Introduction
Osteosarcoma (OS) is the most commonly occurring
type of primary malignant bone tumor; it primarily occurs in
children and adolescents, with a current estimated incidence rate
of 5.4/1,000,000 worldwide (1,2).
Currently, the treatment of OS relies on the application of
comprehensive treatments, including chemotherapy and surgery. The
5-year survival rate of OS has improved over the last 30 years
since 1980, reaching ~70% in the USA (3). However, there is still a considerable
amount of patients with OS who have exhibited clinical
insensitivity to the presently available chemotherapies, and these
patients typically have a poor prognosis (4). Therefore, investigating novel
strategies, such as translational research, is necessary to help
broaden the understanding of OS and determine novel approaches for
therapeutic modalities (5).
Focal adhesion kinase (FAK) is a ~125-kDa protein
that is recruited as a participant in focal adhesion dynamics
between cells, and serves a role in cellular motility and survival.
FAK also serves a role in the protein-protein-interaction adaptor
at the sites of cell attachment to the extracellular matrix (ECM),
contributing to focal-adhesion ‘scaffolding’ and transmitting
adhesion-dependent signals into the cell interior (6). A previous study has reported that FAK is
involved in the regulation of the turnover of adhesion sites, which
are vital in the control of cell migration (7).
In the context of cancer, a considerable amount of
evidence has demonstrated increased FAK expression in tumor cells,
including in cancers of the colon, rectum, oral epithelium and
ovary (8,9). Additionally, the phosphorylation of FAK
at specific sites, for example, phosphorylation of FAK at Y397, has
been demonstrated to be associated with numerous tumor types
(10).
Although the inhibition of FAK function may be a
promising anti-cancer approach, it remains obscure as to what the
most effective modality would be. Due to the fact that FAK is a
non-receptor protein-tyrosine kinase implicated in signaling
pathways that affect cell migration, proliferation and apoptosis,
and potentially serves a role in oncogenic transformation that lead
to increased kinase activity, potential intervention routes have
included the inhibition of kinase activity and the disruption of
vital protein-protein interactions (6). The underlying mechanisms that can
explain the increased expression of FAK in tumor cells have not
been completely elucidated; however, the amplification of the FAK
gene has been reported in a number of cancer cell lines (6–9,11). The aim of the present study was to
explore the role of FAK in the carcinogenesis and development of
OS. Firstly, the expression levels of FAK in human OS tissues and
cell lines were compared with normal adjacent tissues. Secondly,
the expression of FAK was downregulated by transfecting the MG-63
cell line with FAK siRNA. in vitro and in vivo
functional studies, including the Cell Counting Kit-8 (CCK-8)
assay, Transwell invasion assay and flow cytometric analysis were
conducted to measure the proliferation, migration and apoptosis of
the cells, in order to examine the functional effects of FAK
depletion in OS. Thirdly, western blotting analysis was conducted
to evaluate the expression pattern of phosphorylated (p)-AKT,
p-BRAF and p-PDK1 proteins to test their effects on the AKT and
MAPK signaling pathways. It is well-established that the AKT and
MAPK signaling pathways are involved in the biological activities
of human cancer types, including cell survival, proliferation,
differentiation and tumor growth (12,13). FAK
expression was determined using immunohistochemistry, and the
association between FAK expression and clinicopathological
characteristics was measured in the present study.
The data of the present study indicated that FAK
expression is significantly upregulated in OS cell lines and
tissues, compared with those in normal adjacent tissues.
siRNA-induced depletion of FAK inhibited the proliferation and
migration of the MG63 cell line. The increased expression of FAK is
closely associated with advanced OS stage and higher rates of
recurrence; therefore, FAK overexpression predicts an unfavorable
clinical outcome in patients with OS.
Materials and methods
OS cell culture and cell lines
Two OS cell lines, MG-63 and U2-OS, were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). OS cells were maintained in a humidified atmosphere
containing 5% CO2 at 37°C. Cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and
100 mg/ml streptomycin (Sigma-Aldrich; Merck KGaA), according to
the established protocols of the ATCC.
Patient and specimen samples
Human OS samples and matched normal adjacent tissue
samples were obtained from 80 patients, who were diagnosed with OS
and were admitted for treatment at the Department of Orthopedics of
the People's Hospital of Yuyao (Yuyao, China). The inclusion
criteria for OS patients were as follows: i) Patients had readily
available and clear clinical data; ii) patients had not previously
received any antitumor treatments, such as radiotherapy or
chemotherapy; and iii) none of the patients presented with any
genetic disorders or other diseases of the bone. A total of 80 OS
tissue samples and 80 paired normal adjacent tissue samples were
obtained from orthopedic surgery. The collected tissues were
divided into two groups, each group had 40 OS tissue samples and 40
paired normal tissue samples, for different experimental purposes:
i) One was frozen immediately following surgery and was stored at
−80°C until RNA isolation; ii) the other group was fixed in 10%
buffered formaldehyde solution and then was embedded in paraffin
and sectioned, according to the standard protocol of the Society
for Applied Immunohistochemistry (14). The study design complied with the
regulation of the Helsinki Declaration, and the present study was
approved by the Ethics Committee of the People's Hospital of Yuyao
(Yuyao, China). All patients agreed to participate in the study
with informed written consent.
