Introduction
Hepatocellular carcinoma (HCC) is one of the most
common causes of cancer-associated mortality worldwide (1). For patients with resectable HCC and a
moderate liver function reserve, surgical resection is an effective
curative treatment (2). However, the
long-term survival rates following resection remain low due to the
high incidence of postoperative recurrence (3). Transarterial chemoembolization (TACE) is
an adjuvant therapy commonly employed to improve the therapeutic
efficacy of hepatectomy for patients with HCC (4).
A previous meta-analysis (5) demonstrated that adjuvant TACE may
suppress recurrence and improve survival rates following resection.
Adjuvant TACE involves the occlusion of the feeding arteries and
locally administered chemotherapy, the combination of which delays
tumor progression and incites tumor necrosis in HCC. In addition,
the function of postoperative adjuvant TACE in improving early
detection of recurrence has also been demonstrated (6). In contrast, certain studies (7) have indicated that adjuvant TACE may not
significantly reduce recurrence and improve survival, and that the
treatment may damage remnant liver function, particularly in
patients with chronic cirrhosis (8),
and that it increases the risk of hepatitis B virus (HBV)
reactivation (9). These conflicting
results leave the question concerning which subpopulations may
benefit from adjuvant TACE following curative resection for HCC. In
the present study, curative resection with and without TACE were
retrospectively evaluated, to determine the efficacy of
postoperative adjuvant TACE to prevent, treat and detect HCC
recurrence.
Patients and methods
Patients
A total of 356 patients from the Department of
Hepatobiliary and Pancreatic Surgery in the Affiliated Minzu
Hospital of Guangxi Medical University (Guangxi, China) between
January 2010-December 2012 were enrolled. Inclusion criteria were
established at the beginning of the present study and included: i)
Patients who had undergone curative resection of liver malignancies
and were diagnosed with HCC by pathology; ii) the absence of
apparent extrahepatic metastasis determined by preoperative and
postoperative chest X-rays, computerized tomography (CT) or
magnetic resonance imaging (MRI) scans; iii) complete gross
resection achieved with no residual tumor in the remnant liver as
determined by intraoperative visual inspection and a negatively
resected margin by histological examination; iv) patients who were
between 16–75 years old, who had a positive postoperative general
condition as assessed by the Eastern Cooperative Oncology Group
(ECOG) performance status (10)
(score 0–2), and moderate liver function (Child-Pugh A) (11); v) preoperative serum levels of
α-fetoprotein (AFP) decreased to normal within 2 months following
resection. The present study was approved by the Ethics Committee
of Affiliated Minzu Hospital of Guangxi Medical University
(Guangxi, China). Written informed consent was obtained from all
participants.
Study design
A total of 166 patients who underwent curative
resection followed by adjuvant TACE, and 199 patients who underwent
resection alone, met the inclusion criteria and were recruited.
There were four components to the present study which were as
follows: i) Univariate and multivariate analyses were performed to
investigate the risk factors for early (defined as recurrent HCC
developing within the first year following resection) (12), or late recurrence; ii) based on
recurrence risk factor analysis, subgroup studies were performed to
evaluate the efficacy of suppression of recurrence by postoperative
adjuvant TACE following curative resection, compared with curative
resection alone; recurrence rates were compared at 6, 12, 18 and 24
months, respectively; iii) log-rank analysis was performed to
compare the 1-, 2-, and 3-year overall survival between the two
therapeutic strategies; iv) amongst patients who experienced
recurrence, CT/MRI imaging was used to evaluate the potential
function of postoperative adjuvant TACE in enhancing the efficacy
of CT/MRI in detecting recurrence.
Treatments
All patients were treated using curative resection.
