Introduction
Under normal conditions, dying cells are immediately
eliminated by professional phagocytes in a process termed
efferocytosis (from the Latin efferre, meaning ‘to bury’). The
molecular machinery of apoptotic cell recognition and uptake by
phagocytes has previously been reviewed (1–3).
Successful efferocytosis suppresses the activation of nuclear
factor κ-light-chain-enhancer of activated B cells within the
phagocyte, inhibits the secretion of tumor necrosis factor (TNF)
and promotes the release of interleukin-4 (IL-4), IL-10 and
transforming growth factor-β (4–6). These
mediators are known to induce an alternative-activated macrophage
phenotype (7). This cell polarization
suppresses inflammation and promotes tissue remodeling and
angiogenesis, thereby contributing to the quiescent clearance of
apoptotic cells. However, if the clearance of apoptotic cells
fails, these cells may enter the late apoptotic/secondary necrotic
stage (8). This condition is
characterized by a disintegrated cell membrane, leading to the
leakage of inflammatory intracellular danger-associated molecular
patterns (DAMPs), such as calreticulin, high-mobility group box 1
(HMGB1) and nucleotides. The accumulation of late
apoptotic/secondary necrotic cells and the prolonged release of
intracellular components from dying cells contributes to chronic or
autoimmune diseases (2). Accordingly,
impaired efferocytosis has been observed in patients suffering from
systemic lupus erythematosus (SLE) (2), chronic granulomatous disease (9), Sjögren's syndrome (10), chronic obstructive pulmonary disease
(COPD) (11) and non-eosinophilic
asthma (12). A previous study
revealed, using genetically modified mouse models, that the absence
of genes for vital elements of the dead cell recognition and
clearance machinery, such as the C1q complex and milk fat
globule-EGF factor 8, results in an autoimmune phenotype that
resembles SLE (13). This suggests
the presence of a causal association between reduced efferocytosis
and these diseases.
Multiple myeloma (MM) accounts for ~10% of malignant
hematological disorders (14). MM is
characterized by the clonal proliferation of cluster of
differentiation 138 (CD138)+ malignant plasma cells in
the bone marrow, aberrant production of immunoglobulins, osteolytic
lesions, osteopenia and intractable bone pain (14). MM and various other hematological
disorders, including chronic lymphocytic leukemia, non-Hodgkin's
lymphoma and acute myeloid leukemia, are characterized by high
quantities of extracellular vesicles in the blood (15). The number of circulating extracellular
vesicles has been recently proposed to be a novel diagnostic marker
for MM (16). Between 45 and 95% of
these vesicles are positive for phosphatidylserine, which is a
hallmark of apoptosis (15). This
suggests that apoptotic microparticles contribute substantially to
the circulating extracellular vesicles. However, the reason why
apoptotic microparticles accumulate in MM remains unknown. Patients
with MM are treated, dependent on their age, with high-dose
induction chemotherapy with or without subsequent autologous
hematopoietic stem-cell transplantation. The most common induction
chemotherapy regimens are bortezomib-based and can be supplemented
with dexamethasone, doxorubicin, cyclophosphamide, lenalidomide or
thalidomide (17,18). The efficacy of bortezomib-containing
induction regimens, with respect to progression-free and overall
survival has been confirmed in a meta-analysis of various phase III
trials with transplant-eligible myeloma patients (19). Bortezomib is a proteasome inhibitor
that induces caspase activation and apoptotic cell death (20). In addition, it inhibits DNA repair and
restores the sensitivity of plasma cells to DNA-damaging
chemotherapeutic agents. Various in vitro studies have
indicated that bortezomib affects also immune cell function.
Straube et al (21)
demonstrated that bortezomib efficiently impairs the in
vitro maturation of dendritic cells (DCs) and blocks the
release of TNF and IL-12 upon lipopolysaccharide (LPS) stimulation
(20,21). In addition, it was revealed that
bortezomib selectively depletes monocytes in peripheral blood
mononuclear cell (PBMC) cultures, decreases the survival of
purified monocytes and induces apoptosis in monocyte-derived DCs
(22). These in vitro data
indicated that bortezomib-based regimens have, in addition to their
pro-apoptotic effect on malignant plasma cells, an inhibitory
influence on monocytes and macrophages; these cells are
particularly important for the efficient clearance of dead tumor
cells. In a previous study it was demonstrated in vitro that
monocytes eliminate late apoptotic/secondary necrotic cells
considerably faster than the early apoptotic cells (23). In addition, monocytes secrete distinct
cytokines when exposed to late apoptotic/secondary necrotic cell
remnants compared with early apoptotic microparticles (24). Thus, any reduction in efferocytosis is
anticipated to result in the accumulation of DAMP-releasing late
apoptotic/secondary necrotic cells, which in turn induce a
pro-inflammatory cytokine profile. In previous clinical studies of
patients with breast cancer, peripheral blood levels of the
immunogenic cell death marker HMGB1 were increased within 3 days of
the administration of the first chemotherapy cycle (25,26). This
result indicated that chemotherapy has an immediate effect on the
elimination of dead cell remnants.
