)
for 10 min. Samples were then counterstained with Mayer's
hematoxylin and eosin. As a negative control, PBS was used instead
of primary antibodies.
Western blot analysis
Western blot analysis was performed as previously
described (18). Briefly, proteins
were extracted from liver samples using the RIPA-IV type Mammalian
Cell Extraction kit (catalog no. DBI-1017; Bendabio, Shanghai,
China), homogenized at 7,104 × g for 15 min at 4°C and
assayed using the Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). Protein samples (40 µg) were subjected to
SDS-PAGE (80 V for 40 min on a 5% acrylamide stacking gel and 120 V
for 70 min on a 10% running gel) and subsequently trans-ferred to a
nitrocellulose membrane (Hybond-C Extra; GE Healthcare
Bio-Sciences, Uppsala, Sweden). The membranes were blocked with TBS
(10 mmol/l Tris-HCl and 250 mol/l NaCl), 5% non-fat powdered milk
and 0.1% Tween-20 for 2 h, followed by incubation with primary
rabbit anti-human UT antibody (1:1,000; catalog no. sc-20940; Santa
Cruz Biotechnology, Inc., Dallas, Texas, USA) overnight at 4°C. The
blots were washed with TBS containing 0.1% Tween-20 for 10 min
(three times), followed by incubation with anti-β-actin antibody
(1:1,000; catalog no. ab8226; Abcam, Shanghai, China) or HRP-linked
goat anti-rabbit immunoglobulin G secondary antibody (1:1,500;
catalog no. GGHL-15PXSPP; Immunology Consultants Laboratory, Inc.,
Portland, OR, USA) for 2 h at room temperature. Films were scanned
using a Gel Doc imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Proteins were visualized using the SuperSignal™
West Pico Chemiluminescent Substrate kit (catalog no. 34079; Thermo
Fisher Scientific, Inc.), and bands were quantified via scanning
densitometry using the Image Lab™ software version 5.1 (Bio-Rad
Laboratories, Inc.). UT protein expression was normalized to
β-actin expression.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using one way analysis of
variance and the Student's t test. The χ2 test was used
to analyze the associations between UII expression and patient
clinicopathological characteristics. The Kaplan-Meier method was
used for survival analysis, and differences in survival were
estimated using the log-rank test. Prognostic factors were examined
by univariate and multivariate analyses using the Cox proportional
hazards regression model. Correlations between different mRNA
expression levels were analyzed using the Pearson rank sum test.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS 20.0
statistical software (IBM SPSS, Armonk, NY, USA).
Results
Clinical data
Patient characteristics and data are presented in
Table I. The patient cohort included
80 males and 49 females, with a median age of 58.37 years (age
range, 21–73 years). The median follow-up time was 84 months.
Histologically, all patients exhibited evidence of HCC with clear
surgical margins. None of the patients had been administered
somatostatin or vasoactive drugs for 1 week prior to surgery.
