Introduction
Hepatoblastoma (HB) is a prevalent malignancy among
children, which histologically derives from pluripotent stem cells
that may differentiate into liver cells and biliary epithelial
cells, and accounts for almost two-thirds of pediatric malignant
liver tumors (1,2). Although the survival rate of HB has
increased from 35 to 75% during the last 30 years with the
application of surgical excision, adjuvant chemotherapy and liver
transplantation (3), additional
investigation of the underlying molecular mechanism will be
beneficial for the improving diagnosis and treatment of patients
with HB.
Previous studies have supported the hypothesis that
the development of malignancies is closely associated with various
cytokines, in which chemokines appear to have crucial roles.
Chemokines are members of the cytokine super family and are
secreted by various cell types, including immune, mesothelial,
endometrial glandular and stromal cells, and trophoblasts (4). According to the order of conserved
cysteine residues, chemokines are classified as C, CC, CXC and C
(X)3C. Additionally, CXC chemokines are further grouped
into ELR+ CXC and ELR− CXC on the basis of
the presence or absence of the amino-terminal ELR motif (5). In addition to their function in
chemotaxis, chemokines can induce various activation progressions
in physiology through their effects on regulating angiogenesis,
cellular proliferation, differentiation and apoptosis (6–8). However,
previous data have suggested that a variety of chemokines are also
involved in the pathogenesis of malignancies. Milliken et al
(9) reported that high expression of
C-X-C motif chemokine ligand (CXCL) 8 in ovarian cancer epithelial
cells resulted in an increased proliferation rate compared with low
expression of CXCL8 in the cells. As an efficient mediator of
angiogenesis, the expression of CXCL5 in non-small cell lung cancer
was associated with angiogenesis, which is vitally important in the
proliferation, invasion and metastasis of tumor cells (10). In prostatic carcinoma, CXCL12
contributes to the migration potential of tumor cells by activating
the transcription of genes associated with the cytoskeleton,
including microtubule associated protein RP/EB family member 3 and
dedicator of cytokinesis 9, and downregulating the expression of
intercellular adhesion proteins, including cadherin-1 and β-catenin
(11). The biological functions of
chemokines rely mainly on their receptors, a type of G
protein-coupled receptor that mediates the functions of chemokines
and is usually expressed in immune cells and endothelial cell
membrane. Murakami et al (12)
indicated that C-X-C chemokine receptor type 4 is an essential
molecular determinant for the metastatic accumulation of tumor
cells in the lungs of mice. The tumor homing hypothesis also showed
that the specific combination of the chemokine ligand and its
receptor is sufficient to initiate tumor metastasis (13). Previous studies have shown that
overexpression of CXCL5 is present in numerous human tumors
including prostate, squamous cell and stomach tumors. Additionally,
CXCL5 may have an important role in the occurrence and progression
of tumors by cooperating with its receptor C-X-C chemokine receptor
type 2 (CXCR2) (14–16). Although a previous study by Zhou et
al (17) demonstrated that the
expression of CXCL5 in hepatocellular carcinoma tissues was
evidently increased compared with that in para-carcinoma tissues
and overexpression of CXCL5 can promote the growth and invasion of
hepatocellular carcinoma cells, the effects of CXCL5 contributing
to the growth and migration of HB cells through the
autocrine/paracrine pathways have not, to the best of our
knowledge, been reported. Therefore, the current study aimed to
explore whether CXCL5 can affect the oncogenic potential of HB
through autocrine and paracrine signaling.
Materials and methods
Cell culture
The human HB HepG2 cell and human hepatic stellate
LX-2 cell lines were maintained in a 37°C humidified incubator at
5% CO2 in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 U/ml streptomycin (DMEM complete
medium).
Cell transfection
The lentiviral CXCL5 expression vector
(pEZ-Lv203-A1113) and empty vector (pEZ-Lv203-NEG) were constructed
by GeneCopoeia, Inc. (Rockville, MD, USA), which were utilized to
prepare a DNA/EndoFectin Lenti complex, which were transfected into
293Ta lentiviral packaging cells (American Type Culture Collection,
Manassas, VA, USA) using the Lenti-Pac™ HIV Expression Packaging
kit (cat. no. HPK-LvRT-20; GeneCopoeia, Inc.) according to the
manufacturer's protocol. After 48 h of transfection, the
pseudovirus-containing culture medium was collected and purified by
filtering the supernatant through 0.45 µm low protein-binding
filters. HepG2 and LX-2 cells were transfected by incubating them
in DMEM complete medium with 50% diluted viral supernatant for 48
h, following which fresh DMEM complete medium containing puromycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 2 ng/ml was
added for selection. Cells were used for further experimentation 14
days after transfection.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from parental, empty
vector-transfected, as well as CXCL5-transfected HepG2 and LX-2
cells with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and first-strand cDNA was synthesized using Reverse
Transcription System (cat. no. A3500; Promega Corporation, Madison,
WI, USA). PCR primers were synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China), the primer sequences and conditions are
presented in Tables I and II, respectively.
 | Table I.Primer sequences used for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-polymerase chain reaction.
| Gene | Forward primer
sequence, 5′-3′ | Reverse primer
sequence, 5′-3′ |
|---|
| β-actin |
AGAAAATCTGGCACCACACC |
CTCCTTAATGTCACGCACGA |
| CXCL5 |
GCTACCACTTCCACCTTG |
CCACTATGAGCCTVVTGT |
| CXCR2 |
CAGGAATGTGGCCAAAAAT |
GGAAACTCCCTCGTGATG |
| NDRG3 |
GGCGAATTGTCCCCTACCACCAG |
CTGCCTCCTGTTCTTACCCACCTA |
| Bcl-2 |
CGAACTCAAAGAAGGCCACAAT |
TGGGAGAACGGGGTACGATA |
| Bax |
TGAGCACTCCCGCCACAAAG |
TTGTCGCCCTTTTCTACTTTGCC |
| P53 |
TGCAATAGGTGTGCGTCAGAA |
CCCCGGGACAAAGCAAA |
| VEGF |
CAAATCTAGCCAGGAAACGACC |
AAGGAGGAGGGCAGAATCATCACGA |
 | Table II.Reverse transcription-polymerase
chain reaction conditions for each primer set. |
Table II.