The present study investigated the efferocytotic
capacity of the monocytes of patients with MM prior to and during
bortezomib-based induction chemotherapy. Blood was taken prior to
and during the first cycle of therapy and analyzed for
efferocytosis, for the quantity of dead cell remnants, and for a
panel of diverse cytokines, chemokines and soluble immune
checkpoint molecules. The results revealed a clear impairment of
efferocytosis in MM and give an overview of the immunological
consequences.
Materials and methods
Patient population and sample
collection
A total of 13 patients with MM (7 males and 6
females; age range 44–70 years; mean age 62 years) and 12 healthy
volunteers (6 males and 6 females; age range, 42–66 years; mean
age, 59 years) were enrolled between November 2013 and September
2015 at the Vienna General Hospital (Vienna, Austria). Only
patients without prior myeloma-specific therapy for at least 4
weeks were included. Patients receiving any other medications,
ongoing or active HIV or hepatitis (B or C) infection were excluded
from the study. All patients received a bortezomib-based regimen in
various combinations with dexamethasone (n=8), doxorubicin (n=6),
cyclophosphamide (n=4) or thalidomide (n=3), in accordance with the
international guidelines (27) and as
described previously (28). Bone
marrow aspirates (BMA) were collected at the time of diagnosis and
following induction therapy. Mononuclear cells (MNCs) were prepared
from the aspirates. Samples of peripheral blood were collected
immediately prior to and 3, 7 and 10 days after the administration
of the initial therapy cycle. These samples were used to prepare
serum for chemokine and cytokine analysis, and PBMCs for cell
analysis. Furthermore, patient monocytes from day 0 and day 3 were
isolated from the peripheral blood samples and used for
efferocytotic analysis. The study was approved by the Ethics
Committee of the Medical University of Vienna (Vienna, Austria; ECS
1352/2013) and all participants provided written informed
consent.
Cell preparation
MNCs and PBMCs were isolated by Ficoll
centrifugation at 400 × g for 20 min at room temperature from BMA
or peripheral blood samples collected in EDTA-coated syringes or
vacutainers. Cells were then washed in PBS and either used for
direct analysis or for monocyte isolation using CD14+
microbeads (cat. no. 130-050-201; Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany) according to the manufacturer's protocol.
Briefly, PBMCs were incubated with CD14+ microbeads for
15 min on ice. Following a washing step, the cell suspension was
run through a LS column (Miltenyi Biotec GmbH), which was installed
on a MultiMACS Cell Separator (Miltenyi Biotec GmbH). The
flow-through was discarded. Next, the LS column was removed from
the MultiMACS Cell Separator, flushed with buffer and the cell
suspension containing CD14+ monocytes was eluted into a
fresh tube. Monocytes were used for the subsequent efferocytosis
assay.
Cell analysis
Following isolation of PBMCs or MNCs, they were
washed in PBS supplemented with 2% bovine serum albumin (BSA; Merck
KGaG, Darmstadt, Germany). For leukocyte typing, cells were then
incubated with either anti-CD4-fluorescein isothiocyanate
(FITC)/CD8-phycoerythrin (PE) (cat. no. 340039; BD Biosciences,
Franklin Lakes, NJ, USA), anti CD14-FITC (cat. no. 17-0149-42;
eBioscience; Thermo Fisher Scientific, Inc., Waltham, MA, USA) or
anti CD138-FITC (cat. no. 11-1389-42; BD Biosciences) antibodies or
the isotype control (cat. nos. 11-4752-80 and 17-4714-42,
respectively; eBioscience; Thermo Fisher Scientific, Inc.) for 30
min on ice, following which the cells were washed. The antibodies
were diluted according to the manufacturer's recommendations. A
parallel sample was stained with 7-aminoactinomycin D (7-AAD;
eBioscience; Thermo Fisher Scientific, Inc.) for 20 min at room
temperature in the dark. For staining of dead cells, cells were
washed in PBS at 45 g for 5 min at room temperature and then
resuspended in 100 µl PBS supplemented with 0.5 µl Zombie Violet
(BioLegend Inc., San Diego, CA, USA). For intracellular active
caspase-3 staining, cells were fixed and permeabilized using an
IntraPrep kit (Beckman Coulter, Inc., Brea, CA, USA) according to
the manufacturer's protocol. Cells were then stained with
anti-active caspase-3 (cat. no. 559565; BD Biosciences) antibody
for 30 min on ice. Following another wash and a permeabilization
step (IntraPrep Permeabilization Reagent; Beckman Coulter, Inc.),
cells were incubated with an allophycocyanin (APC)-conjugated
detection antibody (cat. no. 47-4714-82; eBioscience; Thermo Fisher
Scientific, Inc.) for 30 min on ice. Subsequently, the cells were
immediately analyzed with a Gallios flow cytometer (Beckman
Coulter, Inc.,) using the Kaluza Analysis software (v.1.3; Beckman
Coulter, Inc.).
