Introduction
Dihydroartemisinin (DHA) is a semisynthetic
derivative of artemisinin (ARS) isolated from the traditional
Chinese herb Artemisia annua L.; it has been frequently used
in the treatment of malaria (1,2). Previous
studies have reported that DHA also has potent antitumor effects;
however, its anticancer mechanism is not well understood (3–6).
To date, a number of antitumor mechanisms that
underlie the effects of DHA have been investigated. Lai et
al (5) reported that ARS forms
cytotoxic free radicals by reacting with iron to kill breast cancer
cells in rats. It was also confirmed that DHA has the ability to
slow the growth of breast tumors via a similar mechanism, as it is
an analog of ARS (5). In a previous
study, Noori and Hassan (6)
demonstrated that DHA inhibited tumor growth through regulation of
the immune response in the RIN cell line, and also inhibited the
growth of tumor tissue in vivo. It was demonstrated that DHA
exerts its anticancer action by increasing the expression of
interferon-γ and decreasing the level of interleukin-4 (6). The majority of reports concerning the
mechanisms underlying DHA have focused on DHA-induced apoptosis
(7,8).
Chen et al (7) reported that
ARS and its derivatives, particularly DHA, were potently cytotoxic
to human ovarian cancer A2780 and OVCAR-3 cells through the death
receptor and mitochondrial-mediated caspase-dependent apoptotic
pathways. Handrick et al (8)
demonstrated that DHA induced the activation of caspases and DNA
fragmentation in Jurkat T-lymphoma cells, which would induce
apoptosis. In addition, DHA has been identified to induce autophagy
in cancer cells: Hu et al (9)
and Jia et al (10)
demonstrated that DHA has the ability to induce autophagy and exert
anticancer activities in cell lines of various types of cancer,
including the human multiple myeloma cancer RPMI 8226 cell line,
promyelocytic leukemia NB4 cell line, human colorectal cancer
HCT116 cell line, human cervical cancer HeLa cell line and the
human pancreatic cancer cell lines BxPC-3 (CRL-1687) and PANC-1
(CRL-1469).
Autophagy is an intracellular degradation process of
dispensable material for cell survival when cells encounter
environmental stresses, including nutrient starvation and pathogen
infection (11–14). In recent years, autophagy has been
considered to serve an important role in carcinogenesis,
development and patient prognosis (12–17). It
has been revealed that several autophagy-related (Atg) genes were
involved in autophagosome formation (12–17).
Autophagy is a protective mechanism that exerts antitumor action
(13–17). For example, mice with the heterozygous
mutant autophagy gene ATG6/BECN1 are prone to developing liver and
lung tumors (13–17). Atg7 is known to serve a role in
forming the autophagic vacuole (15–19).
Conversely, the protective mechanism of autophagy can be utilized
by cancer cells to overcome environmental stresses, including
nutritional deficiency and the therapeutic use of anticancer drugs
(19). Gonzalez et al
(19) demonstrated that the
anticancer action of MitoQ® was inhibited in the
Atg7-deficient human MDA-MB-231 cell line. In the present study,
RNAi technology was utilized to interfere with Atg7 expression to
suppress autophagy.
It was assumed that the anticancer action of DHA
would be affected by the level of autophagy; therefore, the
associated changes to cell viability, expressions of associated
genes and the cell cycle in breast cancer cells were observed when
the level of autophagy was altered. Rapamycin is a lipophilic
macrolide antibiotic that has the ability to induce autophagy in
various cell types (20,21); thus, in the present study, rapamycin
was used to induce autophagy. Although the amount of data
concerning autophagy and DHA is extensive, the association between
the autophagy and DHA response in cancer cells remains unclear. The
present study aimed to investigate the effect of autophagy on the
anticancer action of DHA in MDA-MB-231 cells. Rapamycin and Atg7
small interfering RNA (siRNA) was used as inducer and inhibitor of
autophagy, respectively. Following this, cell viability was
detected by the MTT method (22), the
expression levels of Atg7 and death-associated protein kinase
(DAPK) were measured by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and the cell cycle distribution
was estimated using flow cytometry. The present study revealed that
the anticancer action of DHA is enhanced by rapamycin, but is
reduced following Atg7 knockdown. DAPK is involved in membrane
blebbing and the formation of autophagic vesicles in the process of
cell death (23,24). The expression of the DAPK1 gene was
assessed when autophagy was altered in DHA-treated MDA-MB-231
breast cancer cells. It was demonstrated that rapamycin regulated
the Atg7 gene and alter the expression of DAPK, which inhibited
proliferation or promoted apoptosis in breast cancer MDA-MB-231
cells.