Transient siRNA transfection
Functional analyses used siRNA duplexes specific for
FAK to knockdown FAK expression, which were designed by and
purchased from GenePharma Co., Ltd. (Shanghai, China). The
transient transfections of siRNA were carried out using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham MA, USA). OS cells were plated at ~80%
confluence in DMEM containing 10% serum with no antibiotics.
Transfections were performed within <20 h after plating. FAK
siRNA and Lipofectamine® 2000 were diluted with 100 µl
serum-free DMEM. After 5 min, the diluted FAK siRNA was mixed with
diluted Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), and the mixture was incubated for 30 min at room temperature
to ensure complex formation. FAK siRNA was transfected at the
concentration of 50 nmol/l. The knockdown effect was confirmed
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The FAK siRNA sequence was as follows: FAK siRNA
forward, 5′-GCCTGGTGAAAGCTGTCATC-3′ and reverse,
5′-GCTTCTGTGCCATCTCAATC-3′. A scrambled siRNA that did not target
FAK was used as control. The sequence of the scrambled siRNA was as
follows: Sense, 5′-ACGCGUAACGCGGGAAUUUdTdT-3′ and antisense,
5′-AAAUUCCCGCGUUACGCGUdTdT-3′.
RT-qPCR analysis
Total RNAs were extracted from OS tissues, OS cell
lines and paired normal adjacent tissues using TRIzol®
(Thermo Fisher Scientific, Inc.) and a RecoverAll™ Total Nucleic
Acid Isolation kit (Ambion; Thermo Fisher), according to the
manufacturer's protocol. The experiment was performed as follows:
RNAs were reverse transcribed using the SYBR Green PrimeScript™
RT-PCR kit (cat no. RR066A; Takara Biotechnology Co., Ltd., Dalian,
China). The GAPDH primer sequence used in the study was as follows,
sense 5′-CAGAAGACUGUGGAUGGCC-3′, anti-sense
3′-GGCCAUCCACAGUCUUCUG-5′. RT-qPCR conditions were as follows:
Denaturing at 95°C for 5 min, prior to 40 cycles of amplification
at 95°C for 30 sec, then 60°C for 30 sec and 72°C for 30 sec. Each
sample was assayed in triplicate to quantify the mean
2−ΔΔCq values, ± standard deviation (SD) (15). FAK miRNA expression level was
normalized to the relative quantities of GAPDH.
Western blot analysis
Proteins were quantified using the BCA protein assay
kit (Pierce; Thermo Fisher Scientific Inc.). The following primary
antibodies used in the present study were purchased from Abcam
(Cambridge, MA, USA.); pan-AKT antibody (dilution 1:1,000; catalog
no. ab8805); p-AKT3 (S472) + AKT2 (S474) + AKT1 (S473) antibody
(dilution 1:1,000, catalog no. ab192623); BRAF antibody (dilution
1:1,000; catalog no. ab33899); p-BRAF (T401) antibody (dilution
1:800; catalog no. ab68215); PDK1 antibody (dilution 1:1,000;
catalog no. ab52893); p-PDK1 (S241) antibody (dilution 1:800;
catalog no. ab32800); BAX antibody (dilution 1:1,000; catalog no.
ab32503), Bcl-2 antibody (dilution 1:1,000; catalog no. ab59348). A
β-actin antibody (dilution 1:1,000; catalog no. A2066) purchased
from Sigma-Aldrich (Merck KGaA) was used as the endogenous control.
Total proteins were extracted by lysing cultured cells using RIPA
buffer, and cell debris were removed by centrifuging the lysates at
15,000 × g for 15 min at 4°C. The protein extracts (50 µg per lane)
were separated and fractionated using 12% SDS-PAGE and were then
transferred to PVDF membranes (Merck Millipore). The PVDF membranes
were blocked with PBS buffer supplemented with 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) overnight at 4°C, and were then
incubated with the primary antibodies at room temperature for 2 h,
followed by incubation with horseradish peroxidase-conjugated goat
anti-rabbit IgG antibody (1:10,000; catalog no. ab205718; Abcam) at
room temperature for another 2 h. Following washing with
tris-buffered saline with 0.1% Tween-20, the bands of interest were
visualized using ECL (EMD Millipore, Billerica, MA, USA).