Efferocytosis assay
Following isolation of patient monocytes, the
efferocytosis assay was performed as described previously (23). Briefly, carboxyfluorescein
succinimidyl ester (CFSE)-labeled (Invitrogen; Thermo Fisher
Scientific, Inc.) Jurkat cells (ATCC-LGC Standards GmbH, Wesel,
Germany) were cultured in Dulbecco's modified Eagle's Medium/Ham's
F-12 including GlutaMAX (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal calf serum (Linaris GmbH,
Dossenheim, Germany) at 37°C and in a humidified 5% CO2
atmosphere. Cells were treated with 100 nM bortezomib (kindly
provided by the pharmacy of the General Hospital of Vienna) for 24
h in serum-free Ultra Culture (UC) medium (Lonza Group, Ltd.,
Basel, Switzerland), washed to remove the drug and then incubated
for 1 h in UC medium supplemented with 25% normal human serum (NHS;
PAA Laboratories; GE Healthcare, Chicago, IL, USA). For co-culture,
100,000 apoptotic Jurkat cells were added to 100,000 monocytes and
incubated for 2 h at 37°C in a total volume of 200 µl UC medium.
Next, the cells were washed with PBS supplemented with 2% BSA and
stained with an APC-conjugated anti-CD14 antibody (cat. no.
17-0149-42; eBioscience; Thermo Fisher Scientific, Inc.) for 30 min
on ice. Cells were analyzed immediately using a Gallios flow
cytometer. The efferocytotic index was calculated as follows: The
CFSE+/CD14+ cell-population was divided by
the total CD14+ cell count and multiplied by 100.
Analysis of soluble immune
modulators
Patient serum samples were collected and stored at
−80°C until analysis. IL-1β, IL-6, IL-8, IL-10, IL-12 p70, IL-23,
IFN-γ, TNF-α, C-C motif chemokine ligand 2 (CCL2), chemokine (C-X-C
motif) ligand 9 (CXCL9), CCL24, CXCL10, vascular endothelial growth
factor-A (VEGF-A) and VEGF-C were quantified using a customized
magnetic bead-based ProcartaPlex Multiplex Immunoassay
(eBioscience; Thermo Fisher Scientific, Inc.). Soluble forms of the
checkpoint molecules CD27, CD28, CD80/B7-1, CD137, CD152/CTLA-4,
CD223/LAG-3, herpes virus entry mediator (HVEM), B- and
T-lymphocyte attenuator (BTLA), programmed death ligand 2 (PD-L2),
PD-L1, programmed cell death protein 1 (PD-1),
glucocorticoid-induced TNFR-related protein (GITR),
indoleamine-pyrrole 2,3-dioxygenase (IDO) and T-cell immunoglobulin
and mucin-domain containing-3 (TIM-3) were measured using a
ProcartaPlex Human Immuno-Oncology Checkpoint Panel (cat. no.
EPX140-15803-901; Affymetrix; Thermo Fisher Scientific, Inc.). All
assays were performed according to the manufacturer's protocol and
analyzed with a Luminex analyzer.
Statistical analysis
Multiple groups (Fig.
1A: Healthy volunteers, MM patients prior to therapy, and MM
patients following therapy; Figs. 1B,
2B, 3A-F: Prior to therapy, 3, 7, and 10 days
following therapy) were analyzed by one-way analysis of variance
with the Greenhouse-Geisser correction. Comparisons between two
groups (Fig. 1C and D: Prior to and
following therapy, respectively) were assessed with an unpaired
Student's t-test. Data are expressed as the mean ± the standard
deviation. All statistical analyses were calculated using the
GraphPad Prism software package version 6.04 (GraphPad Software,
Inc., La Jolla, CA, USA).
 | Figure 1.Efferocytosis and dead cell remnants.
(A) Monocytes were isolated from HV and patients with MM prior to
and after 3 days of induction therapy. Cells were co-cultivated
with CFSE-labeled and either bortezomib-treated (apoptotic) or
untreated (viable) Jurkat prey cells for 2 h. Next, the cell
mixtures were stained with anti-CD14 antibody and analyzed by flow
cytometry. The percentage of CFSE+ cells within the
CD14+ monocyte population was taken as the efferocytotic
capacity. (B) PBMCs were isolated from patients with MM prior to
and 3, 7, and 10 days after the administration of the first cycle
of therapy. Cells were washed, stained with 7-AAD and analyzed by
flow cytometry for the surface expression of CD4, CD8 or CD14. (C
and D) Mononuclear cells from prior to and after therapy were
isolated from bone marrow aspirates, washed and stained with (C)
7-AAD or (D) anti-CD138 antibody. The percentage of
CD138+ and 7-AAD+ cells is shown in the
graphs. Each dot represents one patient at a given time point. The
mean is depicted as a horizontal line. HV, healthy volunteer; CFSE,
carboxyfluorescein succinimidyl ester; CD14, cluster of
differentiation 14; PBMC, peripheral blood monocyte; 7-AAD,
7-aminoactinomycin D. |
Results
Efferocytosis by monocytes
Monocytes obtained from patients as well as healthy
volunteers (HVs) exhibited an evidently higher uptake of apoptotic
cell remnants than of viable prey cells (Fig. 1A). However, this difference was much
lower with monocytes from patients with MM than with monocytes from
HVs. The uptake of apoptotic cell remnants was similar prior to and
3 days after the administration of therapy. These data revealed
that the efferocytotic capacity is markedly reduced in patients
with MM compared with in healthy subjects. However, chemotherapy
had no further effect on this monocytic cell function.