Materials and methods
Cell culture and reagents
MDA-MB-231 cells were provided by the National Key
Laboratory of Molecular Biology Department, West China Medical
Centre of Sichuan University (Chengdu, China). Rapamycin was
obtained from Selleck Chemicals (Houston, TX, USA; cat no. S1039)
and DHA was obtained from Shaanxi Sciphar Biotechnology Co., Ltd.
(Xi'an, China). The cell lines were cultured in high-glucose
Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% penicillin and streptomycin (both Invitrogen;
Thermo Fisher Scientific, Inc.). When cells reached ~80%
confluence, the cells were seeded into 96-well plates
(1×105/ml) and cultured for 20 h. All cultures were
maintained at 37°C in a humidified atmosphere containing 5%
CO2. Next, the cells were treated in groups, which
included DHA alone (10 µg/ml), DHA (10 µg/ml) and rapamycin (100
nmol/l) (the rapamycin group), and Atg7-knockdown cells treated
with DHA (10 µg/ml) (the Atg7-knockdown group). Each group was
repeated three times in triplicate.
siRNA and transfection
MDA-MB-231 cells were transfected with 21–25 nt Atg7
siRNA at 100 nM or negative-control (NC) siRNA Guangzhou RiboBio
Co., Ltd., Guangzhou, China) using the Atg7 siRNA kit (cat no.
RN:R10043.4) following the manufacturer's protocol once cells had
been cultured for 24 h, using Lipofectamine® RNAiMAX
Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The target sequence for Atg7 siRNA (cat no. 1294165753) was
GGAGTCACAGCTCTTCCTT, and the primer sequences were as follows:
Sense, 5′-GGAGUCACAGCUCUUCCUUdTdT-3′ and antisense,
5′-AAGGAAGAGCUGUGACUCCTdTd-3′. Transfection efficiency was
determined by detecting Atg7 expression levels with RT-qPCR.
Following transfection for 4 h, the medium was removed from all
groups and the cells were washed twice with 0.01 mol/l PBS. Next,
the cells were re-cultured in DMEM (high-glucose) at 37°C in a
humidified atmosphere containing 5% CO2. Cells were then
separated into 3 groups; i) DHA+Atg7(−) group, MDA-MB-231 cells
transfected with Atg7-siRNA and treated with DHA (10 µg/ml); ii)
DHA group, MDA-MB-231 cells treated with DHA alone (10 µg/ml); iii)
DHA+Rapamycin(−) group, MDA-MB-231 cells treated with DHA (10
µg/ml) and rapamycin (100 nmol/l). Each group was repeated three
times in triplicate. The time between transfection and subsequent
experimentation was 4 h.
RNA preparation and RT-qPCR
analysis
Total RNA was isolated from cultured MDA-MB-231
cells with TRIzol® Reagent (Takara Biotechnology Co.,
Ltd., Dalian, China) following treatment for 24 h. First-strand
cDNA was synthesized using the Revert Aid™ First Strand
cDNA Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. The method of analysis
used in RT-qPCR followed a protocol described in a previous study
(25). To determine the effectiveness
of the Atg7 siRNA transfection, RT-qPCR was performed to detect
Atg7 mRNA levels using the primers described previously (25) (Sangon Biotech Co., Ltd., Shanghai,
China): Human Atg7, forward 5′-CTTTTTGCCAACATCCCTG-3′ and reverse
5′-GGTCTCTGGTTGAATCTCCT-3′; reference gene human β-actin primers,
forward 5′-GAAGATCAAGATCATTGCTCCT-3′ and reverse
5′-TACTCCTGCTTGCTGATCCA-3′. Conditions for the RT-qPCR reactions
were as follows: 2 min at 94°C followed by 40 cycles of 20 sec at
94°C, 16 sec at 54°C and 30 sec at 72°C. The gene expression
results were analyzed using GraphPad Prism software version 5.01
(GraphPad Software, Inc., La Jolla, CA, USA). To detect mRNA levels
of the target gene in autophagy- and cancer-associated pathways,
the following primers (Shanghai Kehua Bio-engineering Co., Ltd.)
were employed for qPCR using the same conditions as described
previously (26): DAPK1, forward
5′-AGAAATTCAAGAAGTTTGCAG-3′ and reverse
5′-GTCTTCCTCATCCAGAGTAT-3′.