Reverse transcription-polymerase
chain reaction conditions for each primer set.
| Gene | Reaction
conditions |
|---|
| β-actin | 94°C for 3 min,
28-cycles of 94°C for 30 sec, 55°C for 25 sec, 72°C for 1 min |
| CXCL5 | 94°C for 3 min,
28-cycles of 94°C for 30 sec, 55°C for 25 sec, 72°C for 1 min |
| CXCR2 | 94°C for 3 min,
28-cycles of 94°C for 30 sec, 55°C for 25 sec, 72°C for 1 min |
| Bcl-2 | 94°C for 3 min,
38-cycles of 94°C for 30 sec, 54°C for 35 sec, 72°C for 1 min |
| Bax | 94°C for 3 min,
30-cycles of 94°C for 30 sec, 55°C for 30 sec, 72°C for 1 min |
| P53 | 94°C for 3 min,
35-cycles of 94°C for 35 sec, 55°C for 25 sec, 72°C for 1 min |
| VEGF | 94°C for 3 min,
34-cycles of 94°C for 30 sec, 61°C for 31 sec, 72°C for 1 min |
Western blot analysis for protein
detection
The parental, empty vector-transfected, as well as
CXCL5-transfected HepG2 cells pellets were harvested and western
blot assays were performed as previously described (18). Anti-CXCR2 mouse polyclonal antibody
(cat. no. sc-30008; dilution, 1:500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-interleukin (IL)-18 rabbit polyclonal
antibody (cat. no. sc-7954; dilution, 1:800; Santa Cruz
Biotechnology, Inc.), anti-IL-1β rabbit polyclonal antibody (cat.
no. YT2342; dilution, 1:1,000; ImmunoWay Biotechnology Company,
Plano, TX, USA), anti-cystathionine-γ-lyase (CSE) rabbit polyclonal
antibody (cat. no. BA3605; dilution, 1:800; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) and anti-β-actin mouse monoclonal
antibody (cat. no. sc-130300; dilution, 1:3,000; Santa Cruz
Biotechnology, Inc.) were utilized in the assays. Goat anti-rabbit
IgG (cat. no. BA1054; dilution, 1:1,000; Wuhan Boster Biological
Technology, Ltd.) and goat anti-mouse IgG (cat. no. BA1050;
dilution, 1:1,000; Wuhan Boster Biological Technology, Ltd.) were
used as secondary antibodies.
ELISA assays
Parental, empty vector-transfected, as well as
CXCL5-transfected HepG2 and LX-2 cells were seeded in a 6-well
plate (1.5×105 cells/well) with DMEM complete medium.
After 48 h of incubation at 37°C, the supernatants were collected
and centrifuged at 22,000 × g at 4°C for 15 min. The secretion
levels of CXCL5 were determined by ELISA using Human CXCL5 Elisa
kit (cat. no. EK0728; Wuhan Boster Biological Technology, Ltd.),
according to the manufacturer's protocol.
Cell proliferation assays
Cell Counting kit-8 (CCK-8; cat. no. AR1160-500;
Wuhan Boster Biological Technology, Ltd.) was utilized to explore
the effect of exogenous, autocrine or paracrine CXCL5 on HepG2 cell
proliferation. HepG2 cells were seeded onto a 96-well plate
(1.5×103 cells/well) with DMEM complete medium
containing 0, 20, 40 or 60 ng/ml exogenous recombinant human CXCL5
(PeproTech, Inc., Rocky Hill, NJ, USA), and proliferation activity
was investigated after 24, 48, 72 and 96 h incubation at 37°C. To
examine the autocrine effects of endogenous CXCL5, the parental,
empty vector-transfected and CXCL5-transfected HepG2 cells were
seeded onto a 96-well plate (2×103 cells/well) with DMEM
complete medium. The proliferation was then determined after 72 h
incubation at 37°C. In order to detect the effect of paracrine
signaling on the growth of HepG2 cells, conditioned medium (CM) was
collected as follows: The parental, empty vector-transfected and
CXCL5-transfected LX-2 cells were seeded into 10-cm plates
(3×106 cells/plate) and maintained in DMEM complete
medium at 37°C for 48 h. The CM was then prepared by collecting the
supernatants. By using different ratios of CM (0, 20, 40, 60 or
80%) dissolved in complete medium, the proliferation of HepG2 cells
was determined.
Colony formation assay
HepG2 cells were plated onto a 24-well plate
(2×102 cells/well) containing DMEM complete medium with
0, 20, 40, 60 or 80 ng/ml exogenous CXCL5. The same number of
parental, empty vector-transfected and CXCL5-transfected HepG2
cells were also seeded in a 24-well plate maintained with DMEM
complete medium. The colonies were stained with crystal violet and
were counted after incubating the cells for 12 days.
Migration assays
HepG2 cells (1×104 cells/well) were
seeded into the upper wells of Transwell® chambers (cat.