Dead cell remnants in blood and bone
marrow
Whether the impaired efferocytosis of monocytes in
patients with MM is associated with the accumulation of dead cells
was then investigated. Considerable numbers of 7-AAD+
PBMCs were observed prior to treatment (ranging between 0.6 and
6.7%; Fig. 1B). This value did not
change within 3 days of the administration of the first dose of
therapy. But on day 7 all patients exhibited low numbers of
7-AAD+ PBMCs (<2.9%), remaining at this low level
until day 10. These data confirm that dead cells are present in the
circulation of untreated patients with MM and indicate that the
majority of these cells are cleared within the first 7 days of
therapy. The 7AAD+ dead cell remnants of untreated
patients with MM could not be attributed to a specific immune cell
subpopulation. They were negative for the three investigated
clusters of differentiation markers (CD4, CD8 and CD14). These cell
surface proteins may have been shed from the cell membrane during
cell death.
The high number of dead cell remnants observed prior
to chemotherapy is evidently due to the disease itself rather than
to the chemotherapy. As MM leads primarily to destruction of the
bone marrow, bone marrow was investigated in the next experiment.
BMA were collected prior to and following therapy, and the dead
cells were stained with 7-AAD and analyzed by flow cytometry (a
collection of bone marrow samples during therapy was not possible
for ethical reasons). The analysis revealed the presence of
considerable levels of 7-AAD+ cells prior to therapy
(median, 8.05% of all cells in the aspirate; Fig. 1C). Notably, the level of
7-AAD+ cells was strongly reduced following therapy.
This reduction was associated with a reduction of the number of
CD138+ plasma tumor cells (Fig. 1D).
The 7-AAD dye stains nucleated cells with a
disintegrated cell membrane irrespective of whether the
permeabilization of the cell membrane was a direct result of cell
rupture (i.e. primary necrosis) or a secondary effect following
apoptosis (i.e. late apoptosis/secondary necrosis). To estimate the
contribution of secondary necrosis to 7-AAD+ cells in
patients with MM, cells were stained with antibodies against
activated caspase 3. The dead cell dye Zombie was used to identify
cells with a disintegrated cell membrane. Fig. 2A describes the gating strategy, the
results are shown in Fig. 2B. A mean
of 18.4% of dead cells with a disintegrated cell membrane were
positive for caspase 3, indicating late apoptosis. The percentage
of activated caspase 3+ dead cells did not change during
the observation period of the therapy. Taken together, these data
indicate that untreated MM is associated with the presence a
considerable number of dead cells in the bone marrow as well as in
the blood flow, indicating a substantial number of late
apoptotic/secondary necrotic cells.
Blood immune cells and serum levels of
immunomodulatory molecules
To determine whether impaired efferocytosis and the
accumulation of dead cells in patients with MM are associated with
a pro-inflammatory response, the main PBMC subpopulations and the
serum levels of a panel of immunomodulatory molecules were
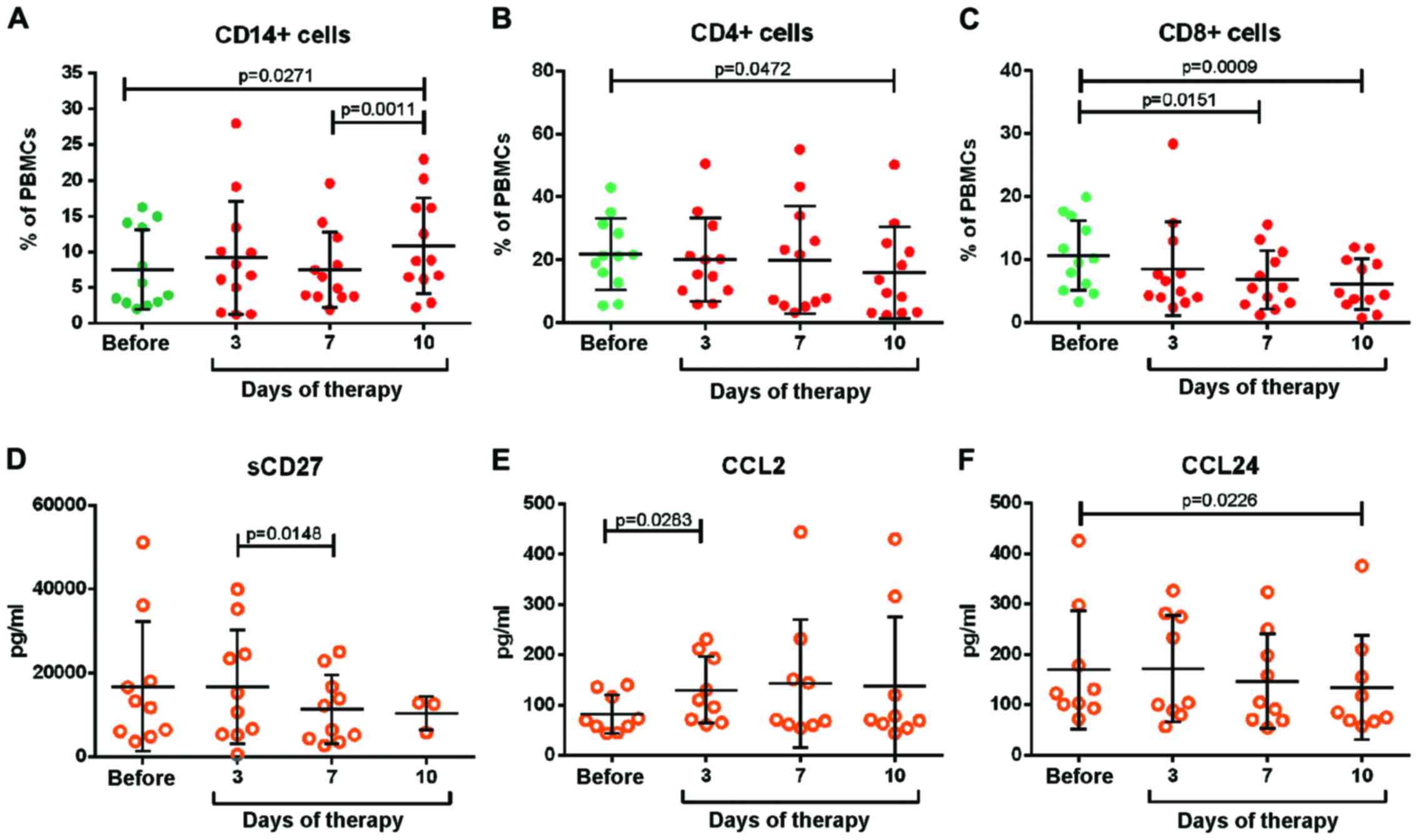
quantified. Fig. 3A demonstrates that
the number of CD14+ monocytes remained unchanged over
the course of chemotherapy, until day 7 when it increased slightly.
