Introduction
Wnt signaling pathways are important in controlling
embryonic development, and are highly conserved in evolution
(1). The members of these pathways
are highly homologous between different species, from lower
organisms such as Drosophila to mammals (1). Previous studies demonstrated that the
processes of cell proliferation, organ formation and cell
determination involve Wnt signal transduction (1,2). In mature
individuals, abnormal activation of Wnt signaling is closely
associated with the occurrence of various diseases such as cancer
(1,2).
It is generally accepted that, as the most important factor of Wnt
signaling pathways in normal mature cells, β-catenin only acts as a
type of intracellular cytoskeleton protein close to the cellular
membrane, and forms a complex with E-cadherin to maintain homotypic
cell adhesion by preventing cell movement (3). β-catenin is not usually present in the
cytoplasm. Only under certain conditions, β-catenin accumulates in
the cytoplasm, and it is then transferred to the nucleus (3). The activation of the Wnt/β-catenin
signaling pathway is considered to have effects on cell
proliferation (3).
Previous studies revealed that the mechanism of
abnormal activity of Wnt signaling in lung cancer is different from
that in colorectal cancer (4,5). One of the most significant differences
is the activation of Wnt signaling pathways. Mutations and
anomalous protein expression of β-catenin and adenomatous polyposis
coli (APC), a tumor-suppressor protein that is a component of the
Wnt/β-catenin signaling pathway, are rarely observed in lung cancer
(4,5).
This suggests that a specific mechanism of Wnt signaling pathways
activation exists in lung tumors.
Multiple factors have been identified to serve a
role in the regulation of Wnt signaling pathways (1). Wnt inhibitory factor-1 (WIF-1) is an
important negative regulatory factor for this pathway, which
belongs to the secreted frizzled-related proteins family (6). As the inhibitory factor upstream of the
Wnt signaling pathways, WIF-1 is a highly conserved gene, and can
inhibit the activation of these pathways by directly combining with
Wnt signaling proteins (6). It is
well known that WIF-1 could act as a tumor suppressor, and WIF-1
has been observed to be epigenetically silenced in various cancers
(6).
Previous studies have shown that disordered
methylation patterns in the regulation of gene expression, such as
high methylation of tumor-suppressor genes, will lead to
tumorigenesis (7). Previous studies
reported that WIF-1 expression was significantly downregulated or
even silenced by high methylation in its promoter region in
non-small cell lung cancer (NSCLC) cell lines (8). Therefore, WIF-1 may be a key antagonist
for Wnt signaling pathways to prevent the occurrence of lung cancer
(9).
The present study aimed to explore the differential
expression of key molecules associated with Wnt signaling pathways
between clinical NSCLC and paracarcinoma tissue samples. In
addition, the present study discusses the role of the activation of
molecules associated with Wnt signaling pathways in the tumorigenic
mechanism of NSCLC.
Materials and methods
Reagents
The RNAprep Pure kit (For Tissue) was purchased from
Tiangen Biotech Co., Ltd. (Beijing, China). The RevertAid First
Strand cDNA Synthesis kit was purchased from Fermentas (Thermo
Fisher Scientific, Inc., Pittsburgh, PA, USA). A reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) kit
(DRR01AM) was purchased from Takara Bio, Inc. (Otsu, Japan).
Antibodies specific to β-catenin (catalog no., sc-7199) and β-actin
(catalog no., sc-130300) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Anti-WIF-1 (catalog no.,
5652) and anti-cyclin D1 (catalog no., 1044S) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Peroxidase-conjugated ImmunoPure® goat anti-rabbit
immunoglobulin G (IgG) [heavy (H) + light (L)] and biotinylated
ImmunoPure® goat anti-mouse IgG (H + L) (catalog no.,
MII0401) were purchased from Pierce (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). All other reagents used were
analytical-grade laboratory chemicals from standard commercial
suppliers.
Clinical cases selection and tissue
samples treatment
Human NSCLC tissue samples and paracarcinoma lung
tissue samples (used as control samples) were obtained from 52
patients [23 squamous carcinomas and 29 adenocarcinomas;
tumor-node-metastasis (TNM) I, 24 cases and TNM II/III, 28 cases]
from January 2012 to October 2013. These patients had undergone
surgical resection of their primary cancer at the Department of
Thoracic Surgery in Tianjin Union Medicine Centre (Hongqiao,
China). Lung tissues were obtained by video-assisted thoracoscopic
surgery to resect the pulmonary lobe containing the tumor, and then
flash frozen in liquid nitrogen and stored at −150°C or fixed in
paraformaldehyde. All patients provided informed consent prior to
sample collection, according to the institutional guidelines of
Tianjin Union Medicine Centre. The present protocol was approved by
the ethics committee of Tianjin Union Medicine Centre. The
histological type and grade of tumor were classified on the basis
of the World Health Organization criteria (10). The stage of each cancer was identified
according to the International Association for the Study of Lung
Cancer and the American Thoracic Society criteria (11). All primary tumor tissues and control
samples were diagnosed by hematoxylin and eosin (H&E) staining.