no. 3422; Corning Incorporated, Corning, NY, USA) in DMEM only
(without FBS); DMEM complete medium (with 10% FBS) containing 0,
20, 40, 60 or 80 ng/ml of exogenous CXCL5 was added to the lower
wells. For the paracrine assay, 0, 20, 40, 60 or 80% of LX-2 CM in
DMEM complete medium was added to the lower wells. To perform the
autocrine investigation, the parental, empty vector-transfected and
CXCL5-transfected HepG2 cells were seeded into
Transwell® chambers (1×104 cells/well) in
DMEM only (without FBS); DMEM complete medium (with 10% FBS) was
added to the lower wells. After cells were incubated for 24 h at
37°C, the upper surface of the membranes was scrubbed with a cotton
swab to remove the cells that had not migrated. The cells attached
to the lower surface of the membrane were stained with crystal
violet for 30 min at room temperature and were counted using a
light microscope (magnification, ×50).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The data were analyzed by q-test using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
CXCL5 and its receptor CXCR2 are
expressed by HepG2 cells
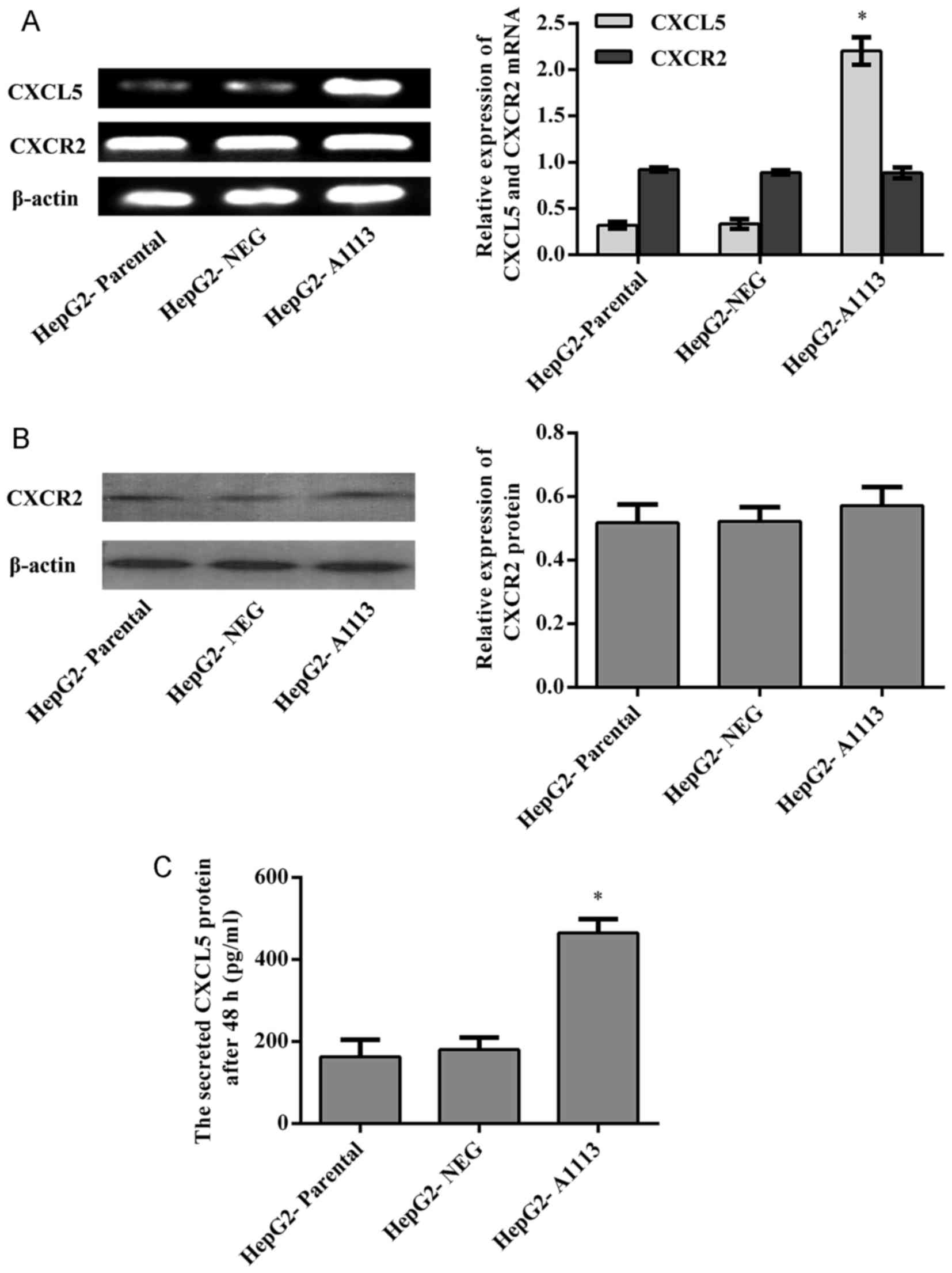
Prior to investigating the functions of CXCL5 in
HepG2 cells, the expression of CXCL5 was examined by RT-PCR and
ELISA, and the expression of CXCR2 was determined by RT-PCR and
western blotting. Both CXCL5 and its receptor CXCR2 were evidently
expressed by HepG2 cells (Fig.
1).
Exogenous CXCL5 promotes carcinogenic
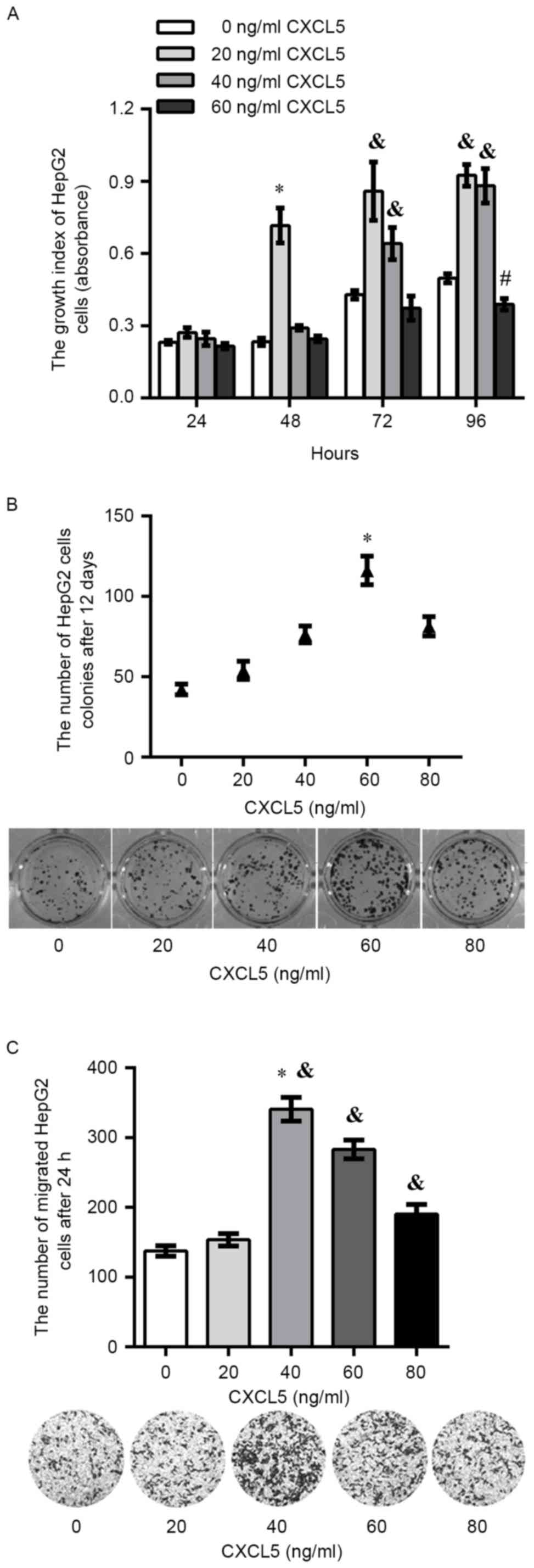
potential of HepG2 cells in vitro
The proliferation assay showed that there was a
significant increase in the proliferation of HepG2 cells treated
with 20 ng/ml exogenous CXCL5 compared with other concentrations of
exogenous CXCL5 after 48 h (P<0.05). In addition, there was a
significant increase in HepG2 cells treated with 20 or 40 ng/ml of
exogenous CXCL5 after 72 or 96 h. It was observed that 60 ng/ml of
exogenous CXCL5 exerted an inhibitory effect on proliferation at
each time point, and a significant decrease on growth in HepG2
cells treated with 60 ng/ml of exogenous CXCL5 was found after 96 h
(Fig. 2A). After 12 days of
incubation, colony formation assay showed that the total colony
number in HepG2 cells treated with 60 ng/ml of exogenous CXCL5 was
significantly increased compared with 0, 20, 40 or 80 ng/ml
exogenous CXCL5 (Fig. 2B).
