Introduction
Triple-negative breast cancers (TNBCs) that lack
expression of estrogen receptor (ER), progesterone receptor (PR)
and human epidermal growth factor receptor 2 (HER2) account for
15–20% of all breast cancers (1).
Clinically, TNBCs are associated with greater aggressiveness and
early metastasis that result in worse patient survival compared to
other breast cancer types (2).
Moreover, absence of well-defined molecular targets currently
limits chemotherapy of TNBC in adjuvant or metastatic settings to
cytotoxic agents (3), whereas newer
targeted drugs have shown disappointing treatment responses
(4). Hence, there is a real need to
develop new therapeutic agents that can improve the survival and
quality of life of patients with TNBC (5).
A compound that shows promising therapeutic action
on TNBC is curcumin, a natural polyphenol with a wide range of
anti-cancer properties that has the added advantage of minimal side
effects (4,6,7). In a
study using MDA-MB-231 breast cancer cells, a cell model widely
used to develop candidate drugs against TNBC, curcumin was
demonstrated to effectively inhibit cell proliferation and induce
apoptosis (6). However, despite the
promising anti-tumor properties of curcumin in vitro, very
low aqueous solubility and poor bioavailability is a major obstacle
for its successful use in vivo (8). Furthermore, curcumin is highly unstable
and is rapidly degraded with a short half-life following
administration into living bodies. These undesirable in vivo
properties render it impractical to deliver curcumin as a free
compound to target tissue in pharmacological concentrations
(9).
Biocompatible nanoparticle (NP) systems are being
extensively investigated as vehicles to stabilize and deliver
anti-tumor drugs in vivo. For example, our group previously
formulated polyethylene glycol-polylactic acid (PEG-PLA) polymeric
NPs loaded with resveratrol and confirmed improved metabolic and
antitumor effects in vivo (10). Similar approaches have also been
applied to improve the pharmacokinetics of curcumin. These include
encapsulating the compound by conjugation to liposomes, polymeric
NPs and micelles (11–13). However, whereas most NP drug-delivery
systems developed to date rely on enhanced permeation and retention
for passive tumor accumulation (14),
newer delivery systems achieve greater treatment effects by
actively targeting cancer cells. A highly promising therapeutic
target for TNBC is the epidermal growth factor (EGF) receptor. EGF
receptors play a crucial role in tumor growth, invasion, and
metastasis (15), and are
overexpressed in over half of TNBCs (16). We and others have previously targeted
EGF receptor-overexpressing cancer cells with its cognate ligand,
EGF (17–19). Growth factor peptides are the most
suited for selective tracing of functionally active high-affinity
surface receptors because they have greater binding affinity and
better tumor penetration compared to antibodies. To our knowledge,
there is only one previous study that synthesized an EGF
receptor-targeted NP for curcumin delivery. Yan et al,
recently prepared a poly (D, L-lactic acid-coglycolic
acid)-block-PEG copolymer conjugated with a synthetic EGF peptide
called GE11 for co-delivery of docetaxel and a curcumin prodrug
(20). However, although significant
antitumor effects were observed in prostate cancer bearing mice,
this was mainly attributed to the effect of docetaxel rather than
to the curcumin prodrug (20). Hence,
there is a need to explore the efficacy of EGF receptor-targeted
curcumin delivery NP systems for the treatment of TNBC.
In this study, we thus developed DSPE-PEG micelle
NPs encapsulating curcumin for improved in vivo
bioavailability. Functional groups of the NP were conjugated with
EGF peptide for EGF receptor-specific targeting. We examined the
anticancer effects of curcumin delivery using these NPs on
MDA-MB-468 TNBC cells in vitro and MDA-MB-468 tumors in
living mice.
Materials and methods
Cell lines and reagents
MDA-MB-468 human breast cancer cells from the
American Type Culture Collection (ATCC; Manassas, VA, USA) were
cultured in a humidified incubator at 37°C and 5% CO2 in
RPMI-1640 media. Media was supplemented with 10% FBS, 2 mM
L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin.
N-hydroxysuccinimide-PEG10000-DSPE
(DSPE-PEG-NHS) was purchased from NANOCS Inc. (New York, NY, USA).
Curcumin and tetrahydrofurane were from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Human EGF was purchased from GenScript
(Piscataway, NJ, USA), and SnakeSkin dialysis tubing was from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Preparation of EGF-conjugated
DSPE-PEG
The lysine residues of EGF peptides were conjugated
to the NHS group of DSPE-PEG-NHS. Briefly, 10 mg/ml of EGF was
mixed with 10 mg of NHS-functionalized DSPE-PEG in 0.1 M HEPES
buffer (pH 7.8) at a molar ratio of 1:2. The mixture was reacted
overnight with gentle shaking at room temperature (RT) and then
transferred into a dialysis membrane with a molecular weight
cut-off of 10 kDa. Unbound EGF was removed by 24 h dialysis with
stirring and change of deionized water every 2 h. EGF-DSPE-PEG was
finally lyophilized on a FDU-1200 freeze dryer (EYELA; Tokyo
Rikakikai Co., Ltd., Tokyo, Japan) and stored at −70°C until
use.