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) whole tissue
specimens were sequentially cut into 4-µm-thick sections. Following
de-paraffinization and rehydration, the sections were cut into 4 µm
thick sections, and were dewaxed in xylene, followed by heating in
a microwave at the temperature of 60°C for 30 min in EDTA buffer
(pH 9.0) for antigen retrieval. FFPE sections were treated with
0.3% H2O2, and then incubated with 10% normal
goat serum (Sigma-Aldrich; Merck KGaA). Antigen retrieval was
conducted using EDTA (pH 8.0) at 100°C for 25 min. Sections were
washed, and then incubated with the FAK antibody (dilution 1:60;
catalog no. ab40794; Abcam) overnight at 4°C. Afterwards, FFPE
sections were incubated at 37°C for 40 min, prior to washing and
incubation with the secondary antibody at a dilution of 1:200 (cat.
no. 9720; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) at room
temperature for 60 min. FFPE sections were washed and incubated
with diaminobenzidine tetrachloride for 15 min, and were
counterstained with hematoxylin for 20 min at room temperature.
Meanwhile, negative controls (NC) were prepared and incubated with
PBS, without the FAK antibody. FAK immunohistochemistry was
independently evaluated by two experienced pathologists from the
People's Hospital of Yuyao (Yuyao, China), and were blinded to
patient characteristics. The percentage of positively stained cells
in the representative fields, were calculated and the images were
photographed using a Leica DM IRB microscope (magnification, ×200
and ×400; Leica Micosystems GmbH, Wetzlar, Germany). FAK levels in
tissue samples (OS tissues and paired normal adjacent tissues) were
measured using a semi-quantitative scoring method (16,17), based
on the intensity and percentage of overall staining. The staining
intensities of FAK were grouped according to the following
criteria: <5% FAK-positive tumor cells was classified as
negative staining; 6–25% FAK-positive tumor cells was classified as
weak staining; 25–50% FAK-positive tumor cells was classified as
moderate staining; and >50% FAK-positive tumor cells was
classified as strong staining.
Cell apoptosis assay using flow
cytometric analysis
Given that the expression of FAK mRNA was lower in
MG-63 cells than that in U2-OS cells, the depletion of FAK mRNA was
greater, and so the MG-63 cell line was used in subsequent
knockdown assays. MG-63 cells were plated on 6-well plates at a
density of 2×105 cells per well, were maintained in the medium
overnight. Afterwards, cells were trypsinized, collected,
centrifuged, and washed three times with cold PBS, and were
harvested and re-suspended in 250 µl 1X binding buffer at a density
of 1×106 cells/ml. Cells were transferred to a 5 ml culture tube,
and then were stained with 5 µl Annexin V (1 mg/ml; Beckman
Coulter, Inc., Brea, CA, USA) at room temperature for 15 min.
Propidium iodide (PI; 1 mg/ml) was subsequently used for cell
staining for 5 min at room temperautre. Cells were vortexed gently
and were incubated at room temperature in the dark for 15 min. A
total of 400 µl of 1X Binding buffer was added into each tube,
prior to assessment of the cell lines with a flow cytometer. Each
experiment was conducted three times, independently.
Cell proliferation assay
The effect of FAK siRNA on the proliferation of OS
cells was measured using CCK-8 (cat. no. 1166; R&D Systems,
Inc., Minneapolis, MN, USA). Cells were seeded into the 96-well
plates (10,000 cells per well). CCK8 was used according to the
manufacturer's protocol, and 24 h after the transfection of FAK
siRNA into the MG-63 cell line, CCK-8 was added into each well and
then was incubated at 37°C for 1.5 h. Optical density was measured
at 450 nm using a microplate spectrophotometer (Tecan Group Ltd.,
Zurich, Switzerland).
Cell invasion assay
The invasive ability of OS cells was determined
using 24-well Transwell plates (Corning Life Sciences, Tewksbury,
MA, USA) pre-coated with Matrigel basement membrane matrix, with a
concentration of 1 mg/ml. A total of 2×104 cells were seeded into
each well in the upper chamber with 0.1% serum. In the lower
chamber, 0.6 ml of medium containing 10% FBS was added to stimulate
invasion. Transwell plates were incubated overnight at 37°C in an
atmosphere of 5% CO2. Non-invading cells were removed
from the top chamber, while invading cells in the bottom chamber
were fixed with 4% paraformaldehyde, stained with 0.1% crystal
violet. The images were photographed using Olympus CKX53 Inverted
Microscope (×200 magnification). Data collected were based on three
independently conducted experiments.
Statistical analysis
Data are expressed as the mean ± SD and were
analyzed using the SPSS statistical software package version 21.0
(IBM Corp., Armonk, NY, USA). One-way analysis of variance followed
Scheffe's test, or the Student's t-test was used to analyze the
differences in the expression levels of FAK mRNA. Pearson's χ2
tests and Fisher's exact test were used to analyze the association
of FAK expression with clinicopathological characteristics.
P<0.05 was considered to indicate a statistical significant
difference.
Results
FAK expression level is upregulated in
human OS tissues and cell lines compared with that in normal
adjacent tissues
To analyze the expression levels of FAK mRNA,
RT-qPCR analysis was conducted in the OS tissue samples, paired
normal adjacent samples, MG-63 and U2-OS cell lines. GAPDH was used
as a reference gene. As shown in Fig.
1A, FAK mRNA expression was significantly lower in paired
normal tissues compared with the levels in OS tissues and cell
lines (P<0.001).