Surgical techniques and perioperative management were performed as
previously described (13). Adjuvant
TACE was performed one month following resection. With the patient
under local anesthesia, contrast medium was injected into the
arteries via a 4.1–5F RC1 catheter (Shanghai Medical Equipment
Works Co., Ltd., Shanghai, China), which was introduced into the
abdominal aorta via the right superficial femoral artery. Hepatic
arterial angiography was performed using fluoroscopy to guide the
catheter into the celiac and superior mesenteric arteries. Then,
the feeding arteries, tumor stain, and vascular anatomy surrounding
the tumor were identified. A microcatheter was introduced through
the 4-5F catheter into the feeding arteries. An emulsion of 3–5 ml
lipiodol and gelfoam particles with doxorubicin (30–50 mg; Shanghai
Yuanye Biotechnology Co., Ltd., Shanghai, China) and cisplatin
(50–100 mg; Beijing Huamaike Biotechnology Co., Ltd., Beijing,
China) was infused into the feeding arteries. Postoperative
adjuvant TACE was repeated for 1–6 cycles at intervals of 1
month.
Anti-virus therapy was routinely given to patients
with HBV or hepatitis C virus following resection, as previously
described (14). Percutaneous ethanol
injection (PEI) or radio-ablation therapy (RA) was performed to
treat recurrent HCC with a diameter of <3 cm. A secondary
resection was only recommended when the remnant liver volume and
functional reserve were considered sufficient. When patients had
contradictions for, or rejected PEI, RA or secondary resection,
subsequent TACE was recommended.
Monitoring for tumor recurrence and
follow-up
Amongst the patients who received curative resection
followed by adjuvant TACE, the serum AFP level was routinely
monitored using radioimmunoassay (125I) (Shanghai Sangon
Biological Engineering Technology And Service Co., Ltd., Shanghai,
China) at the same frequency as the ultrasounds and CT/MRI scans.
The process was automatically completed in GC-1200 radioimmunoassay
gamma counter (Anhui Haoyuan Chemical Group Co., Ltd., Fuyang,
China). Ultrasound evaluation, and dynamic CT or MRI scans were
performed for surveillance of recurrence at the end of the first
month following resection but prior to the first cycle of
postoperative adjuvant TACE, and then at monthly intervals. If the
number of adjuvant TACE cycles was >1, the image scan was
normally performed prior to the next cycle of adjuvant TACE.
Digital subtraction angiography (DSA) was performed concurrent with
the adjuvant TACE. Diagnosis of recurrence was established when
DSA, in addition to any one of the CT/MRI studies, was positive
with or without an increase in serum AFP levels. Images from the
CT/MRI scans prior to and following adjuvant TACE were compared
with each other in order to evaluate whether postoperative TACE
improved the efficacy of CT/MRI to discover recurrent HCC.
Amongst patients who underwent resection alone,
serum AFP level, ultrasound evaluation and dynamic CT or MRI scans
were routinely monitored and performed for the surveillance of
recurrence at the end of the first month subsequent to resection as
well, and then at monthly intervals. DSA was performed following
the diagnosis of suspected recurrence, which was detected by an
increase in serum AFP level or by dynamic contrasted-enhanced CT or
MRI scans. Diagnosis of recurrence was established on the basis of
whether CT/MRI studies or DSA were considered as positive, with or
without an increase in serum AFP level. All patients were followed
up until December 30, 2013, or until mortality.
Statistical analysis
All the data were analyzed using SPSS ver. 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). Normally
distributed data were presented as the mean ± standard deviation
and asymmetrical distributed data were presented as the median
(range). The baseline clinical and pathological characteristics for
patients were analyzed using the χ2 test. Recurrence and
survival curves were determined using the Kaplan-Meier method and
differences between therapeutic strategies were identified using
log-rank analysis. Prognostic factors of recurrence were evaluated
by univariate and multivariate analyses using Cox's regression.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics of
patients
The baseline characteristics of all patients are
summarized in Table I. No statistical
difference was demonstrated in the baseline clinical and
pathological variables, including age, sex, ECOG score, serum AFP
level, hepatitis B surface antigen, liver function tests, tumor
size, tumor capsule, tumor number, macrovascular invasion and tumor
cell differentiation between patients (P>0.05).
 | Table I.Baseline clinical and pathological
characteristics of patients. |
Table I.