UII and UT gene expression is
significantly higher in HCC tissues than in adjacent non-cancerous
tissues
UII and UT mRNA expression was evaluated in 129 HCC
samples and adjacent non-cancerous hepatic tissues by RT-qPCR. The
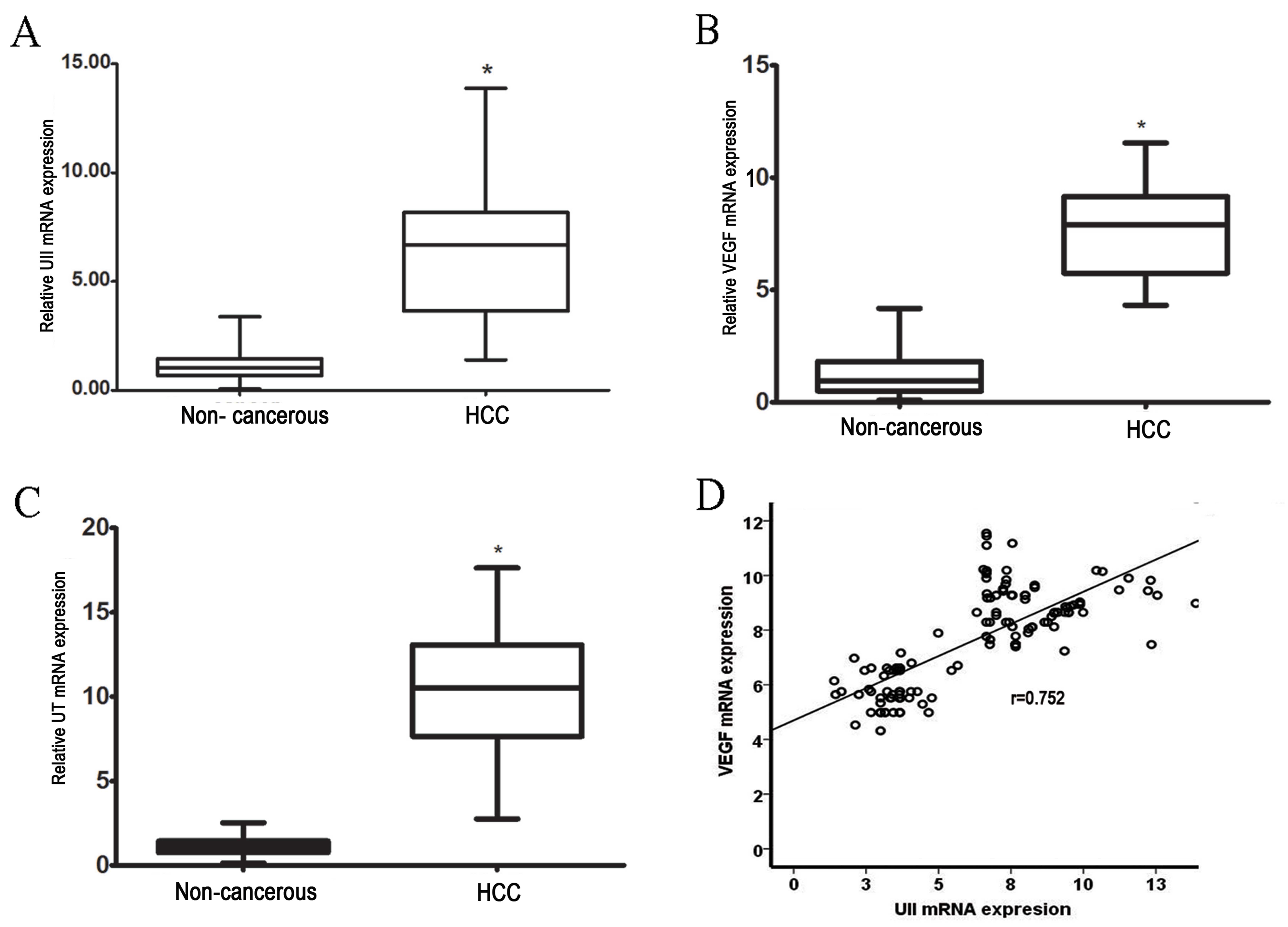
results revealed a 6-fold increase in UII mRNA levels in HCC
tissues compared with adjacent non-cancerous tissues (P<0.01;
Fig. 1A). Similarly, in HCC tissues,
UT expression levels were increased by ~10-fold compared with
adjacent non-cancerous tissues (P<0.01; Fig. 1B). These results revealed that UII and
UT mRNA levels were significantly higher in HCC tissues compared
with adjacent non-cancerous tissues (P<0.01).
VEGF expression is significantly
higher in HCC than in adjacent non-cancerous tissues. The
expression of VEGF mRNA was also examined by RT-qPCR
A 7-fold increase in VEGF mRNA was observed in HCC
tissues when compared with adjacent non-cancerous tissues
(P<0.01; Fig. 1C). Furthermore, a
significant positive correlation was identified between VEGF mRNA
expression and UII expression in HCC (P<0.001; r=0.78; Fig. 1D).