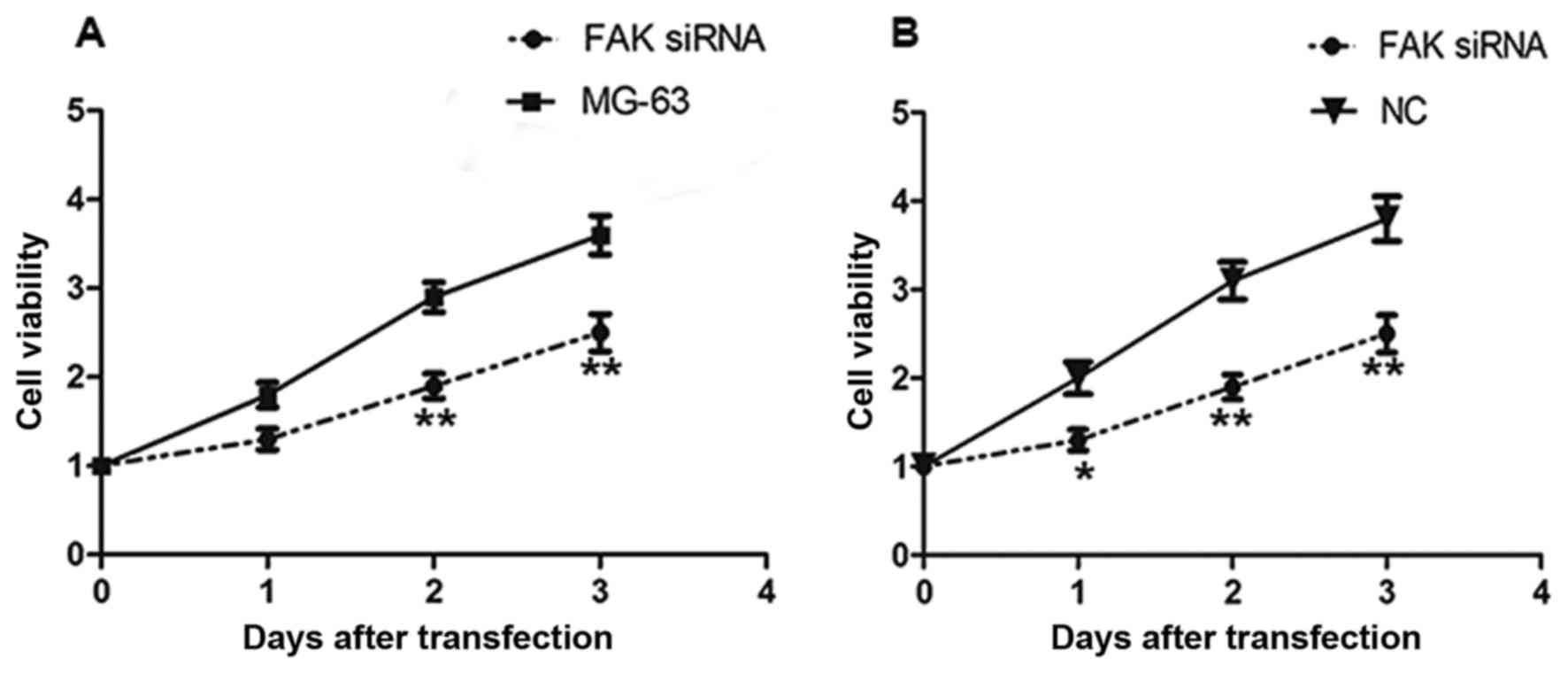
siRNA-induced depletion of FAK and
suppresses the proliferation of MG-63 cells
To investigate the biological role of FAK on the
proliferation of OS, FAK downregulation was conducted by
transfecting MG-63 cells with FAK siRNA. Following transfection,
CCK-8 was used to measure the effect of FAK downregulation on the
proliferation of MG-63 cells (Fig.
2). As illustrated in Fig. 2, the
downregulation of FAK significantly suppressed the growth of MG-63
cells after 48 and 72 h of transfection, compared with that of the
NC group and untrasfected MG-63 cell. The results demonstrated that
the siRNA-mediated inhibition of FAK inhibited the proliferation of
OS cells.