The frozen samples were used for RT-qPCR detection, while the
tissues fixed in paraformaldehyde were used for immunohistochemical
examination. The concentration of RNA was determined by the
absorbance (A) 260/A280 ratio using a spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Assessment of lung histopathology
Histopathological determination was performed as
described previously (12). The lung
tumor tissues were removed from the 10% formaldehyde storage
solution, and then subjected to regular dehydration, paraffin
embedding, sectioning and dewaxing. Upon H&E staining, samples
were subjected again to dehydration and sealing. Next, the tumor's
histopathology was observed under a light microscope, and images
were obtained.
RT-qPCR analysis
Total cellular RNA of lung tissues was isolated
using the RNAprep Pure kit (For Tissue) (Tiangen Biotech Co., Ltd.)
according to the manufacturer's protocol. Total RNA (2.0 µg) was
reverse transcribed into complementary DNA using the RevertAid
First Strand cDNA Synthesis kit with oligo(dT). All primers were
designed and synthesized by Takara Bio, Inc. The primer sequences,
annealing temperatures and expected product sizes are listed in
Table I. All PCR procedures were
conducted in the MJ Research PTC-200 DNA Engine Thermal Cycler
(Bio-Rad Laboratories, Inc.) using the following cycling
parameters: 2 min of initial denaturation at 94°C, followed by 30
sec of denaturation at 94°C, and 35 cycles of 30 sec at 94°C
(denaturing), 30 sec at 58°C (annealing) and 30 sec at 72°C
(elongation), with a final extension at 72°C for 5 min. The
presence of PCR products was confirmed by gel electrophoresis on a
2% agarose gel and staining with ethidium bromide. Bands were
visualized in a Gel Doc 1000 system (Bio-Rad Laboratories, Inc.).
β-actin was used as an internal control in parallel for each
replicate. Gel-Pro Analyzer software version 3.0 (Media
Cybernetics, Inc., Rockville, MD, USA) was used to quantify the
denisty of each band. The experiments were performed three times
independently, and the mean value was used for comparison.
 | Table I.List of primers used for reverse
transcription-quantitative polymerase chain reaction analyses. |
Table I.
List of primers used for reverse
transcription-quantitative polymerase chain reaction analyses.
| Gene name | Primer sequence
(5′-3′)a | Annealing
temperature, °C | Product length,
bp |
|---|
| WIF-1 | F:
ATCCTGCACCTGCGACTACAG | 58.0 | 432 |
|
| R:
GGCGACTTCTCGAAGTAGACC |
|
|
| APC | F:
CGGAACATGCATGACTGATAC | 58.0 | 310 |
|
| R:
GTCACGAGGTACGACCTCAGAT |
|
|
| β-catenin | F:
AAGTTCTTGGCTATTACGACA | 58.2 | 375 |
|
| R:
ACAGCACCTTCAGCACTCT |
|
|
| Cyclin D1 | F:
CAGAAGTGCGAAGCTTAGGTCT | 58.0 | 420 |
|
| R:
GTAGCAGGAGTAGTCCAGCGG |
|
|
| β-actin | F:
CGTTGACATCCGTAACGACTCC | 56.0 | 660 |
|
| R:
ATAGAGCCACCATTCCGACACAG |
|
|
Immunohistochemical staining
analysis
Immunohistochemical staining was used to detect the
protein expression of WIF-1, β-catenin and cyclinD1, as described
previously (13). Paraffin-embedded
tissues were sectioned serially into 4-µm-thick sections, which
were then immersed in 10 mmol/l citrate buffer (pH 6.0) and heated
in a microwave oven at 100°C for 10 min. Endogenous peroxidase
activity was quenched with 3% hydrogen peroxide in methanol for 10
min. Subsequently, the sections were blocked with 5% (v/v) bovine
normal serum (Tiangen Biotech Co., Ltd.) in PBS for 20 min. Next,
sections were incubated for 12 h or overnight at 4°C in a
humidified chamber with the following primary antibodies: Rat
polyclonal anti-human β-catenin (1:50; Santa Cruz Biotechnology,
Inc.), rat polyclonal anti-human β-actin (1:50; Santa Cruz
Biotechnology, Inc.), rat polyclonal anti-human WIF-1 (1:50; Cell
Signal Technology, Inc.), rat polyclonal anti-human APC (1:50; Cell
Signal Technology, Inc.) and rat polyclonal anti-human cyclin D1
(1:50; Cell Signal Technology, Inc.). Thereafter, sections were
washed three times with PBS, incubated with an appropriate
biotinylated secondary antibody (1:1,000; Thermo Fisher Scientific,
Inc.), washed three times and incubated at 4°C for 20 min with
streptavidin-peroxidase (catalog no., CJ31H; Thermo Fisher
Scientific, Inc.). Staining was visualized by adding
3,3′-diaminobenzidine (DAB Substrate; Roche Diagnostics GmbH,
Mannheim, Germany), and then counterstained using hematoxylin.