Additionally, a significant increase in migration was observed in
HepG2 cells treated with 40, 60 or 80 ng/ml exogenous CXCL5
compared with 0, 20 ng/ml exogenous CXCL5 (Fig. 2C).
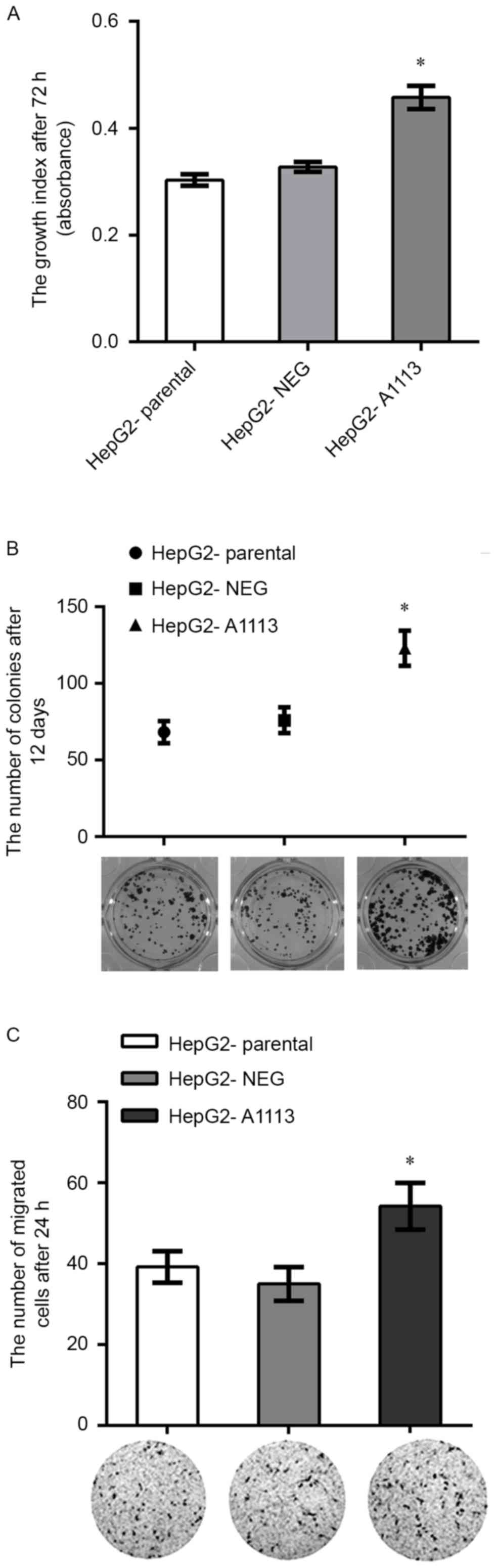
Overexpression of CXCL5 accelerates
proliferation, colony formation and migration of HepG2 cells in
vitro
To study the autocrine roles of CXCL5 on HepG2
cells, the target gene CXCL5 was successfully transfected into
HepG2 cells. RT-PCR and ELISA showed that CXCL5 mRNA and protein
expression in CXCL5 overexpression cells (HepG2-A1113) was
significantly increased in comparison to parental cells
(HepG2-parental) or empty vector expression cells (HepG2-NEG)
(Fig. 1A and C). HepG2-A1113 cells
exhibited increased growth, colony formation and migration compared
with HepG2-parental and HepG2-NEG (Fig.
3A-C).