Baseline clinical and pathological
characteristics of patients.
| Characteristics | Curative resection +
adjuvant TACE (n=166) | Curative resection
alone (n=190) | P-value |
|---|
| Baseline
characteristics |
|
|
|
| Age,
year | 49.12±11.09 | 51.31±11.23 | 0.764 |
| Sex,
male/female | 102/64 | 119/71 | 0.380 |
| Clinical
characteristics |
|
|
|
| HBsAg,
positive/negative | 46/120 | 56/134 | 0.515 |
| Anti-HCV,
positive/negative | 2/164 | 2/188 | 0.274 |
| PLT,
109/l | 198.25±109.17 | 203.94±101.04 | 0.156 |
| TBil,
µmol/l | 16.12
(13.08–26.10) | 15.70
(11.80–25.40) | 0.318 |
| ALB,
g/l | 37.39±3.14 | 37.24±4.74 | 0.936 |
| ALT,
U/l | 50.00
(35.00–69.00) | 51.01
(34.00–61.00) | 0.837 |
| AST,
U/l | 59.00
(49.00–84.00) | 60.00
(50.00–107.50) | 0.883 |
| PT,
s | 13.75±1.52 | 13.84±1.934 | 0.913 |
| AFP,
mg/l | 15.67 (5.79
24) | 12.10
(1.23–26.7) | 0.297 |
| Pathological
characteristics |
|
|
|
| Tumor
capsule, positive/negative | 40/126 | 46/144 | 0.101 |
|
Macro-vascular invasion,
positive/negative | 33/133 | 28/162 | 0.846 |
| Tumor
number, single/multiple | 142/24 | 136/54 | 0.952 |
| Tumor
diameter, cm (≤5/5-10/>10) | 67/85/14 | 74/101/15 | 0.932 |
| Tumor
cell differentiation, high/moderate/low | 10/31/125 | 8/25/157 | 0.671 |
Prognostic factors affecting the
frequency of HCC recurrence following curative resection
During the follow-up period, 251 patients
experienced early recurrence and 65 patients experienced late
recurrence. Univariate analysis and multivariate analysis revealed
that patients with the characteristics of tumor capsule invasion,
vascular invasion, and tumor nodules had the highest tendency to
develop recurrence within the first year, peaking within the first