Cell viability assay
The viability of each group were detected at 6, 12,
24, 48 and 72 h time points following treatment [either DHA alone
(10 µg/ml); DHA (10 µg/ml) and rapamycin (100 nmol/l); or
Atg7-knockdown cells treated with DHA (10 µg/ml)], and experiments
were repeated 3 times. The media were removed from all groups and
replaced with serum-free high-glucose DMEM. Next, MTT (5 mg/ml) was
added into the wells and cultured at 37°C for 4 h.
When the medium of all groups was discarded,
dimethyl sulfoxide was added into the wells (150 µl/well) and
oscillated for 10 min to dissolve the formazan crystals. A
colorimetric assay was performed using an ELISA reader (Supermax
3100 Plus; Shanghai Flash Biotechnology Co., Ltd., Shanghai, China)
to determine the optical density value of each group at a
wavelength of 490 nm (OD490). The number of living cells was
proportional to the OD490, which was used to determine the cell
viability.
Flow cytometry
MDA-MB-231 cells were inoculated on 6 well plates (2
ml/well) and cultured for 24 h at a concentration of
5×104/ml using DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were then cultured at 7°C in 5%
CO2 and 95% saturated humidity following treatment by
group as aforementioned [either DHA alone (10 µg/ml); DHA (10
µg/ml) and rapamycin (100 nmol/l); or Atg7-knockdown cells treated
with DHA (10 µg/ml)] for 24 h. Subsequently, cells were harvested
after 24 and 48 h and fixed with precooling 70% ethanol at 4°C for
1 h. Cells were then centrifuged at 400 × g for 10 min at 4°C,
washed twice with 0.01 mol/l PBS solution, and incubated with 1 ml
0.01 mol/l PBS containing 100 µg/ml RNAase A (cat no. HZB0210;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 30 min.
Subsequently, 75 µl propidium iodide (Sigma-Aldrich; Merck KGaA)
was added to 1 ml 0.01 mol/l PBS at a final concentration of 50
µg/ml and incubated at 4°C in dark room for 30 min. Finally, BD
FACS Calibur Flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) was used and cell cycle data was analyzed using BD FACSDiva