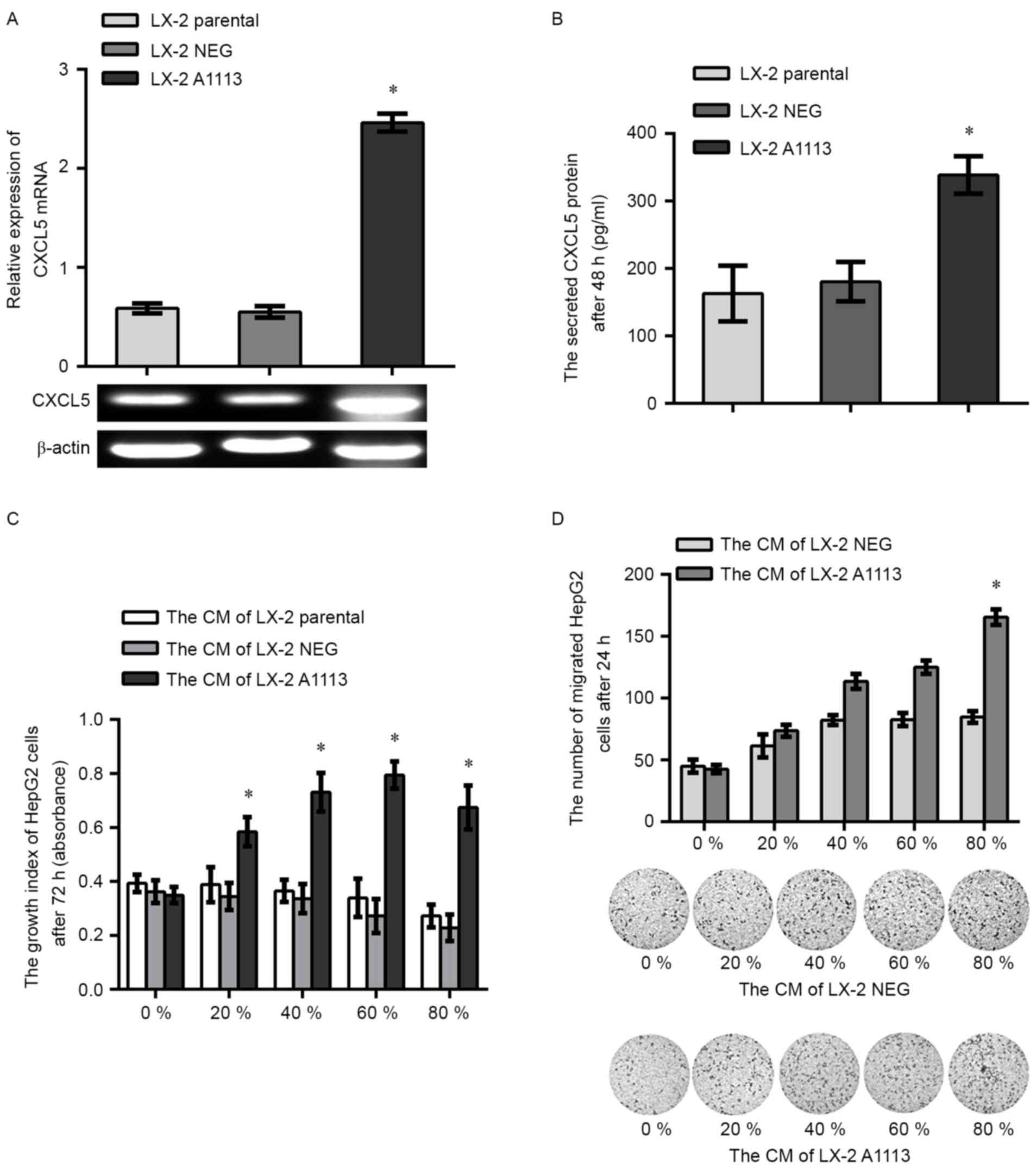
Upregulation of CXCL5 in LX-2 cells
encourages the carcinogenic potential in HepG2 cells by paracrine
signaling
To investigate paracrine role of CXCL5, the identity
of CXCL5 overexpression LX-2 cells (LX-2 A1113) and empty vector
expression LX-2 cells (LX-2 NEG) was confirmed by RT-PCR (Fig. 4A) and ELISA assays (Fig. 4B). CCK-8 and Transwell assays showed
that proliferation and migration of the HepG2 cells treated with
the CM of LX-2 A1113 was significantly increased compared with the
cells treated with CM from LX-2 parental or LX-2 NEG cells
(Fig. 4C and D).
Overexpression of CXCL5 regulates the
expression of genes in HepG2 cells
RT-PCR showed downregulation of N-myc downregulated
gene (NDRG) 3, B-cell lymphoma-2 (Bcl-2) -associated X protein
(Bax) and P53 in HepG2-A1113 cells compared with HepG2-parental or
HepG2-NEG cells. However, overexpression of CXCL5 in HepG2 cells
led to upregulation of Bcl-2 and vascular endothelial growth factor
(VEGF) mRNA (Fig. 5A). Western
blotting indicated that the protein levels of IL-18, IL-1β and CSE
in HepG2-A1113 cells were increased compared with HepG2-parental
and HepG2-NEG cells (Fig. 5B).
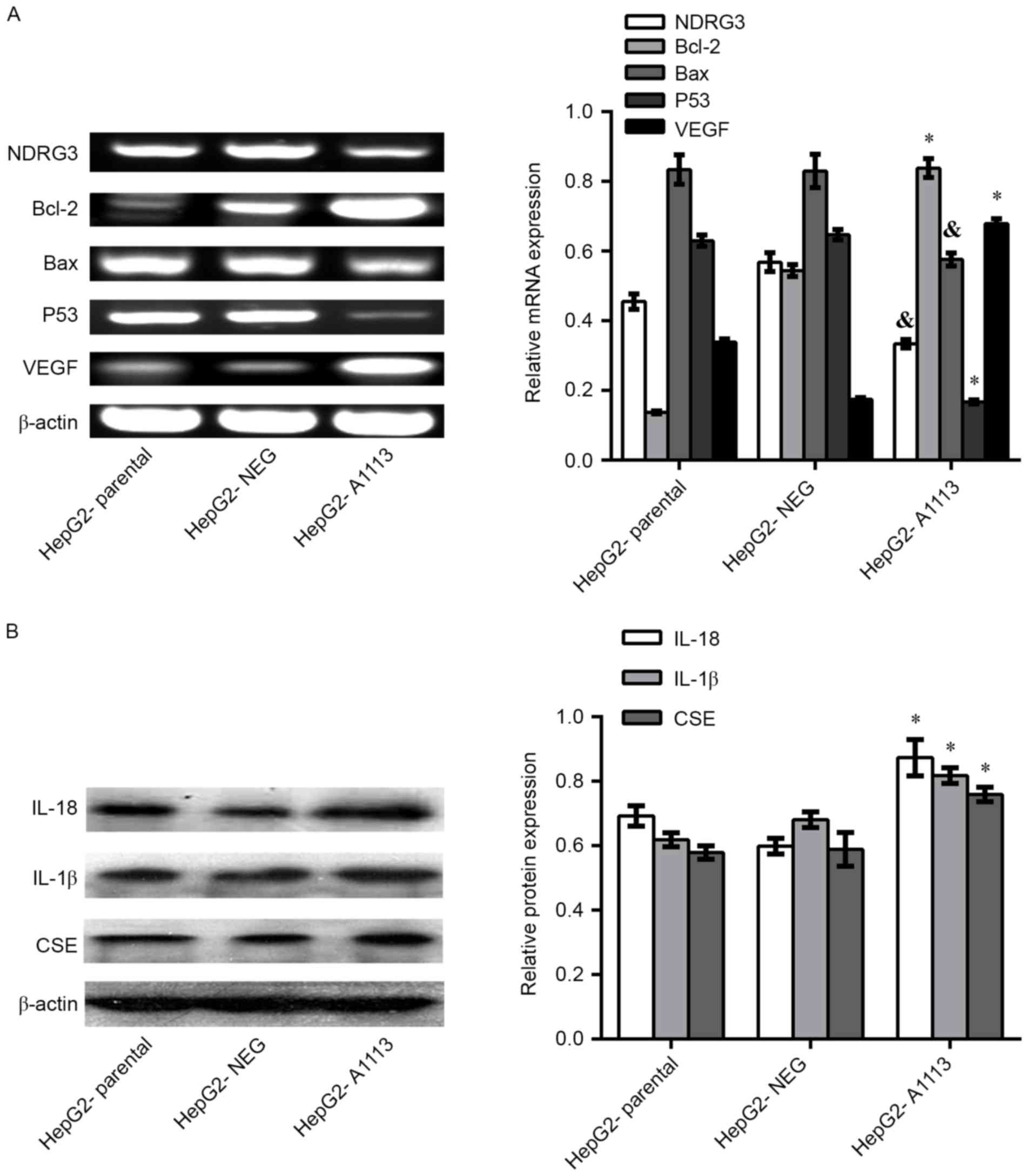 | Figure 5.Overexpression of CXCL5 regulates the
expression of genes in HepG2 cells. (A) In reverse
transcription-polymerase chain reaction assays, downregulation of
NDRG3, Bax and P53 mRNA in HepG2-A1113 cells were detected compared
with HepG2-parental or HepG2-NEG cells. However, the levels of
Bcl-2 and VEGF mRNA in HepG2-A1113 cells were increased. (B)
Western blotting assays showed upregulation of IL-18, IL-1β and CSE
proteins in HepG2-A1113 cells (*P<0.01;
&P<0.05). Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; VEGF, vascular endothelial growth
factor; IL, interleukin; CXCL5, C-X-C motif chemokine ligand 5;
CSE, cystathionine-γ-lyase; HepG2-NEG, empty vector-transfected
HepG2 cells; HepG2-A1113, CXCL5-transfected HepG2 cells. |
Discussion
At present, the biological roles of chemokines in
malignancies is diverse and the views are involved in both
carcinogenesis and tumor inhibition. However, the preponderance of
evidence showed that chemokines contribute mainly to carcinogenesis
in the progress of cancers (19).
Although CXCL5 has been reported to have numerous roles in
carcinomas (20,21), to the best of our knowledge, the
present study was the first to conduct a paracrine secretion assay
to investigate the effects of endogenous CXCL5 secreted by hepatic
stellate LX-2 cells on the oncogenic potential of HB HepG2 cells.
HepG2 was originally thought to be a hepatocellular carcinoma cell
line and was utilized to investigate hepatocellular carcinoma.
However, previous research has shown that HepG2 is a HB-derived
cell line (22), which has a crucial
role in studying the underlying progression and mechanism of HB
(23,24). In the present study, it was confirmed
that CXCL5 and its receptor CXCR2 are expressed in HepG2 cells.
Additionally, an appropriate concentration of exogenous CXCL5
significantly promoted the proliferation and migration of HepG2