Sections were next rinsed in tap water, dehydrated through 70–100%
graded alcohol, cleared in xylene and finally mounted in permanent
mounting medium. Representative micrographs of the
immunohistochemical results were acquired with a microscope camera
system (FSX100; Olympus Corporation, Tokyo, Japan). Three sections
were analyzed for each sample.
Statistical analysis
Data were presented as percentage or as means ±
standard deviation. Student's t-test and analysis of variance were
used to detect differences in the mean values of the variables.
Fisher's exact test or χ2 test was used as appropriately
to analyze the differences in each variable. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed with the software SPSS 13.0
(SPSS, Inc., Chicago, IL, USA).
Results
Clinicopathological characteristics of
cases
A total of 29 cases with adenocarcinoma and 23 cases
with squamous carcinoma were randomly selected and employed in the
present study. The mean age of the patients in the adenocarcinoma
group and in the squamous carcinoma group was 56.3 years (range,
38–73 years) and 53.8 years (range, 35–72 years), respectively.
There were no differences in gender, nodal metastasis, pathological
classification or clinical stage between the adenocarcinoma group
and the squamous carcinoma group (P=0.930, P=0.420, P=0.638 and
P=0.438, respectively). The clinicopathological characteristics and
statistical analysis of these cases are displayed in Table II.
 | Table II.Clinicopathological factors of