Formulation of curcumin-loaded
phospholipid NPs (Cur-NPs)
EGF-conjugated and unconjugated DSPE-PEG
phospholipid micelles were loaded with curcumin to formulate
EGF-Cur-NP and Cur-NP, respectively, using the thin-film hydration
method (21). Briefly, 2 mg of
curcumin and 20 mg of EGF-conjugated or unconjugated DSPE-PEG were
dissolved in 1 ml tetrahydrofurane at RT and mixed in a
round-bottom flask. The organic phase-solution mixture was
evaporated under vacuum at 40°C for 30 min. The film was flushed
with nitrogen gas for 50 min, and the organic solvent was
completely removed overnight at RT in a fume-hood. The thin film
was hydrated with 20 ml of 0.5 mM HEPES buffer (pH 7.5) with
vortexing, and sonicated for 3 min in a 50°C water bath to form
micelles. The NPs were purified by passage through a 0.22 µm
syringe filter to remove unloaded curcumin and transferred to a
fresh 50 ml tube. The resultant nano-suspension was cooled to
−70°C, lyophilized by a freeze dryer, and stored at −70°C until
use. Empty NP was prepared by the same procedure using unconjugated
DSPE-PEG without curcumin loading.
Physicochemical characterization of
NPs
The hydrodynamic diameter of NPs was determined with
a DLS-7000 dynamic light scattering spectrophotometer (Brookhaven
Instruments, Corporation, Holtsville, NY, USA) at 25°C using a
scattering angle of 90°. The zeta potential of NPs in filtered
phosphate-buffered saline (PBS; pH 7.4) was measured by a ZetaPlus
analyzer (Brookhaven Instruments, Corporation). Measurements were
performed twice per sample. The morphological shape of NPs was
assessed by a JEM ARM 200F transmission electron microscope (JEOL,
Peabody, MA, USA). Briefly, a drop of NPs was placed on a
Formvar-coated copper grid, negatively stained with 1% uranyl
acetated solution, and air-dried at RT. Magnifications of 50,000-
to 100,000-fold were used.
Confirmation of EGF conjugation by EGF
receptor activation
EGF conjugation of NPs was confirmed by its ability
to activate EGF receptors on overexpressing MDA-MB-468 cancer cells
by 5 min stimulation with a concentration of 5 nM. Free EGF and
Cur-NP were used as positive and negative controls, respectively.
Cells were lysed, and extracted protein was separated on a 10%
sodium dodecyl sulfate polyacrylamide gel electrophoresis and
transferred to a polyvinylidene difluoride membrane. After
incubation with a rabbit monoclonal antibody against total EGFR or
a mouse monoclonal antibody against phospho-EGFR (Tyr1068; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight, the
membrane was incubated with a secondary antibody at RT for 1 h.
Immuno-reactive protein was detected by chemiluminescence, and band
intensities were quantified using a GS-800 densitometer and
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Radioiodine labeling of NPs and cell
binding assays
Cur-NP and EGF-Cur-NP were radiolabeled with
125I using the Iodo-gen technique. Briefly, Pierce
pre-coated IODO-GEN tubes (Thermo Fisher Scientific, Inc.) were
washed with PBS, after which 100 µl PBS, 17.5 MBq (5 µl) of
Na125I, and 50 µl of NPs (250 µM stock) were added.
After 30 min reaction, the mixture was loaded on a PBS pre-soaked
PD-10 desalting column. PBS elution was performed to separate
125I-labeled NP from free 125I by collecting
0.5 ml fractions that were measured for radioactivity. The first
radioactivity peak fraction was used for cellular uptake
experiments.
For binding experiments, cancer cells were incubated
for 1 h with 110 ~150 kBq of 125I-Cur-NP or
125I-EGF-Cur-NP in Dulbecco-PBS with 1% bovine serum
albumin (BSA) at 37°C and 5% CO2. EGF receptor-specific
binding was determined by adding an excess amount of cold EGF (10
µM). Cells were rapidly washed twice and measured for
cell-associated radioactivity on a γ-counter (Wallac; PerkinElmer,
MA, USA). Uptake levels were expressed as radio-counts relative to
that of controls.