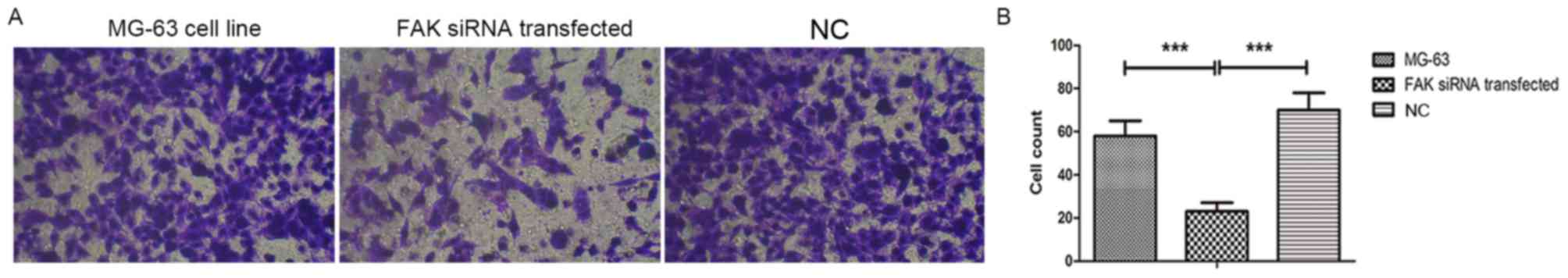
siRNA-mediated knockdown of FAK
inhibites the invasion of OS cells
To evaluate the role of FAK in the invasive
potential of MG-63 OS cells transfected with FAK siRNA, Transwell
assays were performed. As presented in Fig. 3, the invasive potential of OS cells
was suppressed after the downregulation of FAK (MG-63 cell line
transfected with FAK siRNA), compared with those of non-transfected
MG-63 cells and the scrambled siRNA transfection group. The invaded
cell numbers in the FAK siRNA group were the smallest of the three
groups analyzed.
siRNA-mediated downregulation of FAK
induces the apoptosis of OS cells and enhances the expression of
apoptotic proteins
To investigate the mechanism behind the association
of FAK with the regulation of OS cell growth, cell apoptosis was
measured by flow cytometry. As shown in Fig. 4, the percentage of apoptotic OS cells
was significantly lower in the group of untransfected MG-63 cells
(3.45%; Fig. 4A) and the NC siRNA
group (4.07%; Fig. 4B) when compared
with the siRNA-induced FAK knockdown (14.92%) group (Fig. 4C).
Western blot analysis of Bax and Bcl-2
antibodies demonstrated that FAK depletion induces apoptosis
As shown in Fig. 5,
the expression of Bax was increased and the expression of Bcl-2 was
decreased in MG-63 cells transfected with FAK siRNA, when compared
with the negative control (NC) group and untransfected MG-63 cells.
Consistent with the results obtained by flow cytometry, western
blot analysis of Bax and Bcl-2 demonstrated that down-regulation of
FAK induces apoptosis in OS cells.
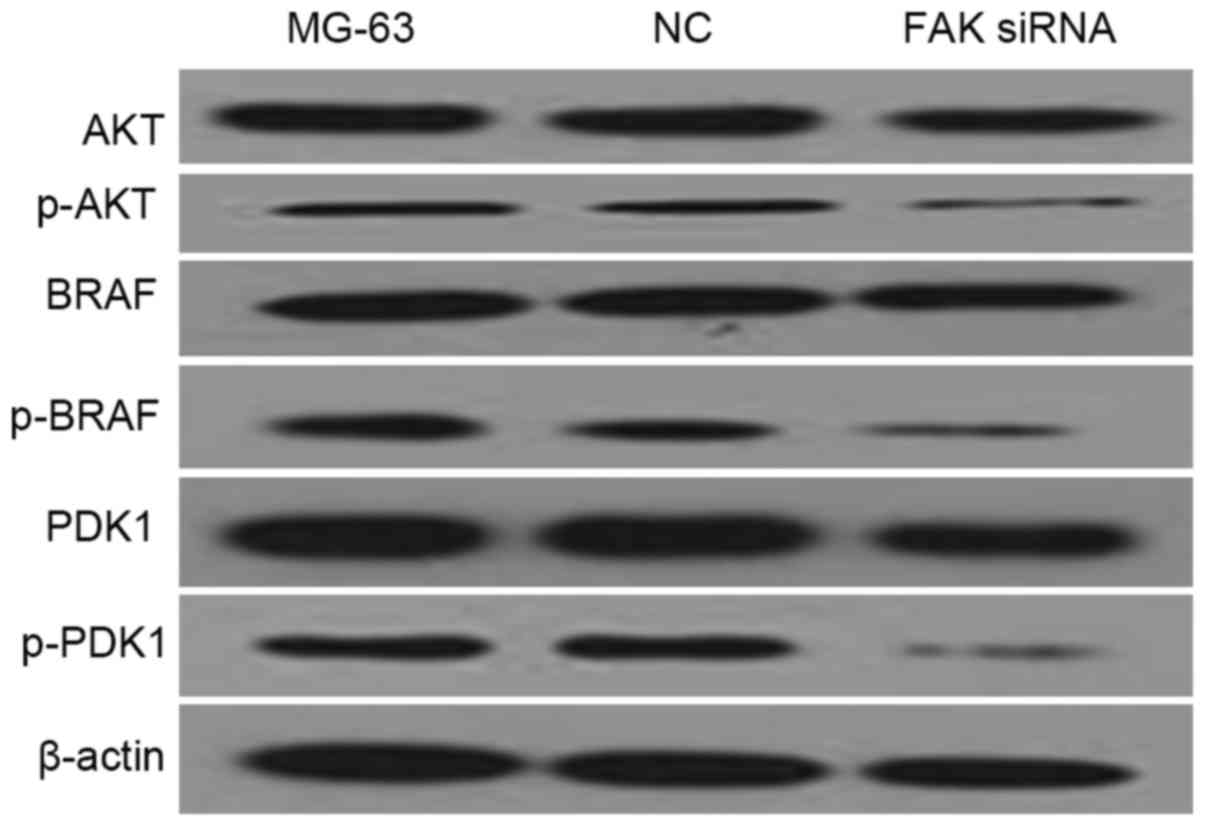
FAK down-regulation is associated with
the deactivation of AKT and MAPK pathways
PDK1 is a component of the AKT pathway, while BRAF
is a component of the MAPK pathway. In the western blot analysis of
the present study, AKT, p-AKT, PDK1 p-PDK1, BRAF, p-BRAF antibodies
were used to measure the protein levels of their corresponding
genes in FAK-depleted MG-63 cells. β-actin was used as endogenous
control. As shown in Fig. 6, p-AKT,
p-PDK1 and p-BRAF protein levels in the FAK-depleted MG-63 cell
line were significantly lower than those in other examined groups.