By contrast, the number of CD4+ regulatory and
CD8+ cytotoxic T cells continuously declined (Fig. 3B and C). The panel of immunomodulatory
molecules consisted of 14 different cytokines, chemokines and
angiogenesis factors (TNF, IFN-α, IL-1β, IL-6, IL-8, IL-10, IL-12
p70, IL-23, CCL2, CCL24, CXCL9, CXCL10, VEGF-A and VEGF-C), as well
as 14 soluble forms of co-stimulatory or co-inhibitory receptors
(sPD-1, sPD-L1, sPD-L2, sTIM-3, sCTL-A4, sIDO, sCD27, sCD28, sCD80,
sCD137, sHVEM, sLAG-3, sBTLA and sGITR). The results of all
parameters are summarized in Table I.
In total, 12 of these molecules were under the detection limit in
all samples. The majority of the remaining molecules were present
at stable levels throughout the therapy. Only sCD27, CCL2 and CCL24
were affected by bortezomib-based therapy (Fig. 3D-F). Prior to therapy, a strong
variation in sCD27 was observed between patients. Notably, the high
levels declined within seven days of therapy. By contrast, CCL2
levels strongly increased and those of CCL24 declined slightly. The
results revealed no induction of classical pro-inflammatory
cytokines or chemokines. Only a few immunomodulatory molecules were
affected by the therapy.
 | Table I.Serum levels of immunomodulatory
factors. |
Table I.
Serum levels of immunomodulatory
factors.
|
|
Concentrationa, pg/ml |
|---|
|
|
|
|---|
| Parameter | Day 0 | Day 3 | Day 7 | Day 10 |
|---|
| TNF | n.d. | n.d. | n.d. | n.d. |
| IFN-α | n.d. | n.d. | n.d. | n.d. |
| IL-1β | n.d. | n.d. | n.d. | n.d. |
| IL-6 | n.d. | n.d. | n.d. | n.d. |
| IL-8 | n.d. | n.d. | n.d. | n.d. |
| IL-10 | n.d. | n.d. | n.d. | n.d. |
| IL-12 p70 | n.d. | n.d. | n.d. | n.d. |
| IL-23 | n.d. | n.d. | n.d. | n.d. |
| CCL2 |
82±44 | 130±74 |
143±128 | 138±136 |
| CCL24 |
169±123 |
172±113 | 147±99 | 135±105 |
| CXCL9 |
117±149 |
154±196 |
198±284 | 176±275 |
| CXCL10 | n.d. | n.d. | n.d. | n.d. |
| VEGF-A |
677±511 |
687±567 |
502±366 | 680±554 |
| VEGF-C |
153±100 | 179±94 |
167±110 | 134±73 |
| sPD-1 |
153±152 |
136±151 |
81±109 | 198±109 |
| sPD-L1 | n.d. | n.d. | n.d. | n.d. |
| sPD-L2 |
32,345±15,105 |
32,731±17,049 |
33,774±14,787 | 47,711±24,709 |
| sTIM-3 | 15,963±5,024 | 16,754±5,396 | 15,847±4,797 |
19,874±10,398 |
| sCTL-A4 |
112±150 |
170±137 |
172±144 | 336±222 |
| sIDO | n.d. | n.d. |
423±1,043 | 680±634 |
| sCD27 |
13,612±14,530 |
14,305±13,459 | 11,509±8,142 | 10,419±6,136 |
| sCD28 |
132±178 |
153±144 |
159±143 | 256±248 |
| sCD80 |
582±572 |
594±570 |
563±501 | 1,322±844 |
| sCD137 |
206±273 |
245±417 |
135±188 | 593±725 |
| sHVEM | n.d. | n.d. | n.d. | n.d. |
| sLAG-3 |
295±292 |
189±166 |
234±273 | 45±67 |
| sBTLA |
21,037±21,630 |
25,758±28,507 |
16,186±17,670 | 29,916±24,860 |
| sGITR | n.d. | n.d. | n.d. | n.d. |
Discussion
The present study revealed that monocytes in
patients with MM have a reduced efferocytotic capacity. Impaired
efferocytosis has also been observed in SLE (2), chronic granulomatous disease (9), Sjögren's syndrome (10), COPD (11), smokers (11), non-small cell lung cancer (29), non-eosinophilic asthma (12), and cystic fibrosis (30). To the best of our knowledge, this is
the first report of diminished efferocytosis in MM. Notably,
bortezomib-based induction had no negative effect on monocyte
function or the monocyte count. However, a clear decline in the
number of CD4+ and CD8+ T-cells was observed.