Fluorescent microscopic evaluation of
curcumin internalization
MDA-MB-468 cells grown on an 8-well chamber slide
(Lab-Tek II; Nalge Nunc International, Rochester, NY, USA) to 80%
confluence were treated for 2 h with 10 µM of free curcumin,
Cur-NP, or EGF-Cur-NP. Cells were then washed twice with PBS and
CC/Mount aqueous solution mounted (Sigma-Aldrich; Merck KGaA).
Curcumin, which fluoresces at approximately 405 nm, was visualized
within cells by a Zeiss confocal laser scanning microscope using
appropriate filters.
In vitro cytotoxicity and colony
formation assay
Cytotoxic effects were evaluated by sulforhodamine B
(SRB) assays. MDA-MB-468 cells were seeded on a 96-well plate at a
density of 4×103 cells per well and treated 24 h later
with graded doses of free curcumin, Cur-NP, or EGF-Cur-NP for 72 h.
Cells were then fixed with 10% (w/v) trichloroacetic acid and
stained with SRB for 30 min. Excess dye was removed by repeated
washing with 1% (v/v) acetic acid, and protein-bound dye was
dissolved in 10 mM Tris solution for optical density determination
at 510 nm on a micro-plate reader.
Colony formation assays were performed on MDA-MB-468
cells seeded on a 6-well plate at a density of 400 cells per well.
Cells were treated 24 h later with 5 µM of free Cur, Cur-NP, or
EGF-Cur-NP for 72 h. Media was changed every 3 days for two weeks
until colony formation. Cells were finally washed twice with cold
PBS and stained with 2.3% (w/v) crystal violet solution for 30 min.
After staining, wells were rinsed with tap water, dried in room air
at RT, and the number of colonies containing >50 cells was
counted.
Tumor-bearing mouse model and NP
treatment
All animal experiments were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals and approved by the Samsung Biomedical
Research Institute ethics committee. Tumor models were prepared in
BALB/c nude mice by subcutaneous injection of 5×106
MDA-MB-468 breast cancer cells into the right shoulder. When tumor
size averaged 50 mm3, mice were randomly allocated into
empty NP, Cur-NP (10 mg/kg), and EGF-Cur-NP (10 mg/kg) treatment
groups (all in 0.3 ml saline, n=4 per group). NPs were
intraperitoneally injected three times per week for a total of 8
injections. Health status of the mice was monitored by observation
of behavior and weighing of body weight. Tumors were measured each
treatment day with a caliper for maximal (length) and minimal
diameter (width). Tumor volume in mm3 was calculated as
length × width2 ×1/2.
In vivo imaging of tumor
18F-FDG uptake
A pilot micro-PET/CT imaging test was performed in 2
separate tumor-bearing mice fasted for 4 h immediately prior to and
24 h after the first dose of either vehicle (saline) or EGF-Cur-NP
(n=1, each). At 1 h after tail vein injection with 7.4 MBq of
18F-FDG, animals were isoflurane anesthetized, and
PET/CT images were acquired on an Inveon scanner (Siemens Medical
Solutions, Erlangen, Germany). On non-attenuation-corrected coronal
PET images, ovoid regions-of-interest (ROIs) were placed to include
all tumor mass while excluding adjacent tissue. A 20% threshold was
then used to automatically delineate the tumor margin. A second ROI
was drawn on the contralateral shoulder as background activity.
Tumor-to-background (Tm/Bkg) ratios of uptake were obtained by
dividing mean standard uptake value of the tumor by that of
background.
Data analysis
All data are presented as mean ± standard deviation.
Student's t-tests were used to compare 2 groups, and two-way
analysis of variance with Fisher's least significant difference
post hoc analysis was used to compare 3 or more groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Characteristics of curcumin-loaded
NPs
Phospholipid NPs successfully formed micellar
structures by thin-film hydration with a curcumin loading
efficiency of 63.3%. Whereas free curcumin was extremely
hydrophobic, Cur-NP and EGF-Cur-NP showed good solubility in PBS.
The physicochemical characteristics of Cur-NP and EGF-Cur-NP are
summarized in Fig. 1A. Mean diameter
measured by dynamic light scattering was 248.9±2.8 nm for Cur-NP
and 229.3±6.0 nm for EGF-Cur-NP. Zeta potential was close to
neutral and similar for Cur-NP and EGF-Cur-NP (Fig. 1A). Particle morphology assessed by
transmission electron microscopy displayed evenly formulated Cur-NP
and EGF-Cur-NP without clustering (Fig.