patients with adenocarcinoma or squamous carcinoma. |
Table II.
Clinicopathological factors of
patients with adenocarcinoma or squamous carcinoma.
| Characteristics | Patients with
adenocarcinoma (n=29), n (%) | Patients with
squamous carcinoma (n=23), n (%) | P-value |
|---|
| Gender |
|
| 0.930 |
| Male | 18 (62.07) | 14 (60.87) |
|
|
Female | 11 (37.93) | 9
(39.13) |
|
| Lymph nodal
metastasis |
|
| 0.420 |
|
Positive | 23 (79.31) | 16 (69.57) |
|
|
Negative | 6
(20.69) | 7
(30.43) |
|
| Pathological
classification |
|
| 0.638 |
| High | 13 (44.83) | 7
(30.43) |
|
|
Middle | 7
(24.14) | 10 (43.48) |
|
| Low | 9
(31.03) | 6
(26.09) |
|
| Clinical stage |
|
| 0.438 |
| I | 12 (41.38) | 12 (52.17) |
|
|
II/III | 17 (58.62) | 11 (47.83) |
|
mRNA expression levels of WIF-1, APC,
β-catenin and cyclin D1
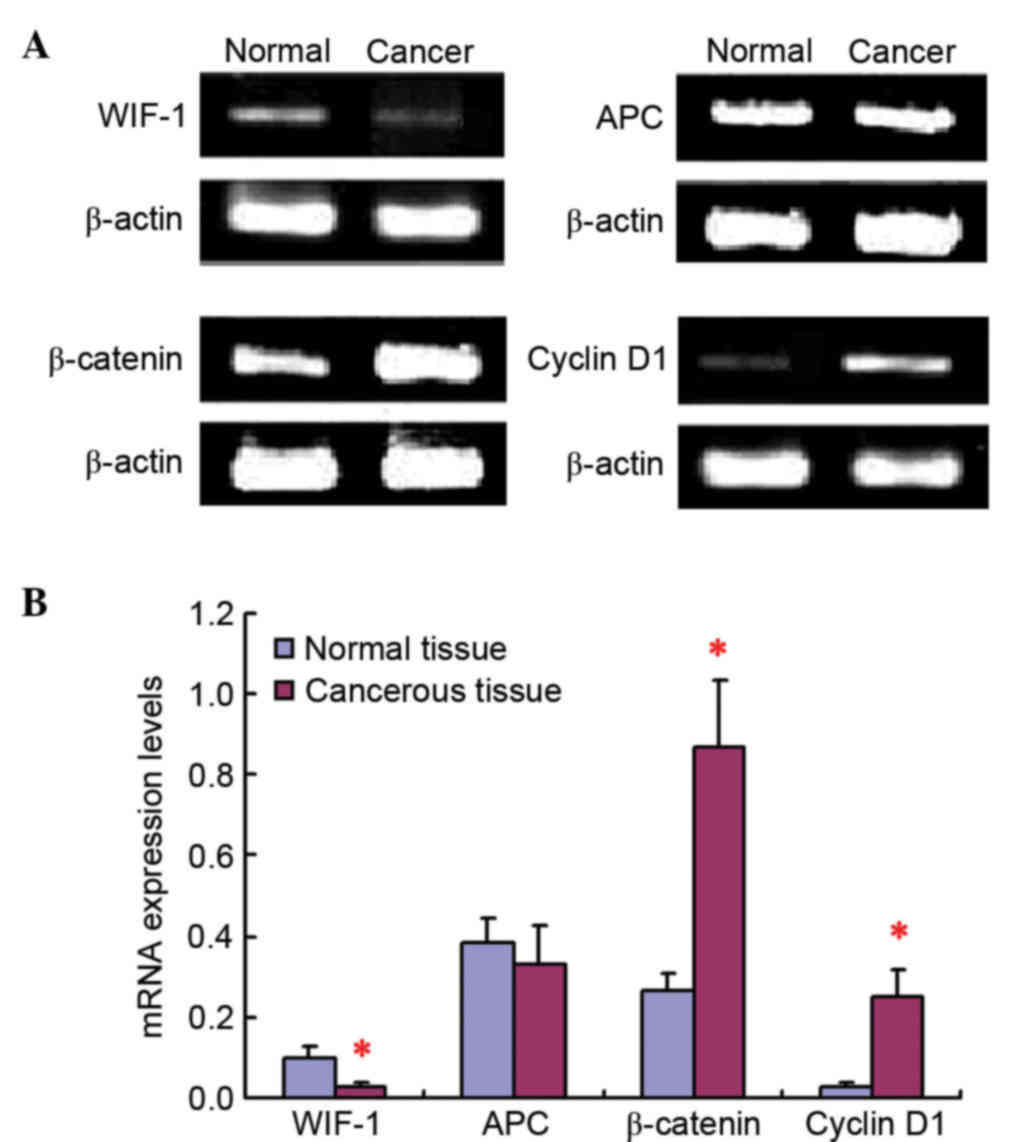
The RT-qPCR results revealed that WIF-1, APC,
β-catenin and cyclin D1 mRNA expression was present both in cancer
tissue samples and in paracarcinoma tissue samples (Fig. 1). No obvious difference could be
observed for APC mRNA expression levels between lung cancer tissues
and adjacent tissues to the carcinoma. However, compared with
normal tissues, the mRNA expression level of WIF-1 was
significantly downregulated, while the β-catenin and cyclin D1 mRNA
expression levels were remarkably increased, in tumor tissues.
Importantly, the mRNA expression of cyclin D1 was remarkably low in
normal lung tissues, but was markedly upregulated in tumor
tissues.