The data demonstrated that the AKT and MAPK pathways are involved
in the down-regulation of FAK.
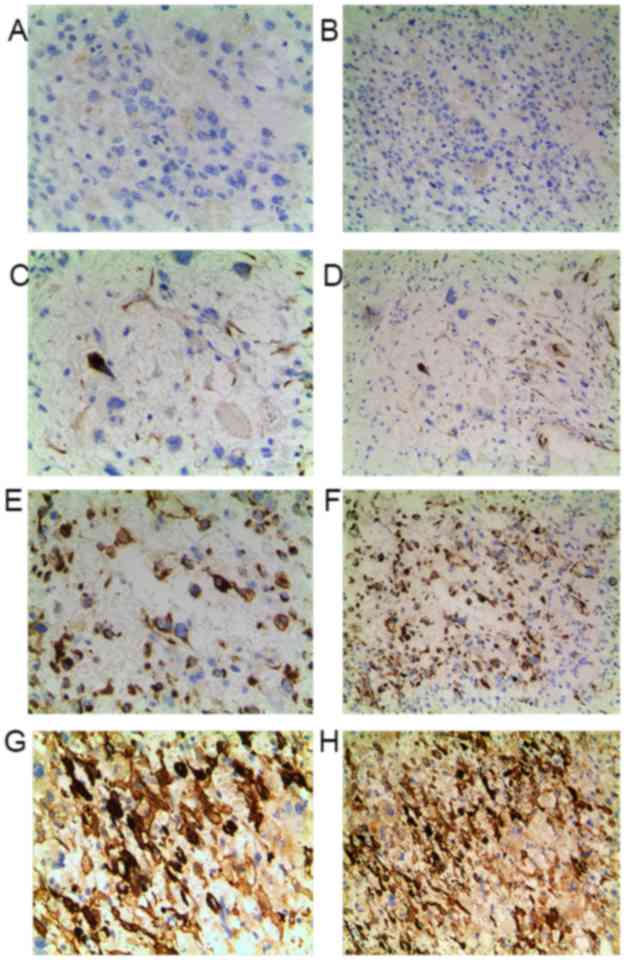
Elevated FAK expression suggests an
unfavorable prognosis in patients with OS
To further determine the clinicopathological
significance of FAK in OS, we performed IHC analysis of FAK in
tissues collected from 80 cases of OS. Representative IHC images of
FAK expression are shown in Fig.
7.
The associations between FAK expression level and
the corresponding clinicopathological characteristics of patients
with OS were calculated using Pearson's χ2 test. Associations
between FAK expression level and the clinicopathological features
of 80 patients with OS patients are summarized in Table I, based on the IHC results of FAK
protein expression (Fig. 7). The
Enneking surgical staging system was consulted when measuring the
grade of OS (18). Stage I represents
low grade, stage II tumors are high grade and stage III represents
tumors with distant metastasis. The present study identified that
clinically advanced Enneking stages (P<0.001) and recurrence
(P=0.041) in patients with OS were associated with higher FAK
expression levels.
 | Table I.Expression levels of FAK and their
associations with the clinicopathological features of the 80
patients with osteosarcoma included in the present study. |
Table I.