software version 4.1 (BD Biosciences).
Statistical analysis
All results, unless otherwise indicated, are
expressed as the mean ± standard error of at least triplicate
experiments. Data were analyzed using the GraphPad Prism software
version 5.01. P<0.05 was considered to indicate a statistically
significant difference. Differences were evaluated using Student's
t-test, or two-way repeated measures analysis of variance with
Bonferroni's post hoc test. Information regarding autophagy
pathways were obtained from the Kyoto Encyclopedia of Genes and
Genomes (KEGG) (27).
Results
Rapamycin promotes the death of
DHA-treated MDA-MB-231 cells whereas Atg7 deficiency increases the
survival rate of DHA-treated MDA-MB-231 cells
MDA-MB-231 cells were treated with DHA-alone (10
µg/ml), DHA (10 µg/ml) and rapamycin (100 nmol/l), or DHA (10
µg/ml) and Atg7 siRNA. MDA-MB-231 cells were examined using an MTT
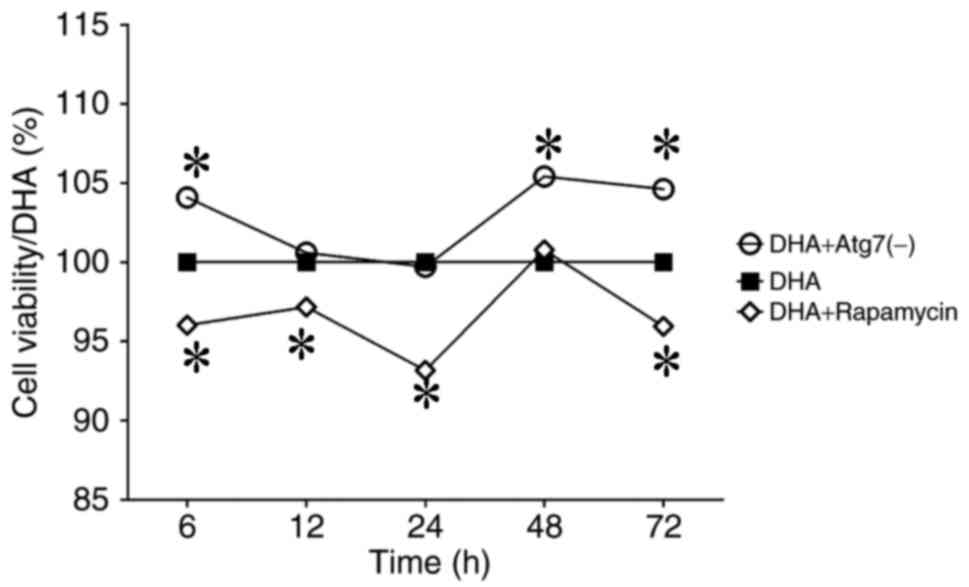
assay (6, 12, 24, 48 and 72 h). Rapamycin decreased the viability
of MDA-MB-231 breast cancer cells, whereas Atg7 knockdown increased
cell viability (Fig. 1). The data
demonstrated that rapamycin enhanced the anticancer action of DHA
on this breast cancer cell line; therefore, rapamycin promoted the
death of DHA-treated MDA-MB-231 cells. Hence, rapamycin-induced
autophagy may promote the anticancer effect of DHA on breast cancer
cells (Fig. 1).
Atg7 may indirectly upregulate DAPK
expression and enhance autophagy
To investigate the effect of autophagy inhibition or
induction on gene expression involved in the cancer and autophagy
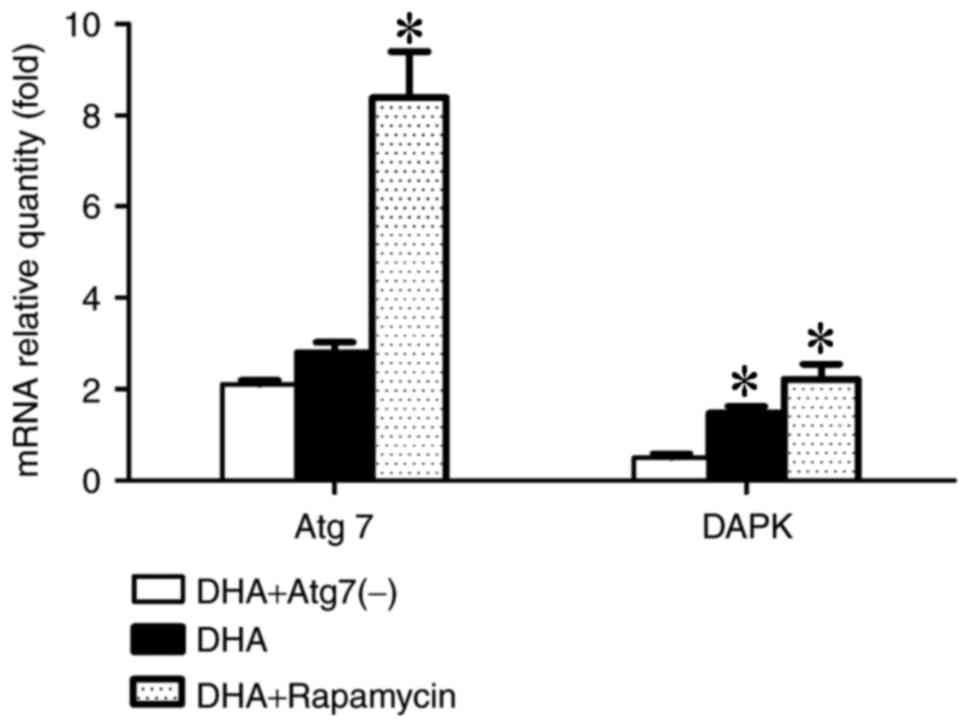
pathways, the mRNA levels of Atg7 and DAPK were examined using
RT-qPCR, with β-actin as the reference gene, following 24 h of the
aforementioned treatments. Expression of Atg7 and DAPK was
increased in the rapamycin group, whereas the expression levels
decreased in the Atg7-knockdown group. The data demonstrated that
the Atg7 gene may positively regulate DAPK expression via an
unknown mechanism (Fig. 2).