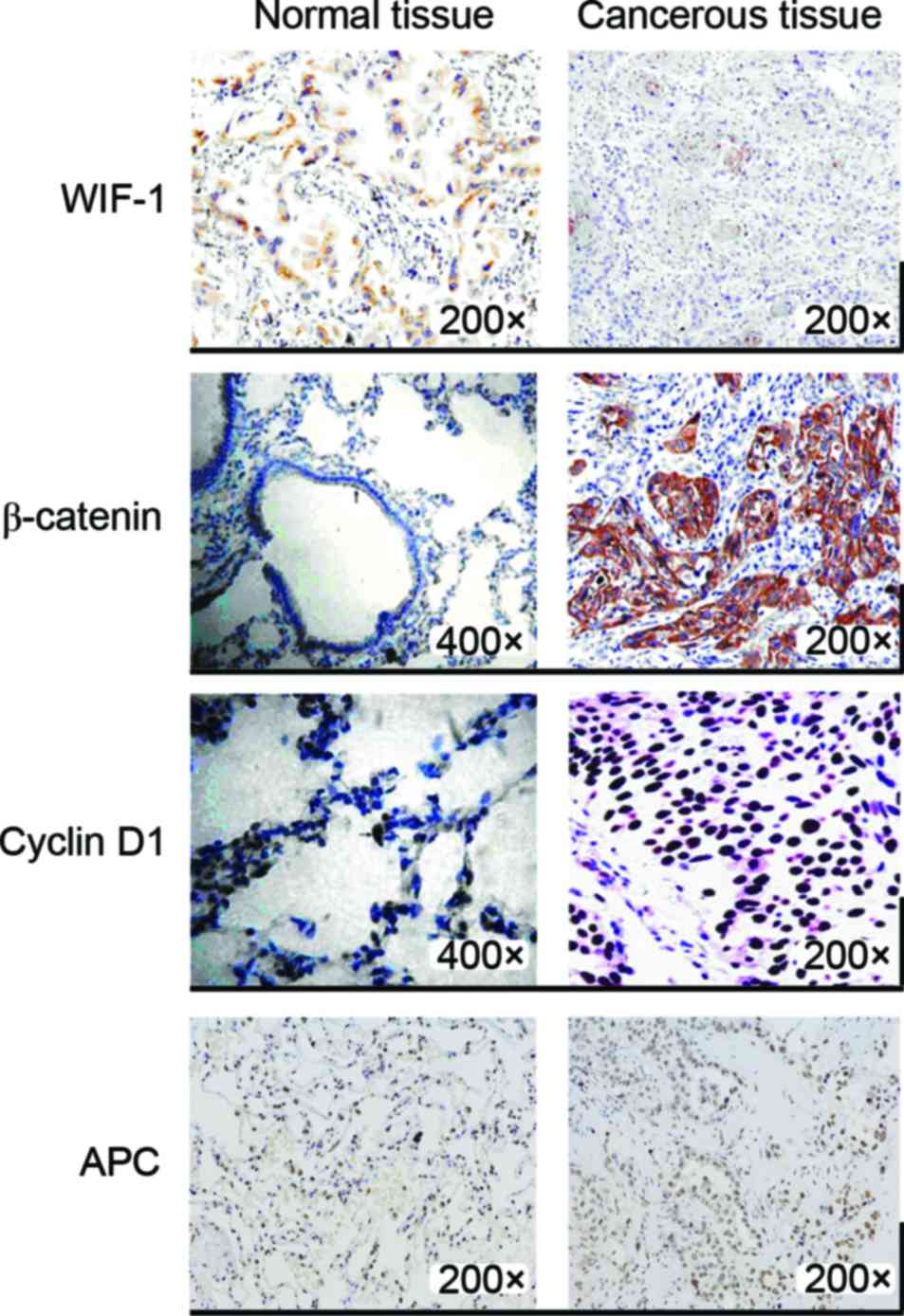
Immunohistochemical staining detection
of the protein expression levels of WIF-1, β-catenin, cyclin D1 and
APC
As shown by immunohistochemical staining, WIF-1,
β-catenin, cyclin D1 and APC exhibited positive expression both in
cancer tissue samples and in paracarcinoma lung tissue samples
(Fig. 2). The proteins levels of
β-catenin and cyclin D1 were much higher in cancer tissues than in
paracarcinoma tissues. β-catenin was mainly expressed in the
cytoplasm. The upregulation rates of β-catenin and cyclin D1 were
76.9 and 67.3% in lung tissue, respectively. WIF-1 expression was
clearly decreased in cancer tissues compared with that in normal
tissues, and its downregulation rate was 92.3%. The same results
were obtained for mRNA expression, and no obvious difference could
be observed for APC protein expression levels between lung cancer
tissues and adjacent tissues to the carcinoma. The results of the
analysis of the association between the protein expression levels
of WIF-1, β-catenin and cyclin D1 detected by immunohistochemical
staining and the clinical characteristics of patients in the NSCLC
group are shown in Table III. The
percentage of patients with downregulated WIF-1 expression and
positive lymph nodal metastasis is significantly higher than the
percentage of patients who are negative for lymph nodal metastasis
or exhibit upregulated WIF-1 expression with positive lymph nodal
metastasis (P=0.006). The percentage of patients with upregulated
cyclin D1 expression and positive lymph nodal metastasis is
significant higher than that of patients with negative lymph nodal
metastasis or those who exhibit downregulated cyclin D1 expression
with positive lymph nodal metastasis (P=0.043). Furthermore, the
percentage of patients with upregulated cyclin D1 expression and
middle or low pathological classification was significantly higher
than the percentage of patients with high pathological
classification or with downregulated cyclin D1 expression and
middle/low pathological classification (P=0.008).
 | Table III.Analysis of the association between
the protein expressiona
of WIF-1, β-catenin and cyclin D1 and the clinical characteristics
of patients with non-small cell lung cancer. |
Table III.