Immunohistochemical analysis of UT
expression in HCC tissues
Immunohistochemistry revealed that non-cancerous
tissues exhibited low UT protein expression levels (Fig. 2A, lower left panel). By contrast,
abundant UT protein expression was identified in HCC tissues
(Fig. 2A, lower right panel).
Furthermore, UT staining was observed in the cytoplasm of tumor
stromal cells (Fig. 2B). Western blot
analysis of six representative HCC tissues identified a significant
increase in the levels of UT protein in cancerous tissues when
compared with non-cancerous tissues (Fig.
2C and D). These findings are consistent with the relative UT
mRNA expression levels detected by RT-qPCR (Fig. 1B).
 | Figure 2.IHC staining of UT protein in HCC and
adjacent non-cancerous tissues. (A) Upper left panel, adjacent
non-cancerous tissue (HE staining); upper right panel, HCC tissue
(HE staining); lower left panel, adjacent non-cancerous tissue (IHC
staining); and lower right panel, HCC tissue (IHC staining). IHC
staining revealed low levels of UT expression in adjacent
non-cancerous tissue; however, increased UT expression was observed
in HCC tissue. Scale bars, 50 µm. (B) Positive UT expression was
identified in the cytoplasm of tumor stromal cells. Scale bar, 50
µm. (C) UT protein expression was analyzed by western blotting in
six representative HCC tissues and the matched adjacent
non-cancerous tissues, A 60-kDa band, indicative of UT expression,
was observed in the liver of 6 patients. (D) Quantification of
western blotting revealed that UT protein expression was
significantly higher in HCC tissues compared with adjacent
non-cancerous tissues. UT protein expression was normalized to
β-actin and expressed as the mean ± standard deviation. *P<0.01,
HCC vs. adjacent non-cancerous tissue. HCC, hepatocellular
carcinoma; UT, urotensin II receptor; IHC, immunohistochemical; HE,
hematoxylin and eosin. |
High UII mRNA expression is associated
with tumor size, histological differentiation and pathological
stage in HCC patients
Associations between UII expression and
clinicopathological parameters in HCC patients were analyzed using
the χ2 test. The median mRNA expression level of UII in
HCC tissues, 6.56-fold, was used as the cut-off value to divide the
129 patients into two groups: A low-expression group (UII mRNA
expression level <6.56; n=53) and a high-expression group (UII
mRNA expression level >6.56; n=76). As presented in Table I, a correlation was identified between
UII expression and tumor size (P<0.001), histological
differentiation (P<0.001) and pathological stage (P=0.026).
However, no significant associations were observed between UII mRNA
expression and gender (P=0.398), age (P=0.421), liver cirrhosis
(P=0.295), expression of hepatitis B surface antigen (HBsAg)
(P=0.139) or serum AFP levels (P=0.854). Furthermore, the
association between UT expression and clinicopathological
parameters was analyzed (Table
III). The mean UT relative mRNA expression level of HCC tissues
was 10.04, which was used as the cut-off value to divide the 129
patients into two groups. Higher UT expression was significantly
associated with tumor size (P<0.001) and pathological stage
(P=0.016); however, no significant association was identified
between UT mRNA expression and gender (P=0.197), age (P=0.543),
liver cirrhosis (P=0.193), HBsAg expression (P=0.183), serum AFP
levels (P=0.724) or histological differentiation (P=0.252)
(Table III).
 | Table III.Associations between UT expression
and clinicopathological parameters in 129 hepatocellular carcinoma
patients. |
Table III.