Rapamycin promotes apoptosis in
DHA-treated MDA-MB-231 breast cancer cells
To investigate the effect of DHA on the cell cycle
and apoptosis following the induction or inhibition of autophagy,
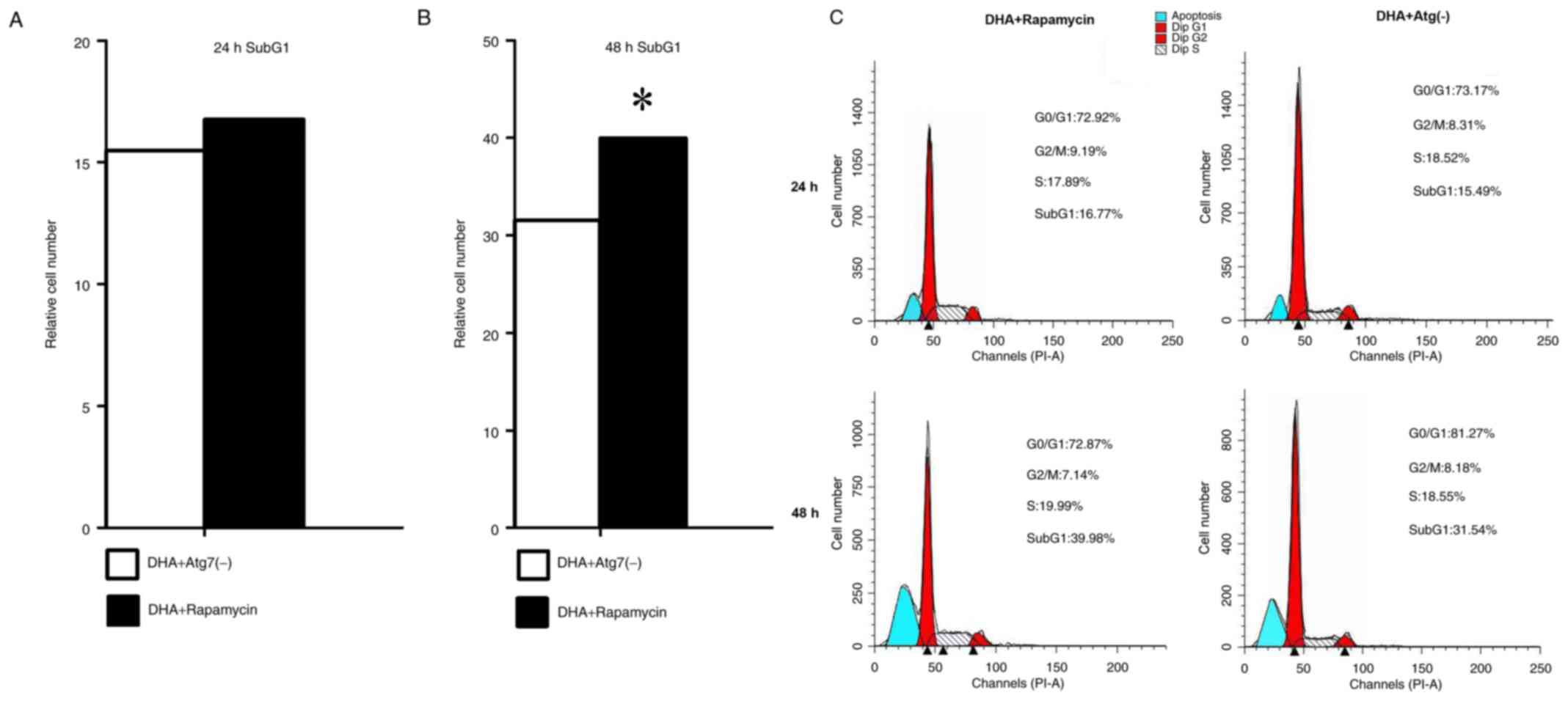
the number of cells in each cell cycle phase was examined using a
flow cytometer at 24 and 48 h. Treatment with rapamycin notably
increased the cell number in the apoptosis phase at 24 and 48 h,
which indicated that the induction of autophagy may be vital for
apoptosis to occur in MDA-MB-231 cells. Compared with the rapamycin
group, the cell number during the apoptosis phase was less in the
Atg7-kncokdown group at each time point. Cell cycle arrest for all
groups at 24 and 48 h occurred in the G0/G1
phase. The results indicate that rapamycin promoted apoptosis in
DHA-treated MDA-MB-231 breast cancer cells (Fig. 3).