The in vivo data contradict those of previous in
vitro studies, which stated that bortezomib specifically
inhibited monocytes, leaving lymphocytes unaffected (21,22). The
reason for this difference is unclear; however, owing to large
differences in the experimental setting, comparisons between in
vitro and in vivo data should be conducted with care.
Corroboration for the results of the present study comes from a
report by Blanco et al (31),
which demonstrated that bortezomib induced selective depletion of
alloreactive T lymphocytes (31);
however, the authors did not include monocytes in their
analysis.
The reduced efferocytosis in patients with untreated
MM was accompanied by an accumulation of dead cells. Approximately
20% of patients were positive for active caspase 3, indicating that
at least a proportion of the circulating dead cells were derived
from late apoptotic/secondary necrotic cells. The
disease-associated tissue damage in MM occurs mainly in the bone
marrow, and an accordingly increased number of dead cell remnants
were observed in this compartment. A previous report demonstrated
an accumulation of apoptotic Annexin
V+/CD138+ plasma cells in the bone marrow of
patients with untreated MM (32). The
authors revealed that a higher number of apoptotic plasma cells in
the bone marrow was associated with an unfavorable prognosis
(32). However, it is unclear whether
the dead cell remnants in the circulation were derived from the
bone marrow or if they were the consequence of a disease-associated
direct effect on blood cells. The impaired efferocytotic capacity
of circulating monocytes may contribute to the accumulation of dead
cell remnants in the circulation, although the present study did
not identify whether this is the only reason. The total number of
7-AAD+ dead cell remnants diminished during the first
week of therapy. As no significant therapeutic effect on
efferocytosis by monocytes could be observed, it was assumed that
the lower number of 7-AAD+ cells results from the
reduced formation of new dead cells in response to therapy. Thus,
the lower degree of efferocytosis in patients with MM is evidently
sufficient to clear the remaining dead cell remnants.
Leaky dead cell remnants release passive
intracellular immunogenic DAMPs, which activate the Toll-like
receptor signaling pathway in monocytes/macrophages, resulting in
the secretion of pro-inflammatory mediators such as TNF, IL-1β,
IL-6, IL-8, IL-12p70, and IL-23 (33). No induction of any these cytokines was
observed; however, an increase in CCL2 following administration of
the first cycle of therapy was observed. Fraser et al
(34) demonstrated that CCL2 is
released from macrophages exposed to apoptotic cells in a
C1q-dependent manner. C1q binds exclusively to a subpopulation of
late apoptotic/secondary necrotic cells (26), indicating that the late
apoptotic/secondary necrotic cells found in the present study may
contribute to CCL2 formation. There is also a report claiming that
CCL2 can be produced by tumor cells and tumor-associated
fibroblasts (35). High CCL2 levels
are associated with increased numbers of TAMs and poor patient
prognosis in various types of cancer (35). Thus, the increased release of CCL2 may
also result from the cytotoxic effects of the induction therapy on
malignant plasma cells or stromal cells in the bone marrow. CCL2 is
known to stimulate the emigration of inflammatory monocytes from
the bone marrow into the circulating (35) to promote the survival of
CD11b+ peripheral blood mononuclear cells (36) and to induce M2-type macrophage
polarization (37). Thus, the
increased CCL2 levels observed during induction therapy in MM may
contribute to the sustained survival of monocytes during bortezomib
treatment. Accordingly, a slight increase in the number of
circulating monocytes was observed after 10 days of therapy.
Notably, CCL2 levels increased during therapy in exactly those
patients exhibiting high sCD27 levels prior to treatment, although
no difference in CCL2 levels between sCD27high and
sCD27low patients were observed prior to therapy, and
the underlying reason remains unclear. The cell surface form of
CD27 is highly expressed on normal plasma cells, T cells, B cells,
NK cells and their precursors. Following cell activation, CD27 can
be shed from the cell surface, thereby giving rise to circulating
sCD27. Therefore, the soluble form is regarded as a marker for
lymphoid cell activation (38). The
shedding mechanism is mediated by the protein ‘a disintegrin and
metalloprotease’, which is activated in response to extracellular
ATP via P2X purinoreceptor 7 (39).
As aforementioned, extracellular ATP is a well-known DAMP released
by dead cell bodies with a disintegrating cell membrane. Thus, the
concomitant reduction in dead cell remnants and sCD27 observed
during the first cycle of therapy may be mutually associated.
Notably, a prior study revealed that the in vitro treatment
of human myeloma cells with bortezomib reduces the cell surface
expression of CD27 (40). However, it
is unclear whether this effect also occurs in vivo.