1B). These findings demonstrate that EGF conjugation does not
significantly influence Cur-NP size, zeta potential, or
morphology.
 | Figure 1.Characteristics and morphologies of
curcumin-loaded DSPE-PEG NP formulations. (A) Particle size, zeta
potential, and polydispersity index of Cur-NP and EGF-Cur-NP
formulations. (B) TEM images of Cur-NP and EGF-Cur-NP. Scale bar=20
nm; TEM magnification, ×100,000. EGF, epidermal growth factor; Cur,
curcumin; NP, nanoparticle; DSPE,
1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine; PEG, polyethylene
glycol. |
EGF receptor-specific targeting of
EGF-Cur-NP
Western blotting of overexpressing MDA-MB-468 cells
showed that 5 min incubations with 5 nM of free EGF or EGF-Cur-NP
were similarly potent stimulators for EGF receptor activation
(phosphorylation), whereas incubation with Cur-NP was not (Fig. 2A).
Competitive binding assays further confirmed EGF
receptor specificity of 125I-EGF-Cur-NP binding. Whereas
125I-Cur-NP binding to MDA-MB-468 cells was not
influenced by excess EGF, 125I-EGF-Cur-NP binding was
significantly reduced to 63.5±0.7% of that of the controls in the
presence of 10 µM of unlabeled EGF (Fig.
2B).
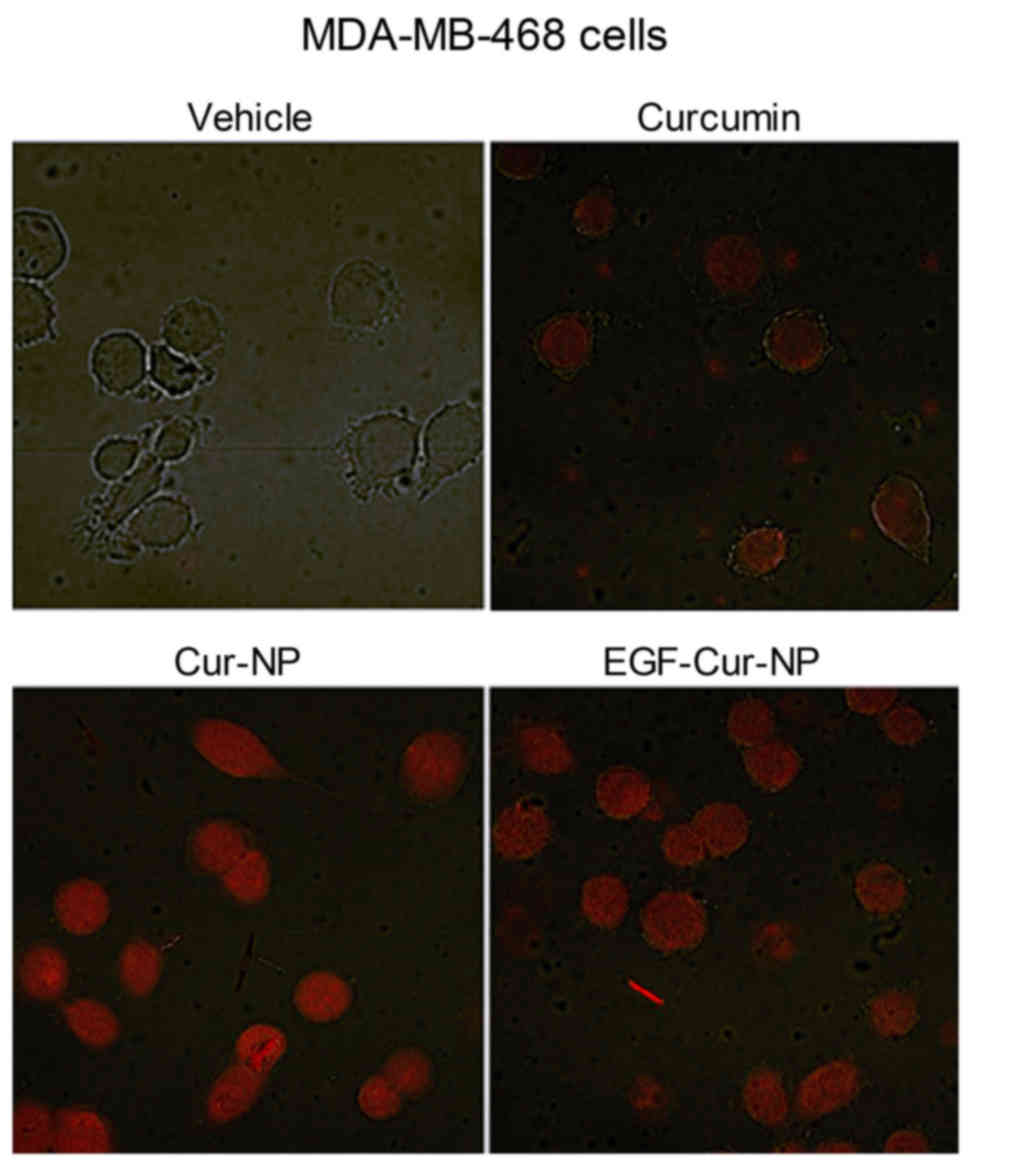
Fluorescent microscopy of NP-mediated
curcumin delivery into cancer cells
The efficiency of cellular delivery of curcumin by
NPs was compared to free curcumin by visualizing fluorescent
signals from the drug under confocal microscopy. The results showed
uniform intracellular delivery by curcumin-loaded NP and EGF-NP
that was more efficient as than free curcumin for MDA-MB-468 cells
(Fig. 3).