6 months following resection. HBV was identified as a risk factor
for late recurrence, primarily occurring more than 1 year following
resection. Other clinicopathological characteristics including
tumor size, AFP level and tumor cell differentiation did not appear
to be associated with recurrence (Table
II).
 | Table II.Predictors of hepatocellular
carcinoma recurrence. |
Table II.
Predictors of hepatocellular
carcinoma recurrence.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | Patients (n) | Recurrence time
(month) | 95% CI | P-value | 95% CI | P-value |
|---|
| Tumor capsule |
|
|
|
|
|
|
|
Positive | 270 | 5.30 | 8.37–9.16 | <0.001 | 1.56–2.64 | <0.001 |
|
Negative | 86 | 17.58 | 9.62–11.13 |
|
|
|
| Macro-vascular
invasion |
|
|
|
|
|
|
|
Positive | 61 | 4.30 | 2.29–9.71 | <0.001 | 0.64–3.78 | <0.001 |
|
Negative | 295 | 19.42 | 8.60–9.14 |
|
|
|
| Tumor number |
|
|
|
|
|
|
|
Single | 278 | 17.99 | 6.74–9.85 | <0.001 | 1.19–3.31 | <0.001 |
|
Multiple | 78 | 6.96 | 9.47–10.99 |
|
|
|
| HBsAg |
|
|
|
|
|
|
|
Positive | 102 | 19.88 | 9.84–10.22 | <0.001 | 1.21–3.37 | 0.008 |
|
Negative | 254 | 25.28 | 7.91–9.87 |
|
|
|
| Tumor diameter,
cm |
|
|
|
|
|
|
| ≤5 | 141 | 26.48 | 7.69–9.88 | 0.086 | 1.67–6.78 | 0.072 |
|
5–10 | 186 | 21.82 | 10.60–11.95 |
|
|
|
|
>10 | 29 | 20.21 | 9.50–11.02 |
|
|
|
| Tumor cell
differentiation |
|
|
|
|
|
|
|
High | 18 | 8.62 | 7.52–9.82 | 0.091 | 1.12–3.68 | 0.077 |
|
Moderate | 56 | 10.55 | 9.69–11.70 |
|
|
|
|
Low | 282 | 12.32 | 8.19–10.36 |
|
|
|
| Postoperative TACE
cycles |
|
|
|
|
|
|
| 0 | 190 | 6.58 | 5.60–9.18 | 0.0065 | 1.06–4.86 | 0.0029 |
| 1 | 98 | 8.82 | 6.79–10.05 |
|
|
|
|
2–3 | 68 | 9.20 | 8.45–11.76 |
|
|
|
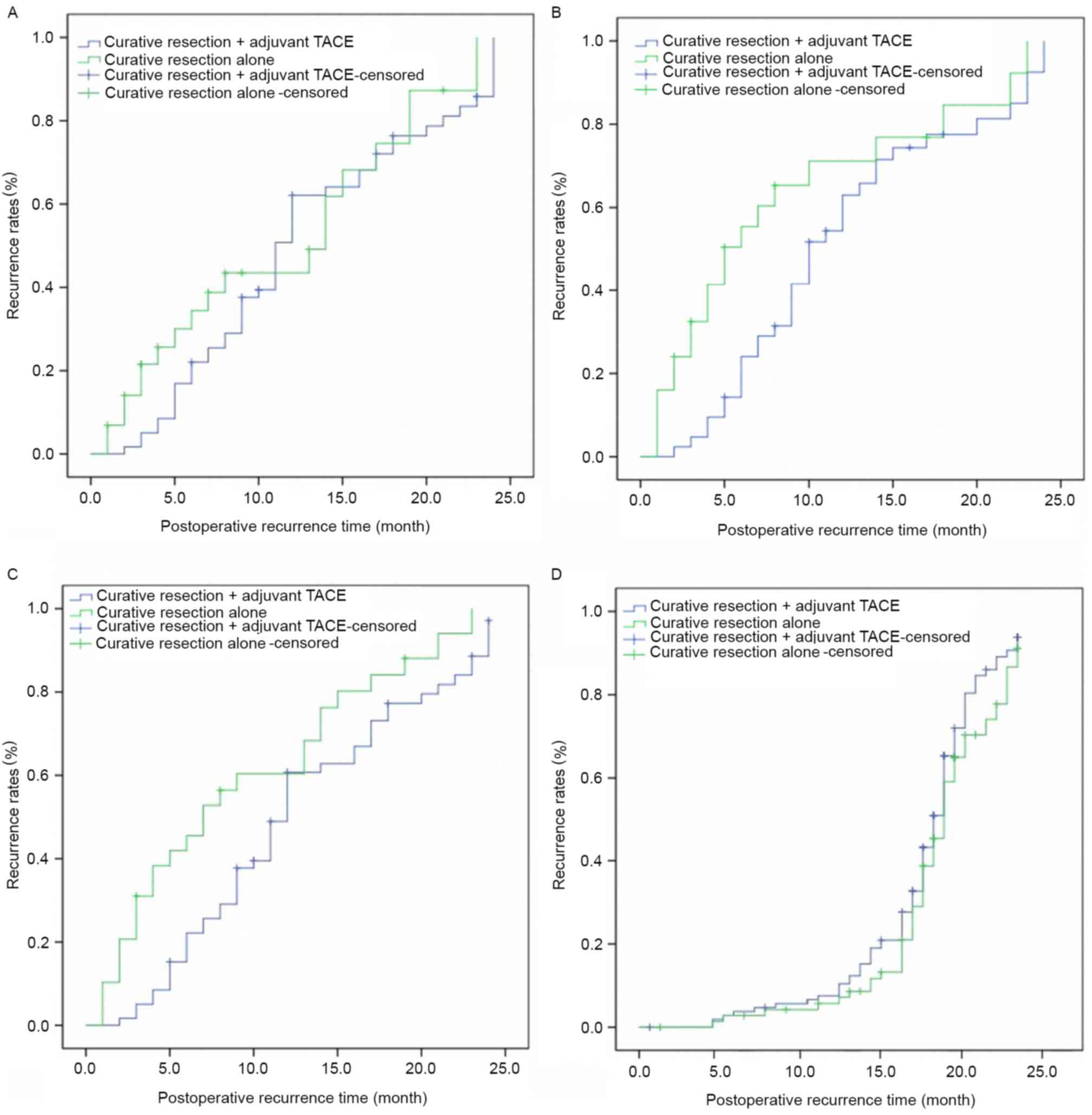
Effect of TACE on recurrence in risk