Associations between UT expression
and clinicopathological parameters in 129 hepatocellular carcinoma
patients.
|
|
| UT mRNA
expression |
|
|---|
|
|
|
|
|
|---|
| Parameter | Patients,
(n=129) | Low (n=54) | High (n=75) | P-value |
|---|
| Age, years |
|
|
| 0.543 |
|
<60 | 59 | 23 | 31 |
|
|
≥60 | 70 | 31 | 39 |
|
| Gender |
|
|
| 0.197 |
|
Male | 80 | 37 | 43 |
|
|
Female | 49 | 17 | 32 |
|
| HBsAg |
|
|
| 0.183 |
|
Positive | 78 | 29 | 49 |
|
|
Negative | 51 | 25 | 26 |
|
| AFP, ng/ml |
|
|
| 0.724 |
|
≤400 | 55 | 24 | 31 |
|
|
>400 | 74 | 30 | 44 |
|
| Liver
cirrhosis |
|
|
| 0.193 |
|
Present | 87 | 33 | 54 |
|
|
Absent | 42 | 21 | 21 |
|
| Esophageal
varices |
|
|
| 0.520 |
|
Present | 77 | 34 | 43 |
|
|
Absent | 52 | 20 | 32 |
|
| Histological
differentiation |
|
|
| 0.252 |
|
Well/moderate | 58 | 39 | 19 |
|
|
Poor | 71 | 15 | 56 |
|
| Tumor size, cm |
|
|
| <0.001 |
| ≤5 | 62 | 42 | 20 |
|
|
>5 | 67 | 12 | 55 |
|
| Pathological
stage |
|
|
| 0.016 |
|
I–II | 58 | 31 | 27 |
|
|
III–IV | 71 | 23 | 48 |
|
High UII mRNA expression is associated
with poor prognosis in HCC patients
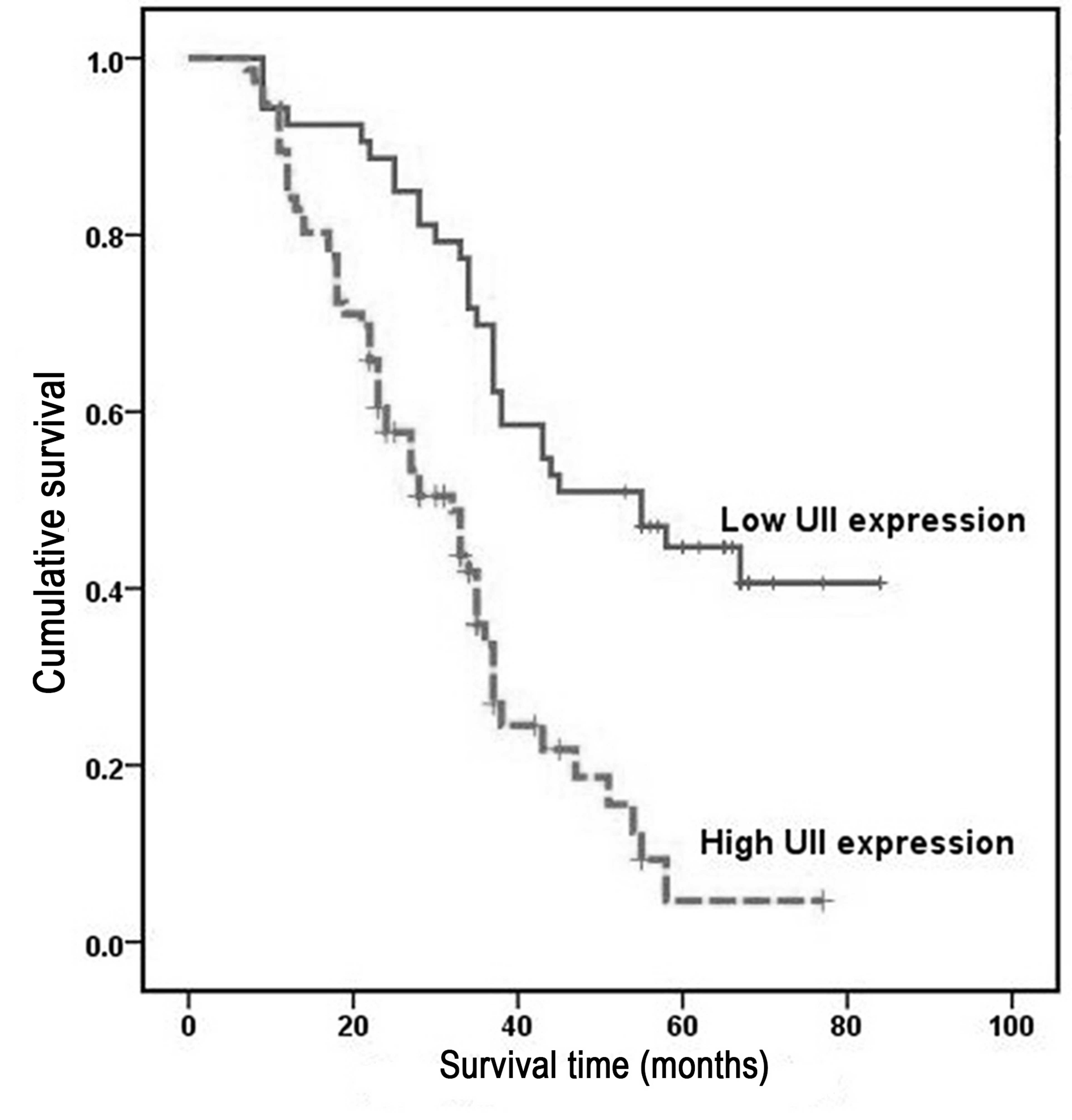
Overall survival was analyzed using the Kaplan-Meier
method, which demonstrated that the survival rates of HCC patients
with high UII expression were significantly lower than those of
patients with low UII expression (P<0.001; Fig. 3). Univariate analysis demonstrated a
significant association between overall patient survival rates and
tumor size (P=0.031), histological differentiation grade (P=0.017),
pathological stage (P=0.017) and UII mRNA expression (P=0.017)
(Table IV). However, no significant
associations were identified between UT mRNA expression (P=0.058),
patient age (P=0.432), gender (P=0.781), HBsAg expression
(P=0.908), serum AFP levels (P=0.407) or patient outcomes.
Multivariate analysis using the Cox proportional hazards model for
all the variables included in the univariate analysis indicated
that histological differentiation grade (P=0.031), pathological