In vitro effect of Cur-NPs and
EGF-Cur-NPs on cancer cell survival
In vitro cytotoxicity assays on MDA-MB-468
cells showed that only the highest dose tested (10 µM) of free
curcumin and Cur-NP significantly reduced cell viability to
41.5±2.8 and 63.1±8.3% of baseline levels, respectively (Fig. 4A). In comparison, EGF-Cur-NPs exerted
a substantially greater dose-dependent antitumor effect that began
at a dose of 312 nM and further suppressed cell survival to
12.2±3.0% of baseline level with a dose of 10 µM (Fig. 4A). The calculated half inhibitory
concentration (IC50) for EGF-Cur-NP was 620 nM.
 | Figure 4.Comparison of cytotoxic effects on
MDA-MB-468 cancer cells. (A) SRB assay-based viable cell content
following 72 h treatment with vehicle (0.5% DMSO) or graded
concentrations of free curcumin, Cur-NP, or EGF-Cur-NP. (B) Number
of cancer cell colonies (>50 cells) assessed by crystal violet
staining. Following 72 h treatment with vehicle or 5 µM free
curcumin, Cur-NP, or EGF-Cur-NP, media was freshly changed, and
cells were further cultured for 2 weeks. Data are mean ± SD of 3
(A) or 2 (B) samples per group expressed as % relative to
vehicle-treated controls. (*P<0.05, †P<0.005,
‡P<0.001, compared to vehicle-treated controls). EGF,
epidermal growth factor; Cur, curcumin; NP, nanoparticle; SRB,
sulforhodamine B. |
The ability to suppress MDA-MB-468 cancer cell
colony formation was also greatest for EGF-Cur-NPF compared to
Cur-NP or free curcumin. Hence, treatment with 5 µM of free
curcumin and Cur-NP for 72 h reduced colony number over 2 weeks to
36.9±7.7 and 13.5±1.5% of controls, respectively (Fig. 4B). Treatment with 5 µM of EGF-Cur-NPs
completely abrogated the colony forming activity of the cells
(Fig. 4B).
Effects of treatment with NPs on in
vivo tumor growth
Piloting of the metabolic response of MDA-MB-468
tumors to EGF-Cur-NP treatment using micro-PET/CT images showed a
48.0% reduction of tumor-to-background ratio of 18F-FDG
uptake compared to baseline level after a single dose of
EGF-Cur-NP, while saline injection caused only a mild 11.5%
reduction of 18F-FDG uptake (Fig. 5A).
Finally, the therapeutic effects of repeated NP
administration on MDA-MB-468 tumor-bearing mice were evaluated.
Negative control animals administered empty NPs showed a steady
growth of tumors to 400±1.5% of baseline volume over 3 weeks (8
repeat injections). Treatment with Cur-NP did not significantly
reduce tumor growth compared to controls (Fig. 5B). In comparison, treatment with
EGF-Cur-NP significantly retarded tumor growth compared to controls
from the 4 to 8th injections (Fig.
5B). After 8 injections, EGF-Cur-NP caused a 59.1% retardation
of tumor growth compared to empty NP. The results showed no
body-weight change in mice treated with empty NP or Cur-NP. The
body-weight of mice treated with EGF-Cur-NP showed a transient mild
(9.2%) reduction at mid treatment (P<0.01; two-way repeat
measures ANOVA), but recovered to the level of other groups by the
end of treatment (Fig. 5C).
Discussion
Despite promising anti-tumor effects in
vitro, the clinical application of curcumin in patients with
cancer including TNBC is severely hampered by difficulty in
achieving pharmacologic concentrations in target tissue due to poor
aqueous solubility and rapid degradation in vivo (8).
In this study, we prepared phospholipid NPs to load
curcumin for improved solubility and in vivo stability.
Phospholipids are naturally occurring amphiphilic molecules that
constitute major structural elements of biological membranes.
Because they are composed of hydrophilic and hydrophobic parts,
phospholipid molecules self-assemble to form micellar NP shells.