Discussion
In recent years, the anticancer effects of DHA have
been demonstrated in a number of studies (28,29). Zhang
et al (30) demonstrated that
DHA can inhibit translationally controlled tumor protein
(TCTP)-dependent cell migration and invasion by inhibiting TCTP and
reducing cell division control protein 42 homolog (Cdc42)
activation in the TCTP-positive cell lines NOZ, GBC-SD, OCUG-1 and
EH-GB-1 in vitro. Additionally, this was also demonstrated
in gallbladder cancer xenograft animal models established using a
spleen-to-liver metastasis model in immunodeficient mice (30). Lemke et al (31) revealed that DHA exerted an anti-glioma
activity, which included promotion of autophagy and induction of
oxidative stress in the glioma cell lines LN-229 and LN-Z308 as
well as in the primary T269 glioma cell line. The study indicated
that DHA mildly inhibited the growth of glioma cells via the
induction of autophagy, and that the anticancer effect of
temozolomide was significantly enhanced following co-treatment with
DHA in vitro and in vivo (31). Zhao et al (32) demonstrated that combined treatment
with DHA and curcumin decreased cell viability, arrested the cell
cycle and promoted apoptosis in human ovarian cancer SKOV3 cells.
It was also identified that treatment with DHA alone has the
ability to induce limited apoptosis, arrest the cell cycle at the S
and G2/M phases and inhibit tumor growth in a xenograft
model established by subcutaneously injecting SKOV3 cells into the
right flank of female BALB/c nude mice, without notable toxicity
(32). Additionally, caspase-3 was
not activated following treatment with DHA, and caspase-3
inhibitors did not inhibit cell apoptosis in any group; therefore,
it was hypothesized that DHA may induce cell apoptosis via other
apoptotic pathways (32). Consistent
with these previous studies, the present study identified that DHA
induced autophagy. Despite the presence of numerous studies
(28–32), there is limited knowledge regarding
the effect of autophagy on the anticancer action of DHA. The
present study revealed the role of autophagy in the anticancer
action of DHA within MDA-MB-231 cells. It was demonstrated that
induction of autophagy using rapamycin promoted the death of
DHA-treated MDA-MB-231 breast cancer cells, and Atg7 knockdown
increased the survival rate of DHA-treated MDA-MB-231 cells.
Furthermore, it was revealed that treatment with rapamycin notably
increased the proportion of apoptotic cells at 24 and 48 h,
indicating that the induction of autophagy may be required for the
apoptosis of MDA-MB-231 cells. Rapamycin has the ability to
increase the expression of Atg7 and DAPK genes to enhance cell
autophagy. Atg7 knockdown decreased the expression levels of DAPK,
thus autophagy was inhibited. Existing data from the associated
autophagy pathway in KEGG (27)
indicated that increased expression of the DAPK gene has the
ability to negatively regulate the ERK signaling pathway and
therefore inhibit cell proliferation (27) and activate autophagy-associated
pathways (27). The data demonstrated
that DHA synergizes with rapamycin and promotes cell death and
apoptosis via the upregulation of DAPK and Atg7, which provides an
indication for its usefulness in anticancer studies and clinical
application. In accordance with the results of other studies
regarding DHA, the effects of DHA on autophagy were further
confirmed (28–32). The present study provides novel
evidence regarding the effects of rapamycin combined with DHA and
Atg7 knockdown; however, owing to the limitations of in
vitro experiments, the associated combined effects should be
investigated in in vivo animal models in the future, with
further cell lines investigated.
In conclusion, the present study demonstrated that
rapamycin had the ability to promote cell death and apoptosis, and
notably increased Atg7 and DAPK gene expression in MDA-MB-231
cells. Conversely, Atg7 knockdown facilitated cell survival and
decreased Atg7 and DAPK gene expression in MDA-MB-231 cells.
Previous studies have demonstrated that increased expression of the
DAPK gene resulted in negative regulation of the ERK signaling
pathway, which would inhibit cell proliferation; therefore,
rapamycin may regulate the proliferation of MDA-MB-231 cells by
increasing Atg7 gene expression, which in turn would increase DAPK
gene expression levels. Consequently, it was considered that
promoting autophagy may be vital in the anticancer effects of DHA,
and that the regulation of the Atg7 expression levels may also
influence DAPK expression levels.