The present study revealed that MM is associated
with reduced efferocytosis by monocytes, and with an increased
number of dead cell remnants, including late apoptotic/secondary
necrotic cells. However, the accumulation of late apoptotic cells
was not associated with an inflammatory response. The
bortezomib-based induction chemotherapy used to treat patients with
MM inhibited neither the number nor the efferocytotic function of
monocytes. However, it reduced the number of dead cell remnants in
the circulation and the bone marrow. The origin and potential role
of dead cell remnants in the pathophysiology of MM remains to be
investigated in future studies.
Acknowledgements
The authors would like to thank Mr. Albert Müller
and Dr Elisabeth Zechtl for their support in patient sample
collection. This work was supported by the Austrian National Bank
OeNB (grant no. 15661).
Glossary
Abbreviations
Abbreviations:
|
MM
|
multiple myeloma
|
|
BMA
|
bone marrow aspirates
|
|
PBMCs
|
peripheral blood mononuclear cells
|
References
|
1
|
Poon IKH, Lucas CD, Rossi AG and
Ravichandran KS: Apoptotic cell clearance: Basic biology and
therapeutic potential. Nat Rev Immunol. 14:166–180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Birge RB, Boeltz S, Kumar S, Carlson J,
Wanderley J, Calianese D, Barcinski M, Brekken RA, Huang X,
Hutchins JT, et al: Phosphatidylserine is a global
immunosuppressive signal in efferocytosis, infectious disease, and
cancer. Cell Death Differ. 23:962–978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Green DR, Oguin TH and Martinez J: The
clearance of dying cells: Table for two. Cell Death Differ.
23:915–926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das A, Ganesh K, Khanna S, Sen CK and Roy
S: Engulfment of apoptotic cells by macrophages: A role of
microRNA-21 in the resolution of wound inflammation. J Immunol.
192:1120–1129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferracini M, Rios FJ, Pecenin M and Jancar
S: Clearance of apoptotic cells by macrophages induces regulatory
phenotype and involves stimulation of CD36 and platelet-activating
factor receptor. Mediators Inflamm. 2013:9502732013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stanford JC, Young C, Hicks D, Owens P,
Williams A, Vaught DB, Morrison MM, Lim J, Williams M,
Brantley-Sieders DM, et al: Efferocytosis produces a prometastatic
landscape during postpartum mammary gland involution. J Clin
Invest. 124:4737–4752. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silva MT: Secondary necrosis: The natural
outcome of the complete apoptotic program. FEBS Lett.
584:4491–4499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernandez-Boyanapalli RF, Falcone EL,
Zerbe CS, Marciano BE, Frasch SC, Henson PM, Holland SM and Bratton
DL: Impaired efferocytosis in human chronic granulomatous disease
is reversed by pioglitazone treatment. J Allergy Clin Immunol.
136:1399–1401.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manoussakis MN, Fragoulis GE, Vakrakou AG
and Moutsopoulos HM: Impaired clearance of early apoptotic cells
mediated by inhibitory IgG antibodies in patients with primary
Sjögren's syndrome. PLoS One. 9:e1121002014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamon R, Homan CC, Tran HB, Mukaro VR,
Lester SE, Roscioli E, Bosco MD, Murgia CM, Ackland ML, Jersmann
HP, et al: Zinc and zinc transporters in macrophages and their
roles in efferocytosis in COPD. PLoS One. 9:e1100562014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simpson JL, Gibson PG, Yang IA, Upham J,
James A, Reynolds PN and Hodge S: AMAZES Study Research Group:
Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin
Exp Allergy. 43:29–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wickman G, Julian L and Olson MF: How
apoptotic cells aid in the removal of their own cold dead bodies.
Cell Death Differ. 19:735–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aggarwal R, Ghobrial IM and Roodman GD:
Chemokines in multiple myeloma. Exp Hematol. 34:1289–1295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caivano A, Laurenzana I, De Luca L, La
Rocca F, Simeon V, Trino S, D'Auria F, Traficante A, Maietti M,
Izzo T, et al: High serum levels of extracellular vesicles
expressing malignancy-related markers are released in patients with
various types of hematological neoplastic disorders. Tumor Biol.
36:9739–9752. 2015. View Article : Google Scholar
|
|
16
|
Canella A, Harshman SW, Radomska HS,
Freitas MA and Pichiorri F: The potential diagnostic power of
extracellular vesicle analysis for multiple myeloma. Expert Rev Mol
Diagn. 16:277–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ludwig H, Avet-Loiseau H, Bladé J,
Boccadoro M, Cavenagh J, Cavo M, Davies F, de la Rubia J, Delimpasi
S, Dimopoulos M, et al: European perspective on multiple myeloma
treatment strategies: Update following recent congresses.