These NPs can trap hydrophobic drugs for stable delivery in
vivo, with avoidance of plasma protein adsorption and prolonged
circulation time (22). In our study,
DSPE-PEG-NHS phospholipid NPs self-assembled into micelles and
could load curcumin in a straightforward and efficient manner
through thin-film hydration. NPs administered in excessive amounts
can potentially exert adverse effects on cellular physiology by
reactive oxygen species generation, cytokine secretion or
inflammatory responses (23). The
in vivo toxicity of NPs is highly dependent not only on
dosage, but also on their chemical composition and physicochemical
properties. The NPs used in this study were biodegradable
phospholipid micelles that have more favorable safety profiles
compared to less biocompatible NPs (24).
In NP formulation, the major challenges to curcumin
are low aqueous solubility and poor in vivo stability
(8). We also found that curcumin was
insoluble in PBS and needed to be dissolved in DMSO or ethanol. In
contrast, our curcumin-encapsulated DSPE-PEG formulation was
readily dissolved in PBS, which indicates improved bioavailability
under systemic administration. Although stability tests were not
performed in the present study, a previous study showed that
curcumin encapsulated in DSPE-PEG phospholipid was highly stable in
PBS for up to 8 h, whereas more than half of free curcumin was
degraded within 10 min (25).
Although most nanometer-sized NPs for drug delivery
are designed to reach tumor cells via leaky tumor vasculature
(26), ligand conjugation allows
specific targeting for increased drug accumulation into tumor
tissue. We therefore conjugated NHS-functionalized PEG molecules
with amine residues of EGF peptide to obtain curcumin-loaded NPs
with EGF receptor-specific targeting capacity. Physicochemical
characteristics of curcumin-loaded NPs were not significantly
altered by EGF ligand conjugation, and specific binding to and
activation of EGF receptors expressed on MDA-MB-468 TNBC cells were
confirmed by Western blots and competitive binding assays,
respectively.
Intracellular uptake and localization of curcumin
after treatment with the free drug or with delivery vehicles can be
quantitatively assessed by laser confocal microscopy using certain
absorption and fluorescence wavelengths (27). In our study, homogenous distribution
of fluorescence demonstrated that curcumin was located uniformly
within cancer cells. The intensity of fluorescent signals from
curcumin was significantly higher in MDA-MB-468 TNBC cells treated
with curcumin-loaded NPs compared to those treated with free
curcumin. Based on the previously verified linear dependency of
fluorescence intensity on intracellular concentration level
(27), this finding indicates greater
cellular uptake and/or retention when curcumin is delivered via our
NPs.
Despite numerous studies on the cytotoxic effects of
curcumin on malignant cells, there is a remarkable paucity of
reports on its effects on normal cells. Although curcumin has been
shown capable of exerting cytotoxicity to normal cells as well as
cancer cells (28), it is suggested
that tumor cells have significantly greater sensitivity (27). In the case of curcumin NPs, even high
doses are considered extremely safe in vivo, making it
suitable as an anticancer agent with minimal toxicity to normal
tissues (29). The NPs used in this
study may further reduce adverse effects on normal tissues because
PEG conjugation decreases uptake by cells of the
reticulo-endothelial system while EGF conjugation allows selective
targeting of cancer cells over normal cells.
Curcumin is reported to negatively regulate various
growth factors, protein kinases, transcription factors, cell
receptors and oncogenic proteins. Meanwhile the compound can induce
cell cycle arrest or apoptotic death of malignant cells (30,31). In
our in vitro cytotoxicity experiments, EGF-Cur-NPs potently
suppressed MDA-MB-468 cancer cell survival, whereas the effects of
free curcumin and Cur-NP were weak. Taurin et al previously
treated MDA-MB-468 cells with polystyrene-co-maleic acid micelles
encapsulating a curcumin analogue (RL71) and observed similar
cytotoxicity compared to free drug (IC50, 1.05 vs. 0.98 µM)
(32). In our results, EGF-Cur-NP
demonstrated a stronger cytotoxic effect (IC50, 0.62 µM). Colony
forming assays similarly demonstrated a significantly greater
anti-tumor effect by EGF-Cur-NP, which completely abrogated the
ability of MDA-MB-468 cells to form colonies. The number of
colonies was also significantly reduced by Cur-NP and free
curcumin, but to a lesser extent.