Acknowledgements
The present study was funded by the Fostering
Project of Sichuan Science and Technology Innovation Seedling
Engineering (grant no. 20132048), the Project of the Education
Department of Sichuan (grant no. 15ZA0159), the National Natural
Science Foundation of China (grant no. H0815) and the Natural
Project of Luzhou Medical College (grant no. 2014ZD-004). The
authors would like to thank Doctor Liuqi Yang (National Key
Laboratory of West China School of Sichuan University) for
providing the MDA-MB-231 cells. The authors thank the Mr Qijie Li
(Department of molecular genetics, West China School of Preclinical
and Forensic Medicine, Sichuan University,) for technical
assistance in flow cytometry.
References
|
1
|
Plucinski MM, Dimbu PR, Macaia AP,
Ferreira CM, Samutondo C, Quivinja J, Afonso M, Kiniffo R, Mbounga
E, Kelley JS, et al: Efficacy of artemether-lumefantrine,
artesunate-amodiaquine, and dihydroartemisinin-piperaquine for
treatment of uncomplicated Plasmodium falciparum malaria in Angola,
2015. Malar J. 16:622017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thanh NV, Thuy-Nhien N, Tuyen NT, Tong NT,
Nha-Ca NT, Dong LT, Quang HH, Farrar J, Thwaites G, White NJ, et
al: Rapid decline in the susceptibility of Plasmodium falciparum to
dihydroartemisinin-piperaquine in the south of Vietnam. Malar J.
16:272017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mao H, Gu H, Qu X, Sun J, Song B, Gao W,
Liu J and Shao Q: Involvement of the mitochondrial pathway and
Bim/Bcl-2 balance in dihydroartemisinin-induced apoptosis in human
breast cancer in vitro. Int J Mol Med. 31:213–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh NP, Lai HC, Park JS, Gerhardt TE,
Kim BJ, Wang S and Sasaki T: Effects of artemisinin dimers on rat
breast cancer cells in vitro and in vivo. Anticancer Res.
31:4111–4114. 2011.PubMed/NCBI
|
|
5
|
Lai H, Nakase I, Lacoste E, Singh NP and
Sasaki T: Artemisinin-transferrin conjugate retards growth of
breast tumors in the rat. Anticancer Res. 29:3807–3810.
2009.PubMed/NCBI
|
|
6
|
Noori S and Hassan ZM: Dihydroartemisinin
shift the immune response towards Th1, inhibit the tumor growth in
vitro and in vivo. Cell Immunol. 271:67–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen T, Li M, Zhang R and Wang H:
Dihydroartemisinin induces apoptosis and sensitizes human ovarian
cancer cells to carboplatin therapy. J Cell Mol Med. 13:1358–1370.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Handrick R, Ontikatze T, Bauer KD, Freier
F, Rübel A, Dürig J, Belka C and Jendrossek V: Dihydroartemisinin
induces apoptosis by a Bak-dependent intrinsic pathway. Mol Cancer
Ther. 9:2497–2510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu W, Chen SS, Zhang JL, Lou XE and Zhou
HJ: Dihydroartemisinin induces autophagy by suppressing NF-κB
activation. Cancer Lett. 343:239–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia G, Kong R, Ma ZB, Han B, Wang YW, Pan
SH, Li YH and Sun B: The activation of c-Jun
NH2-terminal kinase is required for
dihydroartemisinin-induced autophagy in pancreatic cancer cells. J
Exp Clin Cancer Res. 33:82014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin-induced autophagy leads to the apoptosis in breast cancer
stem cells: Molecular mechanisms. Mol Cancer. 12:1712013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Desai S, Liu Z, Yao J, Patel N, Chen J, Wu
Y, Ahn EE, Fodstad O and Tan M: Heat shock factor 1 (HSF1) controls
chemoresistance and autophagy through transcriptional regulation of
autophagy-related protein 7 (ATG7). J Biol Chem 29,. 288:9165–9176.
2013. View Article : Google Scholar
|
|
13
|
Wilson EN, Bristol ML, Di X, Maltese WA,
Koterba K, Beckman MJ and Gewirtz DA: A switch between
cytoprotective and cytotoxic autophagy in the radiosensitization of
breast tumor cells by chloroquine and vitamin D. Horm Cancer.