stage (P=0.006) and UII expression (P=0.0001) were all independent
prognostic factors for overall survival in HCC patients (Table IV).
 | Table IV.Univariate and multivariate analysis
of overall survival for 129 patients with hepatocellular
carcinoma. |
Table IV.
Univariate and multivariate analysis
of overall survival for 129 patients with hepatocellular
carcinoma.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| UII expression | 2.05 | 0.176–1.035 | 0.017 | 1.12 | 0.072–0.811 | <0.001 |
| UT expression | 0.58 | 0.302–1.071 | 0.058 | 0.55 | 0.331–1.075 | 0.080 |
| Age | 1.71 | 1.238–2.794 | 0.432 | 1.53 | 0.275–4.346 | 0.867 |
| Gender | 0.54 | 0.134–1.967 | 0.781 | 0.87 | 0.207–3.675 | 0.450 |
| HBsAg | 1.22 | 0.475–1.557 | 0.908 | 1.26 | 0.296–4.541 | 0.800 |
| AFP | 0.68 | 0.472–2.034 | 0.407 | 1.23 | 0.170–3.043 | 0.877 |
| Liver
cirrhosis | 0.73 | 0.443–2.907 | 0.951 | 1.47 | 0.074–2.693 | 0.203 |
| Histological | 1.33 | 0.479–3.871 | 0.017 | 1.76 | 0.037–0.394 | 0.031 |
| Tumor size | 1.37 | 0.537–3.880 | 0.031 | 2.41 | 0.045–2.364 | 0.440 |
| Pathological
stage | 0.97 | 0.097–3.554 | 0.017 | 1.45 | 1.011–2.093 | 0.006 |
Discussion
Tumor cells produce and secrete a number of
vasoactive peptides such as endothelin-1 and adrenomedullin, which
act as paracrine growth stimulators (27). UII is a somatostatin-like cyclic
undecapeptide that has been identified as a potent mammalian
vasoconstrictor (15). As well as
affecting vascular tone, UII stimulates cell proliferation, and
previous studies have indicated that is involved in the
pathogenesis of certain tumors, including breast, bladder, colon
and prostate cancer (12–14,28). Our
previous study demonstrated that the expression of the UII/UT
system was increased in HCC tissues and cell lines, and that
exogenous administration of UII regulated the proliferation of
cancer cells and increased the expression of various transcription
factors, including ERK, PKC and p38 MAPK (18,20).