We finally compared the anti-tumor effects of our
NPs in MDA-MB-468 tumor-bearing mice. We chose intraperitoneal
injection for our NPs because this allows administration of larger
volumes without burdening the cardiovascular system and has the
advantage of longer circulation time with lower liver uptake
compared to intravenous injection (33). Although our group previously showed
that curcumin can shift cancer cell metabolism toward glycolytic
flux (7), pilot experiments in the
present study suggested reduced MDA-MB-468 tumor glucose metabolism
by EGF-Cur-NP. Because the expected increase of tumor
18F-FDG uptake was not observed, we did not attempt to
measure the precise magnitude of the metabolic effect of EGF-Cur-NP
treatment.
Yu et al previously tested the in vivo
anti-tumor effects in mice repeatedly intravenously injected with
40 mg/kg of methyl ether PEG-poly lactide amphiphilic block
copolymers loaded with curcumin and observed a 47.1% growth
reduction of MCF-7 tumors (34). We
used a much lower dose of 10 mg/kg for curcumin-loaded EGF-Cur-NPs
and still observed a greater 59.1% reduction of MDA-MB-468 tumor
growth compared to control animals.
In conclusion, DSPE-PEG phospholipid NPs conjugated
with EGF can effectively target and activate EGF receptors
expressed on TNBC cells. EGF-DSPE-PEG efficiently encapsulates
curcumin to exert cytotoxic effects in vitro and antitumor
effects in vivo in a manner superior to that of non-targeted
Cur-NP.
Acknowledgements
Not applicable.
Funding
This work was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT, & Future Planning
(grant no. 2014R1A1A3050612).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KHL and KHJ designed experiments and analyzed data;
JHL, JWP and DHK performed experiments. SHM and YSC analyzed the
data. KHJ and KHL wrote the manuscript. All authors read, provided
feedback and approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals and approved by the Samsung Biomedical
Research Institute ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TNBC
|
triple-negative breast cancer
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
NPs
|
nanoparticles
|
|
EGF
|
epidermal growth factor
|
|
NHS-PEG-DSPE
|
N-hydroxysuccinimide-Polyethylene
Glycol-1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine
|
References
|
1
|
Anders CK and Carey LA: Biology,
metastatic patterns, and treatment of patients with triple-negative
breast cancer. Clin Breast Cancer. 9 Suppl 2:S73–S81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palma G, Frasci G, Chirico A, Esposito E,
Siani C, Saturnino C, Arra C, Ciliberto G, Giordano A and D'Aiuto
M: Triple negative breast cancer: Looking for the missing link
between biology and treatments. Oncotarget. 6:26560–26574. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shindikar A, Singh A, Nobre M and
Kirolikar S: Curcumin and resveratrol as promising natural remedies
with nanomedicine approach for the effective treatment of triple
negative breast cancer. J Oncol. 2016:97507852016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jamdade VS, Sethi N, Mundhe NA, Kumar P,
Lahkar M and Sinha N: Therapeutic targets of triple-negative breast
cancer: A review. Br J Pharmacol. 172:4228–4237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun XD, Liu XE and Huang DS: Curcumin
induces apoptosis of triple-negative breast cancer cells by
inhibition of EGFR expression. Mol Med Rep. 6:1267–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung KH, Lee JH, Park JW, Moon SH, Cho YS
and Lee KH: Effects of curcumin on cancer cell mitochondrial
function and potential monitoring with 18F-FDG uptake.
Oncol Rep. 35:861–868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yallapu MM, Jaggi M and Chauhan SC:
Curcumin nanoformulations: A future nanomedicine for cancer. Drug
Discov Today. 17:71–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung KH, Lee JH, Park JW, Quach CHT, Moon
SH, Cho YS and Lee KH: Resveratrol-loaded polymeric nanoparticles
suppress glucose metabolism and tumor growth in vitro and in vivo.
Int J Pharm. 478:251–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naksuriya O, Okonogi S, Schiffelers RM and
Hennink WE: Curcumin nanoformulations: A review of pharmaceutical
properties and preclinical studies and clinical data related to
cancer treatment. Biomaterials. 35:3365–3383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng J, Teply BA, Sherifi I, Sung J,
Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R and
Farokhzad OC: Formulation of functionalized PLGA-PEG nanoparticles
for in vivo targeted drug delivery. Biomaterials. 28:869–876. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan JM, Zhang L, Yuet KP, Liao G, Rhee
JW, Langer R and Farokhzad OC: PLGA-lecithin-PEG core-shell
nanoparticles for controlled drug delivery. Biomaterials.
30:1627–1634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong C, Stylianopoulos T, Cui J, Martin J,
Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG and Fukumura D:
Multistage nanoparticle delivery system for deep penetration into
tumor tissue. Proc Natl Acad Sci USA. 108:2426–2431. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masuda H, Zhang D, Bartholomeusz C,
Doihara H, Hortobagyi GN and Ueno NT: Role of epidermal growth
factor receptor in breast cancer. Breast Cancer Res Treat.