2:272–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:pp. 15077–15082. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nemoto T, Tanida I, Tanida-Miyake E,
Minematsu-Ikeguchi N, Yokota M, Ohsumi M, Ueno T and Kominami E:
The mouse APG10 homologue, anE2-like enzyme for Apg12p conjugation,
facilitates MAP-LC3 modification. J Biol Chem. 278:39517–39526.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizushima N, Yamamoto A, Hatano M,
Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y and Yoshimori
T: Dissection of autophagosome formation using Apg5-deficient mouse
embryonic stem cells. J Cell Biol. 152:657–667. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez Y, Aryal B, Chehab L and Rao VA:
Atg7- and Keap1-dependent autophagy protects breast cancer cell
lines against mitoquinone-induced oxidative stress. Oncotarget.
5:1526–1537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caramés B, Hasegawa A, Taniguchi N, Miyaki
S, Blanco FJ and Lotz M: Autophagy activation by rapamycin reduces
severity of experimental osteoarthritis. Ann Rheum Dis. 71:575–581.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan T, Rawal P, Wu Y, Xie W, Jankovic J
and Le W: Rapamycin protects against rotenone-induced apoptosis
through autophagy induction. Neuroscience. 164:541–551. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Montoro E, Lemus D, Echemendia M, Martin
A, Portaels F and Palomino JC: Comparative evaluation of the
nitrate reduction assay, the MTT test, and theresazurin microtitre
assay for drug susceptibility testing of clinical isolatesof
Mycobacterium tuberculosis. J Antimicrob Chemother. 55:500–505.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harrison B, Kraus M, Burch L, Stevens C,
Craig A, Gordon-Weeks P and Hupp TR: DAPK-1 binding to a linear
peptide motif in MAP1B stimulates autophagy and membrane blebbing.
J Biol Chem. 283:9999–10014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inbal B, Bialik S, Sabanay I, Shani G and
Kimchi A: DAP kinase and DRP-1 mediate membrane blebbing and the
formation of autophagic vesicles during programmed cell death. J
Cell Biol. 157:455–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Q, Shi X, Zhou X, Wang D, Wang L and
Li C: Effect of autophagy inhibition on cell viability and cell
cycle progression in MDA-MB-231 human breast cancer cells. Mol Med
Rep. 10:625–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Overbergh L, Valckx D, Waer M and Mathieu
C: Quantification of murine cytokine mRNAs using real time
quantitative reverse transcriptase PCR. Cytokine. 11:305–312. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kyoto Encyclopedia of Genes and Genomes
(KEGG): hsa05200: Pathways in cancer. KEGG source record: clv04140:
Regulation of autophagy. http://www.kegg.jp/kegg-bin/highlight_pathway?scale=1.0&map=map04140&keyword=autophagyJanuary
17–2018
|
|
28
|
Du XX, Li YJ, Wu CL, Zhou JH, Han Y, Sui
H, Wei XL, Liu L, Huang P, Yuan HH, et al: Initiation of apoptosis,
cell cycle arrest and autophagy of esophageal cancer cells by
dihydroartemisinin. Biomed Pharmacother. 67:417–424. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu GS, Lu JJ, Guo JJ, Huang MQ, Gan L,
Chen XP and Wang YT: Synergistic anti-cancer activity of the
combination of dihydroartemisinin and doxorubicin in breast cancer
cells. Pharmacol Rep. 65:453–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang F, Ma Q, Xu Z, Liang H, Li H, Ye Y,
Xiang S, Zhang Y, Jiang L, Hu Y, et al: Dihydroartemisinin inhibits
TCTP-dependent metastasis in gallbladder cancer. J Exp Clin Cancer
Res. 36:682017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemke D, Pledl HW, Zorn M, Jugold M, Green
E, Blaes J, Löw S, Hertenstein A, Ott M, Sahm F, et al: Slowing
down glioblastoma progression in mice by running or the
anti-malarial drug dihydroartemisinin? Induction of oxidative
stress in murine glioblastomatherapy. Oncotarget. 7:56713–56725.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao J, Pan Y, Li X, Zhang X, Xue Y, Wang
T, Zhao S and Hou Y: Dihydroartemisinin and curcumin
synergistically induce apoptosis in SKOV3 cells via upregulation of
MiR-124 targeting midkine. Cell Physiol Biochem. 43:589–601. 2017.
View Article : Google Scholar : PubMed/NCBI
|