cells. It continues to be uncertain why exogenous CXCL5 at a high
concentration suppressed the proliferation and migration of HepG2
cells. In the present results, overexpression of CXCL5 in HepG2 did
not change the expression of CXCR2, suggesting the suppressive
effects may be a result of the high concentration of CXCL5 blocking
the affinity of CXCR2 to CXCL5. Since tumor cells and surrounding
stromal cells may secrete chemokines that stimulate proliferation
or inhibit the apoptosis of tumor cells by activating chemokine
receptors on tumor cells (19), HepG2
cells overexpressing CXCL5 and LX-2 cells overexpressing CXCL5 were
constructed in the present study to conduct autocrine and paracrine
assays. The autocrine results showed that overexpression of CXCL5
augmented the proliferation, colony formation and migration of
HepG2 cells. Similarly, in paracrine assays, the condition medium
of LX-2 cells overexpressing CXCL5 stimulated the growth and
migration capacities of HepG2 cells.
Both Bax and Bcl-2, which are members of the Bcl-2
family, have multiple roles in the carcinogenesis of tumors. As
cells were exposed to adverse factors, Bax can induce the process
of apoptosis, by which the permeabilization of mitochondrial outer
membrane is strengthened. In contrast, Bcl-2 is a potent inhibitor
of apoptosis for the reason of suppressing the activity of Bax. It
was apparent that Bax and Bcl-2 had opposite effects on cell
apoptosis, the balance between Bax and Bcl-2 determined the cell
fate (25,26). Since both downregulation of Bax and
upregulation of Bcl-2 at mRNA levels were detected in HepG2 cells
overexpressing CXCL5, CXCL5 in HepG2 cells might help protect
against apoptosis and further exert its function on
proliferation.
In addition, the present findings showed that
another apoptosis-associated gene, P53, which is the upstream gene
of Bax and Bcl-2, was upregulated in HepG2 cells overexpressing
CXCL5. Previous studies suggested that in AGS human cancer cells
treated with polyphenols from lyophilized A. cepa Linn,
upregulation of P53 was found, which further increased the
Bax/Bcl-2 ratio by disrupting the balance between Bax and Bcl-2
(27,28). Based on the aforementioned findings
for Bax, Bcl-2 and p53, the present study hypothesized that CXCL5
in HepG2 cells participates in the malignant transformation of HB
by downregulating P53, which decreases the ratio of Bax and
Bcl-2.
The present findings indicated that overexpression
of CXCL5 can downregulate and upregulate the expression of NDRG3
and VEGF at mRNA level, respectively. NDRG3 is a member of the NDRG
family, which contains 4 paralogs, consisting of NDRG1, −2, −3 and
−4 (29). At present, a limited
number of studies about NDRG3 have been produced. It has been found
that NDRG3 may have a role in spermatogenesis, since it is found in
the outer layers of the seminiferous epithelium (30). In our previous study, we identified
that NDRG3 was associated with the proliferation and migration
ability of prostatic carcinoma cells in vitro and in a nude
mouse xenograft model (18).