Expression levels of FAK and their
associations with the clinicopathological features of the 80
patients with osteosarcoma included in the present study.
|
|
| FAK expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Total, n | High, n | Low, n | P-value |
|---|
| Sex |
|
|
| 0.739 |
| Male | 42 | 17 | 25 |
|
|
Female | 38 | 14 | 24 |
|
| Age, years |
|
|
| 0.183 |
|
<18 | 47 | 20 | 27 |
|
|
≥18 | 33 | 19 | 14 |
|
| Recurrence |
|
|
| 0.041a |
|
Yes | 51 | 35 | 16 |
|
| No | 29 | 10 | 19 |
|
| Enneking stage |
|
|
| <0.001a |
| II | 31 | 12 | 19 |
|
|
III | 49 | 35 | 14 |
|
Discussion
OS ranks as the third most common type of cancer in
adolescents, following lymphomas and brain tumors (11). Despite advancements in the diagnosis
and treatment of OS in recent years, its clinical prognosis has not
considerably improved. Furthermore, due to the high mortality and
morbidity rates, rapid progression and poor prognosis of OS caused
by the migration of OS cells, OS continues to pose a severe threat
to the health of patients (3,6). Metastasis remains the principal cause
for the mortality of OS patients (19). Therefore, determining an accurate
biomarker for OS by extensive investigation may help direct the
development of novel therapies. The metastasis of tumors is a
complex biological and pathological process, in which the studies
by Liotta et al (20,21) summarized that three steps are
included: i) Adhesion; ii) degradation; iii) migration and
invasion.
FAK is a non-receptor protein tyrosine kinase that
is widely expressed in the cytoplasm. The phosphorylation of FAK
can mediate numerous downstream signaling pathways, incurring
various biological processes within the cells. Phosphorylation of
FAK mediates signaling pathways and is widely involved in various
biological processes within cells, and contributes to tumor
progression, including invasion, metastasis and angiogenesis
(22–25). The study by Ilić et al
(26) demonstrated that FAK is
involved in the turnover of focal adhesion contacts during cell
migration in FAK-deficient mice. It was also reported that the
association of FAK with signaling proteins, such as PI3-kinase and
paxillin, enables FAK to function in a network of
integrin-stimulated signaling pathways, giving rise to the
activation of ERK and JNK/MAPK (27).
The present study demonstrated the down-regulation of p-AKT, p-PDK1
and p-BRAF proteins in the MG-63 cell line following transfection
with FAK siRNA. These results demonstrated that decreased
expression of FAK results in the deactivation of the AKT and MAPK
pathways.
Considerable evidence has indicated that the
pro-apoptotic regulator Bax serves a role in accelerating
programmed cell death by antagonizing or binding to the apoptosis
repressor Bcl-2 (28). Bcl-2 is
involved in variety of cell systems, and regulates cell death by
regulating the permeability of the mitochondrial membrane.
Consequently, changes in the expression patterns of Bax and Bcl-2
are indicative of cell apoptosis (29). In the present study, the data revealed
that the expression of the pro-apoptotic protein Bax was increased
in the FAK-depleted cell line, while the expression level of the
anti-apoptotic protein Bcl-2 was decreased. Furthermore, flow
cytometric analysis demonstrated that the down-regulation of FAK in
the MG-63 cell line resulted in an increased percentage of early
apoptotic cells. These observations demonstrated that depleted FAK
expression stimulated apoptosis in OS cells.
Clinicopathological parameters, including Enneking
stage, metastasis and recurrence rates, are key indicators for the
progression and deterioration of OS (18,30). The
in vitro and in vivo data of the present study are in
agreement with the results of a previous study, which reported that
increased expression of FAK was associated with tumor progression
and a worse prognosis (31). The
present study postulates that overexpressed FAK promotes malignancy
and eventually results in poorer clinical outcomes in patients with
OS. Consequently, OS patients with a higher expression level of FAK
are anticipated to have a shorter overall survival time.
The limitations of the present study is that the
data acquired were based on in vitro experiments, and the
present study explored the biological role FAK in OS cells by
depleting FAK expression, while the effect of FAK upregulation on
OS cells was not addressed. The comparison of FAK mRNA expression
was conducted using RT-qPCR; and the FAK protein levels in
untransfected MG-63 cells and cells transfected with FAK siRNA or
scrambled control were not assessed by western blotting.
To conclude, the present study comprehensively
demonstrated that FAK plays a role in the proliferation, migration
and apoptosis of OS cells through the AKT and MAPK pathways, and
that enhanced FAK expression was positively associated with
aggressive clinicopathological characteristics of patients with OS.
These observations may provide basis for novel therapeutic
strategies targeting FAK in the treatment of OS.
Acknowledgements
The authors thank Professor Juan Huang (Department
of Oncology, People's Hospital of Yuyao, Yuyao, China) for revising
the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 53048352) and the
People's Hospital of Yuyao.
Availability of data and materials
All data generated or analyzed in the present study
to establish the conclusion are available and included in this
article.
Authors' contributions
BZ obtained the funding and designed the study. HJG
performed the experiments, collected, analyzed and interpreted the
data. BZ wrote the manuscript. All authors have read and approved
the manuscript.
Ethics approval and consent to
participate
The study design complied with the regulation of the
Helsinki Declaration and the present study was approved by the
Ethics Committee of the People's Hospital of Yuyao (Yuyao, China).
All patients provided written informed consent prior to their
inclusion in the study.