However, the association between the UII/UT system and the
clinicopathological behavior of HCC remains unclear.
For a more comprehensive insight into the clinical
value of the UII/UT system in HCC, in the current study, RT-qPCR
was performed to measure UII/UT mRNA expression, and the
association between UII/UT mRNA expression and patient
clinicopathological features was analyzed. The results revealed
that UII mRNA expression was significantly upregulated in HCC
tissues, and that its expression was correlated with tumor stage,
size and differentiation. Furthermore, it was determined that UII
mRNA expression was an independent prognostic factor for overall
patient survival. In addition, UT mRNA expression was analyzed by
RT-qPCR, and it was demonstrated that UT mRNA was significantly
overexpressed in HCC tumor tissue samples when compared with
adjacent non-tumor tissue samples. Western blot analysis confirmed
these results, demonstrating elevated UT protein expression in six
representative HCC tissues. Taken together, these results support
the hypothesis that UII may function as an oncogene in HCC, and
thus may exhibit an important function in the tumorigenesis of
HCC.
Univariate and multivariate analyses demonstrated
that UII expression and prognostic factors, including tumor size,
histological differentiation and pathological stage, are
independent prognostic factors for HCC patients. Univariate
analyses demonstrated a significant association between increased
UII mRNA expression in HCC tissues and decreased overall survival
rates. Kaplan-Meier survival analysis indicated that the overall
survival rate of HCC patients with high UII expression was
significantly lower than that of patients with low UII expression.
These results indicate that UII expression may represent a novel
prognostic marker for HCC patients.
VEGF is one of the most potent angiogenic factors
and an essential mediator of both angiogenesis and endochondral
ossification (29). Previous studies
have demonstrated that it serves a critical function in HCC tumor
angiogenesis (30–32). Furthermore, it has been reported that
the pro-angiogenic cytokine UII directly stimulates an angiogenic
phenotype in endothelial cells following exposure to UII, and
enhances the process indirectly by delaying the production of other
pro-angiogenic factors such as VEGF (10). It is also hypothesized that different
genetic backgrounds of patients and etiological factors may affect
the development of HCC, and thus, different mechanisms of
transformation may occur (29,33). As a
single-agent therapy, the multi-kinase inhibitor sorafenib appears
to have limited efficacy in HCC (9).
However, using sorafenib in combination with other agents that
control HCC-derived symptoms may be clinically beneficial for
patients with HCC (34). UII is a
non-classical angiogenic factor (10,11), and
may therefore regulate the endothelial expression of VEGF in HCC.
Notably, in the present study, a significant positive correlation
was identified between UII and VEGF expression. Therefore, UII may
be involved in tumor angiogenesis by stimulating the production of
VEGF and by enhancing tumor growth and progression in HCC.
Furthermore, UT staining was observed in tumor stromal cells. UII
is a vasoactive cyclic neuropeptide that activates UT and exhibits
various effects; therefore, it has been postulated that UII may
stimulate the proliferation of tumor stromal cells (35). However, further studies are required
to confirm this hypothesis.