factor subgroups
Amongst patients who had the characteristics of
tumor capsule invasion, vascular invasion and multiple nodules,
recurrence rates decreased significantly following curative
resection when adjuvant TACE was added to therapy. This was true at
6 months (tumor capsule invasion: 23.2 vs. 59.4%, P=0.0061;
vascular invasion: 26.3 vs. 64.5%, P=0.0059; multiple nodules: 12.5
vs. 31.2%, P=0.0090) and 12 months (tumor capsule invasion: 68.4
vs. 72.5%, P=0.0093; vascular invasion: 69.8% vs. 78.4%, P=0.0067;
multiple nodules: 36.3% vs. 47.4%, P=0.0270) follow up, but not at
18 months (tumor capsule invasion: 83.1 vs. 89.2%, P=0.2610;
vascular invasion: 87.4 vs. 91.2%, P=0.1900; multiple nodules: 50.3
vs. 60.1%, P=0.0920) and 24 months (tumor capsule invasion: 100.0
vs. 100.0%, P=0.3010; vascular invasion: 100.0 vs. 100.0%,
P=0.4080; multiple nodules: 56.8 vs. 65.3%, P=0.1080; Table III and Fig. 1A-C). Amongst patients with HBV-HCC,
there was no significant difference in recurrence rates at 6 months
(0.0 vs.0.0%, P=0.4910), 12 months (4.3 vs. 4.1%, P=0.4160), 18
months (14.6 vs. 15.3%, P=0.6290), and 24 months (22.4 vs. 24.6%,
P=0.7500) following curative resection with adjuvant TACE compared
with curative resection alone (Table
III and Fig. 1D). These results
supported the hypothesis that postoperative adjuvant TACE was
effective in delaying recurrence in patients with HCC with risk
factors for early recurrence, but not in patients with HBV as a
risk factor for late recurrence.
 | Table III.Hepatocellular carcinoma recurrence
rates following curative resection with and without adjuvant
TACE. |
Table III.
Hepatocellular carcinoma recurrence
rates following curative resection with and without adjuvant
TACE.