136:331–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung KH, Choe YS, Paik JY and Lee KH:
99mTc-Hydrazinonicotinamide epidermal growth factor-polyethylene
glycol-quantum dot imaging allows quantification of breast cancer
epidermal growth factor receptor expression and monitors receptor
downregulation in response to cetuximab therapy. J Nucl Med.
52:1457–1464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung KH, Park JW, Paik JY, Quach CH, Choe
YS and Lee KH: EGF receptor targeted tumor imaging with
biotin-PEG-EGF linked to (99m)Tc-HYNIC labeled avidin and
streptavidin. Nucl Med Biol. 39:1122–1127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Chen L, Liu N, Li S, Hao Y and Zhang
X: EGF-coated nano-dendriplexes for tumor-targeted nucleic acid
delivery in vivo. Drug Deliv. 23:1718–1725. 2016.PubMed/NCBI
|
|
20
|
Yan J, Wang Y, Jia Y, Liu S, Tian C, Pan
W, Liu X and Wang H: Co-delivery of docetaxel and curcumin prodrug
via dual-targeted nanoparticles with synergistic antitumor activity
against prostate cancer. Biomed Pharmacother. 88:374–383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao BJ, Ke XY, Huang Y, Chen XM, Zhao X,
Zhao BX, Lu WL, Lou JN, Zhang X and Zhang Q: The antiangiogenic
efficacy of NGR-modified PEG-DSPE micelles containing paclitaxel
(NGR-M-PTX) for the treatment of glioma in rats. J Drug Target.
19:382–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weingart J, Vabbilisetty P and Sun XL:
Membrane mimetic surface functionalization of nanoparticles:
Methods and applications. Adv Colloid Interface Sci. 197–198:68–84.
2013. View Article : Google Scholar
|
|
23
|
Pandey RK and Prajapati VK: Molecular and
immunological toxic effects of nanoparticles. Int J Biol Macromol.
107:1278–1293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gothwal A, Khan I and Gupta U: Polymeric
micelles: Recent advancements in the delivery of anticancer drugs.
Pharm Res. 33:18–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gülçür E, Thaqi M, Khaja F, Kuzmis A and
Önyüksel H: Curcumin in VIP-targeted sterically stabilized
phospholipid nanomicelles: A novel therapeutic approach for breast
cancer and breast cancer stem cells. Drug Deliv Transl Res. 3:2013.
View Article : Google Scholar
|
|
26
|
Perrault SD, Walkey C, Jennings T, Fischer
HC and Chan WC: Mediating tumor targeting efficiency of
nanoparticles through design. Nano Lett. 9:1909–1915. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kunwar A, Barik A, Mishra B, Rathinasamy
K, Pandey R and Priyadarsini KI: Quantitative cellular uptake,
localization and cytotoxicity of curcumin in normal and tumor
cells. Biochim Biophys Acta. 1780:673–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Strojny B, Grodzik M, Sawosz E, Winnicka
A, Kurantowicz N, Jaworski S, Kutwin M, Urbańska K, Hotowy A,
Wierzbicki M and Chwalibog A: Diamond nanoparticles modify curcumin
activity: In vitro studies on cancer and normal cells and in ovo
studies on chicken embryo model. PLoS One. 11:e01646372016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yallapu MM, Jaggi M and Chauhan SC:
Curcumin nanomedicine: A road to cancer therapeutics. Curr Pharm
Des. 19:1994–2010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deguchi A: Curcumin targets in
inflammation and cancer. Endocr Metab Immune Disord Drug Targets.
15:88–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shanmugam MK, Rane G, Kanchi MM, Arfuso F,
Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP and Sethi G:
The multifaceted role of curcumin in cancer prevention and
treatment. Molecules. 20:2728–2769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taurin S, Nehoff H, Diong J, Larsen L,
Rosengren RJ and Greish K: Curcumin-derivative nanomicelles for the
treatment of triple negative breast cancer. J Drug Target.
21:675–683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung C, Kaul MG, Bruns OT, Dučić T, Freund
B, Heine M, Reimer R, Meents A, Salmen SC, Weller H, et al:
Intraperitoneal injection improves the uptake of
nanoparticle-labeled high-density lipoprotein to atherosclerotic
plaques compared with intravenous injection: a multimodal imaging
study in ApoE knockout mice. Circ Cardiovasc Imaging. 7:303–311.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu Y, Zhang X and Qiu L: The anti-tumor
efficacy of curcumin when delivered by size/charge-changing
multistage polymeric micelles based on amphiphilic poly(β-amino
ester) derivates. Biomaterials. 35:3467–3479. 2014. View Article : Google Scholar : PubMed/NCBI
|