In conclusion, the results of the present study
indicated that the UII/UT system is overexpressed in HCC, and that
high UII mRNA expression is associated with poor overall survival
rates. These findings suggest that the UII/UT system may represent
a potential therapeutic target for HCC treatment in the future.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (Beijing, China; grant no.
81170408), The China Postdoctoral Science Special Foundation
(Beijing, China; grant no. 2013T60386) and The China Postdoctoral
Science Foundation (Beijing, China; grant no. 2012M510094).
References
|
1
|
Chow PK, Choo SP, Ng DC, Lo RH, Wang ML,
Toh HC, Tai DW, Goh BK, Wong JS, Tay KH, et al: National cancer
centre singapore consensus guidelines for hepatocellular carcinoma.
Liver Cancer. 5:97–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bodzin AS and Busuttil RW: Hepatocellular
carcinoma: Advances in diagnosis, management, and long term
outcome. World J Hepatol. 7:1157–1167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peck-Radosavljevic M: Drug therapy for
advanced-stage liver cancer. Liver cancer. 3:125–131. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albertin G, Guidolin D, Sorato E,
Oselladore B, Tortorella C and Ribatti D: Urotensin-II-stimulated
expression of pro-angiogenic factors in human vascular endothelial
cells. Regul Pept. 172:16–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yumrutas O, Oztuzcu S, Büyükhatipoglu H,
Bozgeyik I, Bozgeyik E, Igci YZ, Bagis H, Cevik MO, Kalender ME,
Eslik Z and Arslan A: The role of the UTS2 gene polymorphisms and
plasma Urotensin-II levels in breast cancer. Tumor Bio.
36:4427–4432. 2015. View Article : Google Scholar
|
|
12
|
Franco R, Zappavigna S, Gigantino V, Luce
A, Cantile M, Cerrone M, Facchini G, Perdonà S, Pignata S, Di
Lorenzo G, et al: Urotensin II receptor determines prognosis of
bladder cancer regulating cell motility/invasion. J Exp Clin Cancer
Res. 33:482014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Federico A, Zappavigna S, Romano M, Grieco
P, Luce A, Marra M, Gravina AG, Stiuso P, D'Armiento FP, Vitale G,
et al: Urotensin-II receptor is over-expressed in colon cancer cell
lines and in colon carcinoma in humans. Eur J Clin Invest.
44:285–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grieco P, Franco R, Bozzuto G, Toccacieli
L, Sgambato A, Marra M, Zappavigna S, Migaldi M, Rossi G, Striano
S, et al: Urotensin II receptor predicts the clinical outcome of
prostate cancer patients and is involved in the regulation of
motility of prostate adenocarcinoma cells. J Cell Biochem.
112:341–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ames RS, Sarau HM, Chambers JK, Willette
RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF,
Coatney RW, et al: Human urotensin-II is a potent vasoconstrictor
and agonist for the orphan receptor GPR14. Nature. 401:282–286.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi K, Totsune K, Murakami O,
Arihara Z, Noshiro T, Hayashi Y and Shibahara S: Expression of
urotensin II and its receptor in adrenal tumors and stimulation of
proliferation of cultured tumor cells by urotensin II. Peptides.
24:301–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takahashi K, Totsune K, Murakami O and
Shibahara S: Expression of urotensin II and urotensin II receptor
mRNAs in various human tumor cell lines and secretion of urotensin
II-like immunoreactivity by SW-13 adrenocortical carcinoma cells.
Peptides. 22:1175–1179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Dong K, Xue X, Feng P and Wang X:
Elevated expression of urotensin II and its receptor in
diethylnitrosamine-mediated precancerous lesions in rat liver.
Peptides. 32:382–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu D, Chen J, Wang J, Zhang Z, Ma X, Jia
J and Wang Y: Increased expression of urotensin II and GPR14 in
patients with cirrhosis and portal hypertension. Int J Mol Med.