Furthermore, overexpression of NDRG3 in PCa can significantly
upregulate the expression of CXCL5, and the results of this study
indicated that the effect of NDRG3 on tumorigenesis of PCa is
partly mediated through the NDRG3/CXCL5 pathway (18). By contrast, it was also shown that
overexpression of CXCL5 decreased the expression of NDRG3,
suggesting there is a negative feedback mechanism in the
NDRG3/CXCL5 pathway. Since NDRG3 is an androgen-dependent gene
(18), it is possible that the
deactivation process of estrogen may be delayed as the normal
functions of hepatic cells are damaged with the development of HB
and further lead to an increase in the estrogen/androgen ratio. As
a result, the expression of the androgen-dependent gene NDRG3 will
be suppressed.
VEGF has a variety of biological functions and has
important roles in angiogenesis, but the significance of VEGF in
tumors has yet to be fully elucidated. As demonstrated in a
previous study, VEGF and its receptor kinase insert domain receptor
stimulated the proliferation of gastric adenocarcinoma cells via an
autocrine mechanism (31). On the
basis of the aforementioned findings and the present RT-PCR data,
which found overexpression of VEGF in HepG2 cells overexpressing
CXCL5, the proliferation activity of CXCL5 in HB may be mediated
through VEGF.
As an endogenous enzyme, CSE is crucial to the
generation of H2S. Previous studies have shown that CSE
is involved in a variety of physiological and tumor processes
(32–34), and the knockdown of CSE by shRNA can
decrease cell proliferation, migration and tumor xenograft growth
in nude mice (35). Consistent with
this, the present data showed that the upregulation of the CSE
protein in HepG2 cells overexpressing CXCL5 is positively
associated with the proliferation and migration of HepG2 cells.
It has been recognized that IL-18 has an important
role in the invasion and migration of tumors by contributing to the
evasion of immune recognition, producing tumor growth-stimulating
factors and promoting angiogenesis (36). In a study investigating IL-1β, Tu
et al (37) demonstrated that
IL-1β in transgenic mice promotes spontaneous inflammation,
metaplasia, dysplasia and carcinoma; activating NF-κB through IL-1β
enhanced gastric inflammation and promoted carcinogenesis in
myeloid-derived suppressor cells. In addition, the present western
blotting assay showed that the IL-18 and IL-1β proteins were
upregulated in HepG2 cells overexpressing CXCL5. These findings
support the importance of CXCL5 in immune and inflammatory
reactions of HB.
In summary, the present study demonstrated that the
CXCL5/CXCR2 axis is involved in the carcinogenesis of HB by
regulating the expression of several genes. In particular, the
results of the present study demonstrated that conditional medium
from CXCL5-overexpressing hepatic stellate LX-2 cells, a major
stromal cell type, stimulated HB HepG2 cell proliferation and
migration in a paracrine fashion, suggesting that
stromal-epithelial interactions, by which cancer cells interact
with their surrounding cells, are critical events in tumor
microenvironment.
Acknowledgements
The present study was supported by grants from
National Nature Science Foundation of China (grant no. 81272854),
Nature Science Youth Foundation of Heilongjiang Province (grant no.
QC2013C101), Key Research Program of Jiamusi University (grant no.
Sz2009-008), Science and Innovation Team Building Project of
Department of Education of Heilongjiang Province (grant no.
cxtd-2016-03), President Innovation and Entrepreneurship Foundation
of Jiamusi University (grant no. xzyf2014-12), and Innovation and
Entrepreneurship Training Program for College Students of
Heilongjiang Province (grant no. 201410222036)
References
|
1
|
Stocker JT: Hepatoblastoma. Semin Diagn
Pathol. 11:136–143. 1994.PubMed/NCBI
|
|
2
|
Khaderi S, Guiteau J, Cotton RT, O'Mahony
C, Rana A and Goss JA: Role of liver transplantation in the
management of hepatoblastoma in the pediatric population. World J
Transplant. 4:294–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hiyama E: Pediatric hepatoblastoma:
Diagnosis and treatment. Transl Pediatr. 3:293–299. 2014.PubMed/NCBI
|
|
4
|
Kayisli UA, Mahutte NG and Arici A:
Uterine chemokines in reproductive physiology and pathology. Am J
Reprod Immunol. 47:213–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murdoch C and Finn A: Chemokine receptors
and their role in inflammation and infectious diseases. Blood.
95:3032–3043. 2000.PubMed/NCBI
|
|
6
|
Kollet O, Vagima Y, D'Uva G, Golan K,
Canaani J, Itkin T, Gur-Cohen S, Kalinkovich A, Caglio G, Medaglia
C, et al: Physiologic corticosterone oscillations regulate murine
hematopoietic stem/progenitor cell proliferation and CXCL12
expression by bone marrow stromal progenitors. Leukemia.
27:2006–2015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koch AE: Review: Angiogenesis:
Implications for rheumatoid arthritis. Arthritis Rheum. 41:951–962.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selam B, Kayisli UA, Garcia-Velasco JA,
Akbas GE and Arici A: Regulation of fas ligand expression by IL-8
in human endometrium. J Clin Endocrinol Metab. 87:3921–3927. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milliken D, Scotton C, Raju S, Balkwill F
and Wilson J: Analysis of chemokines and chemokine receptor
expression in ovarian cancer ascites. Clin Cancer Res.