Oncologist. 17:592–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ludwig H, Beksac M, Bladé J, Boccadoro M,
Cavenagh J, Cavo M, Dimopoulos M, Drach J, Einsele H, Facon T, et
al: Current multiple myeloma treatment strategies with novel
agents: A European perspective. Oncologist. 15:6–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nooka AK, Kaufman JL, Behera M, Langston
A, Waller EK, Flowers CR, Gleason C, Boise LH and Lonial S:
Bortezomib-containing induction regimens in transplant-eligible
myeloma patients: A meta-analysis of phase 3 randomized clinical
trials. Cancer. 119:4119–4128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ribatti D, Nico B and Vacca A: Importance
of the bone marrow microenvironment in inducing the angiogenic
response in multiple myeloma. Oncogene. 25:4257–4266. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Straube C, Wehner R, Wendisch M,
Bornhäuser M, Bachmann M, Rieber EP and Schmitz M: Bortezomib
significantly impairs the immunostimulatory capacity of human
myeloid blood dendritic cells. Leukemia. 21:1464–1471. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arpinati M, Chirumbolo G, Nicolini B,
Agostinelli C and Rondelli D: Selective apoptosis of monocytes and
monocyte-derived DCs induced by bortezomib (Velcade). Bone Marrow
Transplant. 43:253–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang YY, Arnold T, Michlmayr A,
Rainprecht D, Perticevic B, Spittler A and Oehler R:
Serum-dependent processing of late apoptotic cells for enhanced
efferocytosis. Cell Death Dis. 5:e12642014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang YY, Rainprecht D, Eichmair E,
Messner B and Oehler R: Serum-dependent processing of late
apoptotic cells and their immunogenicity. Apoptosis. 20:1444–1456.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arnold T, Michlmayr A, Baumann S,
Burghuber C, Pluschnig U, Bartsch R, Steger G, Gnant M, Bergmann M,
Bachleitner-Hofmann T and Oehler R: Plasma HMGB-1 after the initial
dose of epirubicin/docetaxel in cancer. Eur J Clin Invest.
43:286–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Exner R, Sachet M, Arnold T,
Zinn-Zinnenburg M, Michlmayr A, Dubsky P, Bartsch R, Steger G,
Gnant M, Bergmann M, et al: Prognostic value of HMGB1 in early
breast cancer patients under neoadjuvant chemotherapy. Cancer Med.
5:2350–2358. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bird JM, Owen RG, D'Sa S, Snowden JA,
Pratt G, Ashcroft J, Yong K, Cook G, Feyler S, Davies F, et al:
Guidelines for the diagnosis and management of multiple myeloma
2011. Br J Haematol. 154:32–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamm W, Wohlfarth P, Bojic M,
Schörgenhofer C, Drach J, Gisslinger H, Worel N, Schiefer A,
Schulenburg A, Agis H, et al: Outcome in multiple myeloma patients
eligible for stem cell transplantation: A Single-center experience.
Oncology. 89:196–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dehle FC, Mukaro VR, Jurisevic C, Moffat
D, Ahern J, Hodge G, Jersmann H, Reynolds PN and Hodge S: Defective
lung macrophage function in lung cancer ± chronic obstructive
pulmonary disease (COPD/emphysema)-mediated by cancer cell
production of PGE2? PLoS One. 8:e615732013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McCubbrey AL and Curtis JL: Efferocytosis
and lung disease. Chest. 143:1750–1757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blanco B, Pérez-Simón JA, Sánchez-Abarca
LI, Carvajal-Vergara X, Mateos J, Vidriales B, López-Holgado N,
Maiso P, Alberca M, Villarón E, et al: Bortezomib induces selective
depletion of alloreactive T lymphocytes and decreases the
production of Th1 cytokines. Blood. 107:3575–3583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Minarik J, Scudla V, Ordeltova M, Bacovsky
J, Pika T and Langova K: Prognostic significance of apoptotic index
in multiple myeloma patients treated by conventional therapy and
novel agents, thalidomide and bortezomib. Eur J Haematol.
83:528–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krysko DV, Garg AD, Kaczmarek A, Krysko O,
Agostinis P and Vandenabeele P: Immunogenic cell death and DAMPs in
cancer therapy. Nat Rev Cancer. 12:860–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fraser DA, Laust AK, Nelson EL and Tenner
AJ: C1q differentially modulates phagocytosis and cytokine
responses during ingestion of apoptotic cells by human monocytes,
macrophages, and dendritic cells. J Immunol. 183:6175–6185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yadav A, Saini V and Arora S: MCP-1:
Chemoattractant with a role beyond immunity: A review. Clin Chim
Acta. 411:1570–1579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roca H, Varcos ZS, Sud S, Craig MJ and
Pienta KJ: CCL2 and interleukin-6 promote survival of human CD11b+
peripheral blood mononuclear cells and induce M2-type macrophage
polarization. J Biol Chem. 284:34342–34354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kobayashi M, Jeschke MG, Shigematsu K,
Asai A, Yoshida S, Herndon DN and Suzuki F: M2b monocytes
predominated in peripheral blood of severely burned patients. J
Immunol. 185:7174–7179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lens SM, Tesselaar K, van Oers MH and van
Lier RA: Control of lymphocyte function through CD27-CD70
interactions. Semin Immunol. 10:491–499. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moon H, Na HY, Chong KH and Kim TJ: P2X7
receptor-dependent ATP-induced shedding of CD27 in mouse
lymphocytes. Immunol Lett. 102:98–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tagoug I, Plesa A and Dumontet C:
Bortezomib influences the expression of malignant plasma cells
membrane antigens. Eur J Pharmacol. 706:11–16. 2013. View Article : Google Scholar : PubMed/NCBI
|