25:845–851. 2010.PubMed/NCBI
|
|
20
|
Yu XT, Wang PY, Shi ZM, Dong K, Feng P,
Wang HX and Wang XJ: Up-regulation of urotensin ii and its receptor
contributes to human hepatocellular carcinoma growth via activation
of the PKC, ERK1/2, and p38 MAPK signaling pathways. Molecules.
19:20768–20779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shariff MI, Cox IJ, Gomaa AI, Khan SA,
Gedroyc W and Taylor-Robinson SD: Hepatocellular carcinoma: Current
trends in worldwide epidemiology, risk factors, diagnosis and
therapeutics. Expert Rev Gastroenterol Hepatol. 3:353–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei HJ, Chau GY, Lui WY, Tsay SH, King KL,
Loong CC and Wu CW: Prognostic value and clinical relevance of the
6th Edition 2002 American joint committee on cancer staging system
in patients with resectable hepatocellular carcinoma. J Am Coll
Surg. 203:426–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pirisi M, Leutner M, Pinato DJ, Avellini
C, Carsana L, Toniutto P, Fabris C and Boldorini R: Reliability and
reproducibility of the edmondson grading of hepatocellular
carcinoma using paired core biopsy and surgical resection
specimens. Arch Pathol Lab Med. 134:1818–1822. 2010.PubMed/NCBI
|
|
24
|
Wang SB, Wang JH, Chen J, Giri RK and Chen
MH: Natural history of liver cirrhosis in south China based on a
large cohort study in one center: A follow-up study for up to 5
years in 920 patients. Chin Med J (Engl). 125:2157–2162.
2012.PubMed/NCBI
|
|
25
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bagnato A and Catt KJ: Endothelins as
autocrine regulators of tumor cell growth. Trends Endocrinol Metab.
9:378–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou CH, Wan YY, Chu XH, Song Z, Xing SH,
Wu YQ and Yin XX: Urotensin II contributes to the formation of lung
adenocarcinoma inflammatory microenvironment through the NF-κB
pathway in tumor-bearing nude mice. Oncol Lett. 4:1259–1263.
2012.PubMed/NCBI
|
|
29
|
Dai J and Rabie AB: VEGF: An essential
mediator of both angiogenesis and endochondral ossification. J Dent
Res. 86:937–950. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mukozu T, Nagai H, Matsui D, Kanekawa T
and Sumino Y: Serum VEGF as a tumor marker in patients with
HCV-related liver cirrhosis and hepatocellular carcinoma.
Anticancer Res. 33:1013–1021. 2013.PubMed/NCBI
|
|
32
|
Marra M, Sordelli IM, Lombardi A, Lamberti
M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R,
Accardo M, et al: Molecular targets and oxidative stress biomarkers
in hepatocellular carcinoma: An overview. J Transl Med. 9:1712011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colombino M, Sperlongano P, Izzo F,
Tatangelo F, Botti G, Lombardi A, Accardo M, Tarantino L, Sordelli
I, Agresti M, et al: BRAF and PIK3CA genes are somatically mutated
in hepatocellular carcinoma among patients from South Italy. Cell
Death Dis. 3:e2592012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caraglia M, Giuberti G, Marra M, Addeo R,
Montella L, Murolo M, Sperlongano P, Vincenzi B, Naviglio S, Prete
SD, et al: Oxidative stress and ERK1/2 phosphorylation as
predictors of outcome in hepatocellular carcinoma patients treated
with sorafenib plus octreotide LAR. Cell Death Dis. 2:e1502011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scognamiglio PL, Di Natale C, Perretta G
and Marasco D: From peptides to small molecules: An intriguing but
intricated way to new drugs. Curr Med Chem. 20:3803–3817. 2013.
View Article : Google Scholar : PubMed/NCBI
|