Analysis of the association between
the protein expressiona
of WIF-1, β-catenin and cyclin D1 and the clinical characteristics
of patients with non-small cell lung cancer.
|
|
| WIF-1
expression | β-catenin
expression | Cyclin D1
expression |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | N | +, n (%) | −, n (%) | +, n (%) | −, n (%) | +, n (%) | −, n (%) |
|---|
| Histopathology |
|
|
Adenocarcinoma | 29 | 7 (24.14) | 22 (75.86) | 23 (79.31) | 6 (20.69) | 20 (68.97) | 9 (31.03) |
|
Squamous carcinoma | 23 | 5 (7.70) | 18 (92.30) | 17 (73.91) | 6 (26.09) | 15 (65.22) | 8 (34.78) |
| Lymph nodal
metastasis |
|
|
Positive | 39 | 3 (7.69) | 36
(92.31)b | 31 (79.49) | 8 (20.51) | 29
(74.36)b | 10 (25.64) |
|
Negative | 13 | 6 (46.15) | 7 (53.85) | 6 (46.15) | 7 (53.85) | 5 (38.46) | 8 (61.54) |
| Pathological
classification |
|
|
High | 20 | 8 (40.00) | 12 (60.00) | 12 (60.00) | 8 (40.00) | 11 (55.00) | 9 (45.00) |
|
Middle/low | 32 | 6 (18.75) | 26 (81.25) | 25 (78.13) | 7 (21.87) | 28
(87.50)a | 4 (12.50) |
| Clinical stage |
|
| I | 24 | 7 (29.17) | 17 (70.83) | 14 (58.33) | 10 (41.67) | 12 (50.00) | 12 (50.00) |
|
II/III | 28 | 4 (14.29) | 24 (85.71) | 21 (75.00) | 7 (25.00) | 18 (64.29) | 10 (35.71) |
Discussion
During the tumorigenesis of lung cancer, more
attention should be paid to the regulatory effect of WIF-1, which
is the upstream factor in the Wnt signaling pathway (9), as well as to the activation and
regulation of Wnt signal transduction under different physiological
and pathological conditions. Exploring the role of Wnt signaling
pathways in the activating process could provide a theoretical
basis and novel targets for the clinical diagnosis and treatment of
lung cancer.
It has been confirmed that the abnormality of the
Wnt signaling pathway is closely associated with malignant tumors,
including colorectal cancer, melanoma, NSCLC, head and neck cancer,
leukemia and mesothelioma (14).
Mainly absence or downregulation of WIF-1 expression could be
observed in these tumors, which correlates with signal transduction
(15). It was reported that, compared
with normal tissues, decreased WIF-1 expression could happen in 80%
of esophageal cancer cases, 75% of gastric cancer cases, 74.6% of
colon cancer cases and 66% of pancreatic cancer cases (15). However, there was no obvious
correlation between reduced WIF-1 expression and the
clinicopathological characteristics of the tumor (15). In NSCLC, the downregulated or missing
expression of WIF-1 could be detected in ~72% of cases.
In the present study, the expression of various
important factors in the Wnt signaling pathway was detected,
including β-catenin, APC and cyclin D1. Furthermore, the negative
regulatory factors of WIF-1 expression were also detected. The
results indicated that, compared with normal tissues, a marked
decrease in WIF-1 expression and an increase in β-catenin and
cyclin D1 expression could be observed in tumor tissues, indicating
Wnt signaling activation. In lung cancer tissues, β-catenin protein
with position transfer is mainly expressed in cytoplasm other than
in cytomembrane as normal lung tissue. The activation of the Wnt
signaling pathway may be associated with human lung cancer
(16). Repressing WIF-1 expression
could act as an initial promoter in the activation of Wnt signal
transduction. Since the expression of APC was not altered in the
present study, it is possible to conclude that the Wnt signaling
pathways activated in NSCLC may differ from those in other
tumors.
With the in-depth study of Wnt signaling pathways,
WIF-1 as an inhibitory factor and its association with the
signaling pathway is attracting more and more attention. WIF-1
inhibits the activity of Wnt signaling pathways by directly binding
to Wnt proteins (17). In normal
tissues of the human body, with the exception of the retina (which
is the tissue where WIF-1 was initially identified), the presence
of WIF-1 was detected in a variety of organs such as the lung,
prostate, brain and skeletal muscle (17).