| Variables | Curative resection
+ adjuvant TACE | Curative resection
alone |
|---|
| Recurrence time,
months | 6 | 12 | 18 | 24 | 6 | 12 | 18 | 24 |
| Tumor capsule
invasion, % | 23.2 | 68.4 | 83.1 | 100.0 | 59.4 | 72.5 | 89.2 | 100.0 |
| Macro-vascular
invasion, % | 26.3 | 69.8 | 87.4 | 100.0 | 64.5 | 78.4 | 91.2 | 100.0 |
| Multiple nodules,
% | 12.5 | 36.3 | 50.3 | 56.8 | 31.2 | 47.4 | 60.1 | 65.3 |
| HBV positive,
% | 0.0 | 4.3 | 14.6 | 22.4 | 0.0 | 4.1 | 15.3 | 24.6 |
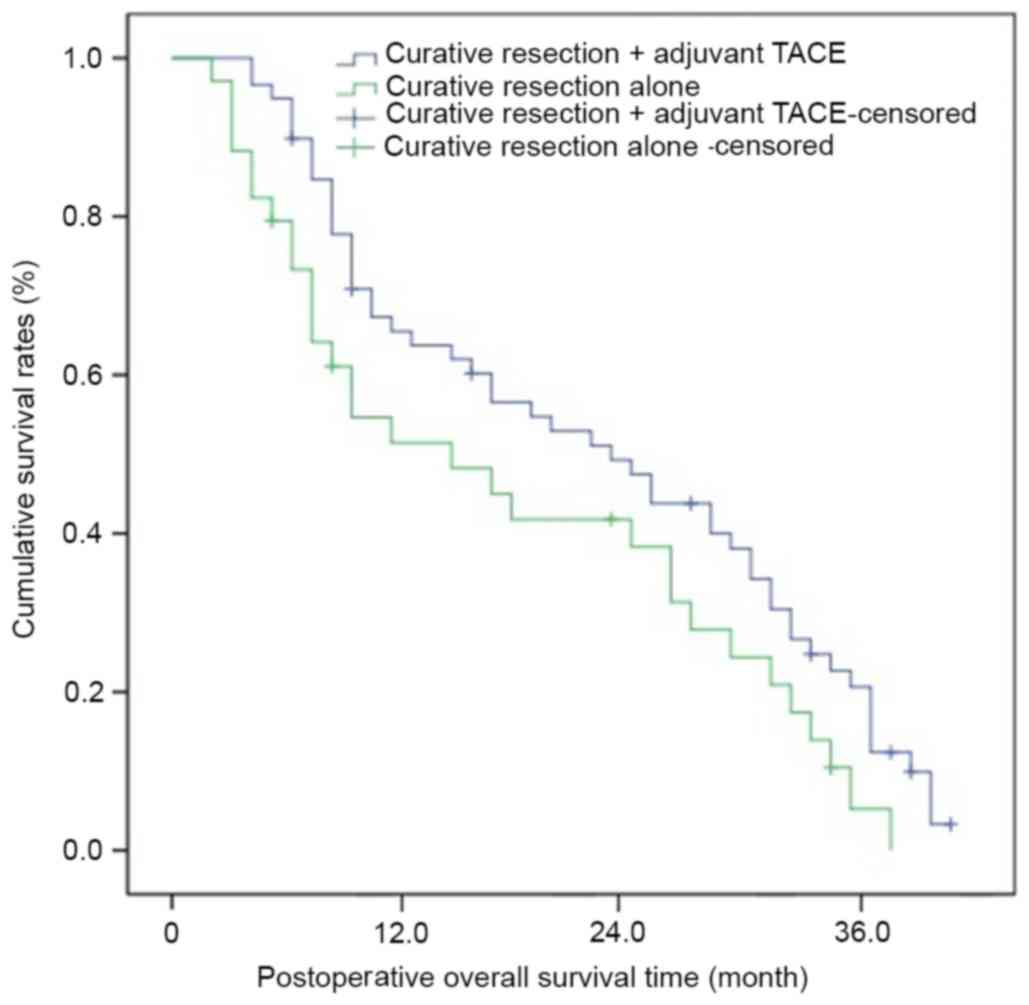
Overall survival following curative
resection with and without adjuvant TACE
Overall survival increased significantly following
curative resection with adjuvant TACE compared with curative
resection alone. The mean survival period of patients following
curative resection + TACE was 25.3 months, and the 1-, 2- and
3-year overall survival rates were 68.5, 49.3 and 27.6%
respectively; the mean survival period of patients who underwent
curative resection alone was 20.5 months, and the 1-, 2- and 3-year
overall survival rates were 50.3, 31.2 and 20.3% respectively
(P=0.0091; Table IV and Fig. 2). In addition, no apparent adverse
events occurred in patients who underwent postoperative adjuvant
TACE (Table V).
 | Table IV.Mean survival periods and survival
rates following curative resection with and without adjuvant
TACE. |
Table IV.