84:1108–1114. 2002.
|
|
10
|
Arenberg DA, Keane MP, DiGiovine B, Kunkel
SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD and
Strieter RM: Epithelial-neutrophil activating peptide (ENA-78) is
an important angiogenic factor in non-small cell lung cancer. J
Clin Invest. 102:465–472. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Begley LA, MacDonald JW, Day ML and
Macoska JA: CXCL12 activates a robust transcriptional response in
human prostate epithelial cells. J Biol Chem. 282:26767–26774.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami T, Maki W, Cardones AR, Fang H,
Kyi Tun A, Nestle FO and Hwang ST: Expression of CXC chemokine
receptor-4 enhances the pulmonary metastatic potential of murine
B16 melanomacells. Cancer Res. 15:7328–7334. 2002.
|
|
13
|
Hirbe AC, Morgan EA and Weilbaecher KN:
The CXCR4/SDF-1 chemokine axis: A potential therapeutic target for
bone metastases? Curr Pharm Des. 16:1284–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Begley LA, Kasina S, Mehra R, Adsule S,
Admon AJ, Lonigro RJ, Chinnaiyan AM and Macoska JA: CXCL5 promotes
prostate cancer progression. Neoplasia. 10:244–254. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyazaki H, Patel V, Wang H, Edmunds RK,
Gutkind JS and Yeudall WA: Down-regulation of CXCL5 inhibits
squamous carcinogenesis. Cancer Res. 66:4279–4284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JY, Park KH, Bang S, Kim MH, Lee JE,
Gang J, Koh SS and Song SY: CXCL5 overexpression is associated with
late stage gastric cancer. J Cancer Res Clin Oncol. 133:835–840.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH,
Wang Z, Huang XW, Fan J and Zhou J: Overexpression of CXCL5
mediates neutrophil infiltration and indicates poor prognosis for
hepatocellular carcinoma. Hepatology. 56:2242–2254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Li Y, Li Y, Hong A, Wang J, Lin B
and Li R: NDRG3 is an androgen regulated and prostate enriched gene
that promotes in vitro and in vivo prostate cancer cell growth. Int
J Cancer. 124:521–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rollins BJ: Inflammatory chemokines in
cancer growth and progression. Eur J Cancer. 42:760–767. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Speetjens FM, Kuppen PJ, Sandel MH, Menon
AG, Burg D, van de Velde CJ, Tollenaar RA, de Bont HJ and
Nagelkerke JF: Disrupted expression of CXCL5 in Colorectal cancer
is associated with rapid tumor formation in rats and poor prognosis
in patients. Clin Cancer Res. 14:2276–2284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia J, Xu X, Huang P, He M and Wang X: The
potential of CXCL5 as a target for liver cancer - what do we know
so far? Expert Opin Ther Targets. 19:141–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
23
|
Yumnam S, Hong GE, Raha S, Saralamma VV,
Lee HJ, Lee WS, Kim EH and Kim GS: Mitochondrial dysfunction and
Ca(2+) overload contributes to hesperidin induced paraptosis in
hepatoblastoma cells, HepG2. J Cell Physiol. 231:1261–1268. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishikawa T, Tanaka Y, Nishikawa M, Ogino
Y, Kusamori K, Mizuno N, Mizukami Y, Shimizu K, Konishi S,
Takahashi Y and Takakura Y: Optimization of albumin secretion and
metabolic activity of cytochrome P450 1A1 of human hepatoblastoma
HepG2 cells in multicellular spheroids by controlling spheroid
size. Biol Pharm Bull. 40:334–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leibowitz B and Yu J: Mitochondrial
signaling in cell death via the Bcl-2 family. Cancer Biol Ther.
9:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khodapasand E, Jafarzadeh N, Farrokhi F,
Kamalidehghan B and Houshmand M: Is Bax/Bcl-2 ratio considered as a
prognostic marker with age and tumor location in colorectal cancer?
Iran Biomed J. 19:69–75. 2015.PubMed/NCBI
|
|
27
|
Zeren T, Inan S, Vatansever HS and Sayhan
S: Significance of apoptosis related proteins on malignant
transformation of ovarian tumors: A comparison between Bcl-2/Bax
ratio and p53 immunoreactiviy. Acta Histochem. 116:1251–1258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee WS, Yi SM, Yun JW, Jung JH, Kim DH,
Kim HJ, Chang SH, Kim G, Ryu CH, Shin SC, et al: Polyphenols
isolated from allium cepa L. Induces apoptosis by induction of p53
and suppression of Bcl-2 through inhibiting PI3K/Akt signaling
pathway in AGS human cancer cells. J Cancer Prev. 19:14–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Li F, Liu X, Shen L, Liu J, Su J,
Zhang W, Deng Y, Wang L, Liu N, et al: The repression of human
differentiation-related gene NDRG2 expression by Myc via
Miz-1-dependent interaction with the NDRG2 core promoter. J Biol
Chem. 281:39159–39168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao W, Tang R, Huang Y, Wang W, Zhou Z,
Gu S, Dai J, Ying K, Xie Y and Mao Y: Cloning and expression
pattern of the human NDRG3 gene. Biochim Biophys Acta.
1519:134–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian X, Meng L, Shou C and Dong Z:
Coexpression of vascular endothelial growth factor and its receptor
KDR on gastric adenocarcinoma MGC803 cell line and stimulation of
exogenous VEGF (165) to MGC803 cells. Sci China C Life Sci.
43:88–95. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu D, Si W, Wang M, Lv S, Ji A and Li Y:
Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 50:38–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y,
Li N, Qi J, Wang L, Shi Y, et al: Sp1 is involved inregulation of
cystathionine γ-lyase gene expression and biological function by
PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell
Signal. 24:1229–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang XH, Wang F, You SJ, Cao YJ, Cao LD,
Han Q, Liu CF and Hu LF: Dysregulation of cystathionine γ-lyase
(CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced
inflammation in macrophage. Cell Signal. 25:2255–2262. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan K, Li N, Qi J, Yin P, Zhao C, Wang L,
Li Z and Zha X: Wnt/β-catenin signaling induces the transcription
of cystathionine-γ-lyase, a stimulator of tumor in colon cancer.
Cell Signal. 26:2801–2808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Palma G, Barbieri A, Bimonte S, Palla M,
Zappavigna S, Caraglia M, Ascierto PA, Ciliberto G and Arra C:
Interleukin 18: Friend or foe in cancer. Biochim Biophys Acta.
1836:296–303. 2013.PubMed/NCBI
|
|
37
|
Tu S, Bhagat G, Cui G, Takaishi S,
Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl
O, Fox JG and Wang TC: Overexpression of interleukin-1beta induces
gastric inflammation and cancer and mobilizes
myeloid-derivedsuppressor cells in mice. Cancer Cell. 14:408–419.
2008. View Article : Google Scholar : PubMed/NCBI
|