At present, the interaction mechanism of WIF-1 and
Wnt is still not fully clear. A previous study reported that WIF-1
displayed different inhibition mechanisms with different Wnt
molecules (18). There are at least
two types of Wnt proteins that could interact with human WIF-1 to
form a non-covalent complex with highly specific affinity in
vitro (18). WIF-1 and Wnt4 are
both components of the extracellular matrix (18). It was reported that WIF-1 could
combine and form complex with Wnt4, thereby inhibiting Wnt4 to
transduce any intracellular signal. Wnt signaling pathways could be
inhibited due to the action of WIF-1, which consequently inhibits
cell growth and differentiation (19). By reducing WIF-1 expression, Wnt
signaling can be activated, which promotes cell growth and
proliferation (19–21).
The structure of WIF-1 is known. Although it has
been confirmed that WIF-1 can be used as a negative feedback
regulatory factor for Wnt signaling pathways (22), its own regulation and the interaction
mechanism between Wnt signaling pathways and WIF-1 expression
regulation are still not fully clear. Previous studies have
confirmed that certain reactive elements of T-cell factor (TCF)
cells exist in the gene promoter region of WIF-1, and that TCF
cells are important nuclear target factors for Wnt signaling
pathways (23). The activation of Wnt
signaling could induce β-catenin accumulation in the cytoplasm and
nuclei entrance, thereby enabling the identification of
transcription factors such as lymphatic enhancement factor, T
cell-related factors and activating target genes, which ultimately
control embryonic development, cell growth, differentiation and
apoptosis (24,25). Notably, the time and spatial
distribution of extracellular WIF-1 may be associated with its
different affinity towards different Wnt molecules (26). Therefore, Wnt signaling may serve a
role in the regulation of the extracellular space distribution of
WIF-1, although this must be confirmed by further studies.
Hypermethylation of the promoter region of the gene coding for
WIF-1 could lead to the post-transcriptional silencing of the WIF-1
gene, and thereby could induce absent or downregulated WIF-1
expression, eventually causing abnormal activation of Wnt signaling
pathways and the corresponding changes (26). Whether hypermethylation of the WIF-1
promoter can be used as an effective detection marker for related
diseases such as cancer and osteoarthrosis, and whether
demethylation of the WIF-1 gene to restore its expression could be
a potential target for the treatment of these diseases, requires
future investigation.
The present study demonstrated that WIF-1, which is
the upstream gene in the Wnt/β-catenin signaling pathway, acts as
the reverse suppressor of Wnt, and its expression is significantly
decreased in lung cancer tissues. In addition, the expression
levels of β-catenin and cyclin D1, which are an important
transcription factor and the downstream target gene of Wnt,
respectively, were increased in lung cancer tissues. These changes
indicate that Wnt signaling pathways can be activated in NSCLC, and
may be closely associated with lymph nodal metastasis and lower
pathological classification.
In summary, the present study demonstrated that the
activation of the Wnt/β-catenin signaling pathway in lung cancer
tissues is initiated by WIF-1 gene inhibition. Since no differences
in APC expression in NSCLC and non-cancerous tissues were observed,
the activation of this signaling pathway may be different between
NSCLC and other tumors.
Acknowledgements
The present study was supported by the Science and
Technology Support Project of Xinjiang Uygur Autonomous Region
(grant no. 2016E02071) and the Tianjin Health and Family Planning
Commission of Science and Technology Project Foundation (grant no.
16KG153).
References
|
1
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen X, Meng J, Yue W, Yu J, Yang J, Yao Z
and Zhang L: Fibulin-3 suppresses Wnt/β-catenin signaling and lung
cancer invasion. Carcinogenesis. 35:1707–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pacheco-Pinedo EC and Morrisey EE: Wnt and
Kras signaling-dark siblings in lung cancer. Oncotarget. 2:569–574.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wend P, Holland JD, Ziebold U and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Semin
Cell Dev Biol. 21:855–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubin EM, Guo Y, Tu K, Xie J, Zi X and
Hoang BH: Wnt inhibitory factor 1 decreases tumorigenesis and
metastasis in osteosarcoma. Mol Cancer Ther. 9:731–741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esteller M: Cancer epigenomics: DNA
methylomes and histone-modification maps. Nat Rev Genet. 8:286–298.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YL, Yang HP, Gong L, Tang CL and Wang
HJ: Hypomethylation effects of curcumin, demethoxycurcumin and
bisdemethoxycurcumin on WIF-1 promoter in non-small cell lung
cancer cell lines. Mol Med Rep. 4:675–679. 2011.PubMed/NCBI
|
|
9
|
Gao Z, Xu Z, Hung MS, Lin YC, Wang T, Gong
M, Zhi X, Jablons DM and You L: Procaine and procainamide inhibit
the Wnt canonical pathway by promoter demethylation of WIF-1 in
lung cancer cells. Oncol Rep. 22:1479–1484. 2009.PubMed/NCBI
|
|
10
|
Travis WD, Brambilla E, Nicholson AG, et
al: WHO Panel: The 2015 World Health Organization classification of
lung tumors: Impact of genetic, clinical and radiologic advances
since the 2004 classification. J Thorac Oncol. 10:1243–1260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Warth A, Muley T, Meister M, Stenzinger A,
Thomas M, Schirmacher P, Schnabel PA, Budczies J, Hoffmann H and
Weichert W: The novel histologic International Association for the
Study of Lung Cancer/American Thoracic Society/European Respiratory
Society classification system of lung adenocarcinoma is a
stage-independent predictor of survival. J Clin Oncol.