Consent for publication
All patients consented to the publication of their
data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OS
|
osteosarcoma
|
|
FAK
|
focal adhesion kinase
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Siclari VA and Qin L: Targeting the
osteosarcoma cancer stem cell. J Orthop Surg Res. 5:782010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pakos EE and Ioannidis JP: The association
of P-glycoprotein with response to chemotherapy and clinical
outcome in patients with osteosarcoma. A meta-analysis. Cancer.
98:581–589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Golubovskaya MV: Focal adhesion kinase as
a cancer therapy target. Anticancer Agents Med Chem. 10:735–741.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer-a new therapeutic opportunity. Nat Rev Cancer. 5:505–515.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weiner TM, Liu ET, Craven RJ and Cance WG:
Expression of focal adhesion kinase gene and invasive cancer.
Lancet. 342:1024–1025. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kornberg LJ: Focal adhesion kinase and its
potential involvement in tumor invasion and metastasis. Head Neck.
20:745–752. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lark AL, Livasy CA, Calvo B, Caskey L,
Moore DT, Yang X and Cance WG: Overexpression of focal adhesion
kinase in primary colorectal carcinomas and colorectal liver
metastases: Immunohistochemistry and real-time PCR analy. Clin
Cancer Res. 9:215–222. 2003.PubMed/NCBI
|
|
10
|
Grisaru-Granovsky S, Salah Z, Maoz M,
Pruss D, Beller U and Bar-Shavit R: Differential expression of
protease activated receptor 1 (Par1) and pY397FAK in benign and
malignant human ovarian tissue samples. Int J Cancer. 113:372–378.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wittig JC, Bickels J, Priebat D, Jelinek
J, Kellar-Graney K, Shmookler B and Malawer MM: Osteosarcoma: A
multidisciplinary approach to diagnosis and treatment. Am Fam
Physician. 65:1123–1132. 2002.PubMed/NCBI
|
|
12
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakr RA, Barbashina V, Morrogh M,
Chandarlapaty S, Andrade VP, Arroyo CD, Olvera N and King TA:
Protocol for PTEN expression by immunohistochemistry in
formalin-fixed paraffin-embedded human breast carcinoma. Appl
Immunohistochem Mol Morphol. 18:371–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lassmann S, Bauer M, Soong R, Schreglmann
J, Tabiti K, Nährig J, Rüger R, Höfler H and Werner M:
Quantification of CK20 gene and protein expression in colorectal
cancer by RT-PCR and immunohistochemistry reveals inter- and
intratumour heterogeneity. J Pathol. 198:198–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhargava R, Beriwal S and Dabbs DJ:
Mammaglobin vs GCDFP-15: An immunohistologic validation survey for
sensitivity and specificity. Am J Clin Pathol. 127:103–113. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jawad MU and Scully SP: In brief:
Classifications in brief: Enneking classification: Benign and
malignant tumors of the musculoskeletal system. Clin Orthop Relat
Res. 468:2000–2002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Zeng B, Ma J and Wan C: Comparative
proteomic analysis of osteosarcoma cell and human primary cultured
osteoblastic cell. Cancer Invest. 27:345–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mak M, Reinhart-King CA and Erickson D:
Microfabricated physical spatial gradients for investigating cell
migration and invasion dynamics. PLoS One. 6:e208252001. View Article : Google Scholar
|
|
23
|
Zachary I: Focal adhesion kinase. Int J
Biochem Cell Biol. 29:929–934. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beviglia L, Golubovskaya V, Xu L, Yang X,
Craven RJ and Cance WG: Focal adhesion kinase N-terminus in breast
carcinoma cells induces rounding, detachment and apoptosis. Biochem
J. 373:201–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakurai S, Sonoda Y, Koguchi E, Shinoura
N, Hamada H and Kasahara T: Mutated focal adhesion kinase induces
apoptosis in a human glioma cell line, T98G. Biochem Biophys Res
Commun. 293:174–181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ilić D, Furuta Y, Kanazawa S, Takeda N,
Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M and Yamamoto T:
Reduced cell motility and enhanced focal adhesion contact formation
in cells from FAK-deficient mice. Nature. 377:539–544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlaepfer DD, Hauck CR and Sieg DJ:
Signaling through focal adhesion kinase. Prog Biophys Mol Biol.
71:435–478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Angelis PM, Stokke T, Thorstensen L,
Lothe RA and Clausen OP: Apoptosis and expression of Bax, Bcl-x,
and Bcl-2 apoptotic regulatory proteins in colorectal carcinomas,
and association with p53 genotype/phenotype. Mol Pathol.
51:254–261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parkes SE, Parke S, Mangham DC, Grimer RJ,
Davies P and Morland BJ: Fifty years of paediatric malignant bone
tumours in the West Midlands, UK, 1957–2006: Incidence, treatment
and outcome. Paediatr Perinat Epidemiol. 24:470–478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyazaki T, Kato H, Nakajima M, Sohda M,
Fukai Y, Masuda N, Manda R, Fukuchi M, Tsukada K and Kuwano H: FAK
overexpression is correlated with tumour invasiveness and lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 89:140–145. 2003. View Article : Google Scholar : PubMed/NCBI
|