30:1438–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Hu X, Bai Y, Alattar M, Ma D, Cao Y,
Hao Y, Wang L and Jiang C: The oxidative damage and inflammatory
response induced by lead sulfide nanoparticles in rat lung. Food
Chem Toxicol. 60:213–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Jiang C, Liu H, Guan Z, Zeng Q,
Zhang C, Lei R, Xia T, Gao H, Yang L, et al: Fluoride-elicited
developmental testicular toxicity in rats: Roles of endoplasmic
reticulum stress and inflammatory response. Toxicol Appl Pharmacol.
271:206–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le PN, McDermott JD and Jimeno A:
Targeting the Wnt pathway in human cancers: Therapeutic targeting
with a focus on OMP-54F28. Pharmacol Ther. 146:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Surmann-Schmitt C, Sasaki T, Hattori T,
Eitzinger N, Schett G, von der Mark K and Stock M: The Wnt
antagonist Wif-1 interacts with CTGF and inhibits CTGF activity. J
Cell Physiol. 227:2207–2216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li ZL, Shao SH, Jiao F, Yue Z and Ma Y:
Cyclin D1 regulates lung cancer invasion and metastasis. Sheng Li
Xue Bao. 64:55–61. 2012.(In Chinese). PubMed/NCBI
|
|
17
|
Xu X, Sun PL, Li JZ, Jheon S, Lee CT and
Chung JH: Aberrant Wnt1/β-catenin expression is an independent poor
prognostic marker of non-small cell lung cancer after surgery. J
Thorac Oncol. 6:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hunter DD, Zhang M, Ferguson JW, Koch M
and Brunken WJ: The extracellular matrix component WIF-1 is
expressed during, and can modulate, retinal development. Mol Cell
Neurosci. 27:477–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin YC, You L, Xu Z, et al: Wnt inhibitory
factor-1 gene transfer inhibits melanoma cell growth. Hum Gene
Ther. 18:379–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alamgeer M, Peacock CD, Matsui W, Ganju V
and Watkins DN: Cancer stem cells in lung cancer: Evidence and
controversies. Respirology. 18:757–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang Y, Wang L, Zhang Y, Ge C and Xu C:
Wif-1 methylation and β-catenin expression in colorectal serrated
lesions. Zhonghua Bing Li Xue Za Zhi. 43:15–19. 2014.(In Chinese).
PubMed/NCBI
|
|
22
|
Yang TM, Leu SW, Li JM, et al: WIF-1
promoter region hypermethylation as an adjuvant diagnostic marker
for non-small cell lung cancer-related malignant pleural effusions.
J Cancer Res Clin Oncol. 135:919–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cadigan KM and Waterman ML: TCF/LEFs and
Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol.
4(pii): a0079062012.PubMed/NCBI
|
|
24
|
Beyer C, Schramm A, Akhmetshina A, et al:
β-catenin is a central mediator of pro-fibrotic Wnt signaling in
systemic sclerosis. Ann Rheum Dis. 71:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts DM, Pronobis MI, Poulton JS, Kane
EG and Peifer M: Regulation of Wnt signaling by the tumor
suppressor adenomatous polyposis coli does not require the ability
to enter the nucleus or a particular cytoplasmic localization. Mol
Biol Cell. 23:2041–2056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bányai L, Kerekes K and Patthy L:
Characterization of a Wnt-binding site of the WIF-domain of Wnt
inhibitory factor-1. FEBS Lett. 586:3122–3126. 2012. View Article : Google Scholar : PubMed/NCBI
|