Mean survival periods and survival
rates following curative resection with and without adjuvant
TACE.
| Treatment | Mean survival
period (months) | 1-year survival
rate (%) | 2-year survival
rate (%) | 3-year survival
rate (%) |
|---|
| Curative resection
+ adjuvant TACE | 25.3 | 68.5 | 49.3 | 27.6 |
| Curative resection
alone | 20.5 | 50.3 | 31.2 | 20.3 |
 | Table V.Complications and adverse events
following postoperative adjuvant transarterial
chemoembolization. |
Table V.
Complications and adverse events
following postoperative adjuvant transarterial
chemoembolization.
| Complication | No. of patients
(%) |
|---|
| Nausea, vomiting,
n, (%) | 29 (17.5) |
| Fever, n (%) | 22 (13.3) |
| Pain, n (%) | 31 (18.7) |
| Alopecia, n
(%) | 3 (1.8) |
| Liver failure, n,
(%) | 0 (0.0) |
| Bleeding of
esophageal venous plexus, n (%) | 0 (0.0) |
| Gastrointestinal
hemorrhage | 0 (0.0) |
| Heart failure, n
(%) | 0 (0.0) |
| Infection, n
(%) | 0 (0.0) |
| Ectopic embolism
syndrome, n (%) | 0 (0.0) |
| Refractory ascites,
n (%) | 0 (0.0) |
| Pulmonary
complication, n (%) | 1 (0.6) |
| Therapy-related
death, n (%) | 0 (0.0) |
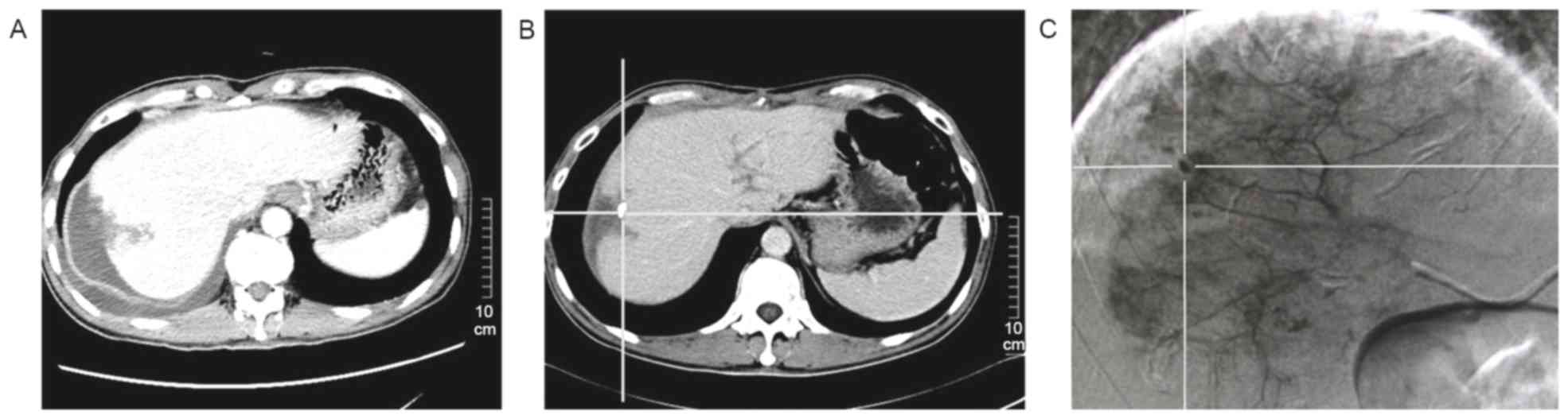
Enhancement of CT/MRI efficacy by
postoperative adjuvant TACE for detecting the recurrence
During the follow-up period, amongst the patients
who experienced recurrence, CT/MRI imaging was used to evaluate the
potential function of postoperative adjuvant TACE in enhancing the
efficacy of CT/MRI in detecting recurrence. In the present study,
when the diameter of recurrent HCC was not >1 cm, nothing was
discovered by dynamic contrasted-enhanced CT in the arterial
enhancement period (Fig. 3A). In
contrast, subsequent to adjuvant TACE being performed, a recurrent
HCC with diameter <1 cm with lipiodol uptake was revealed using
non-contrast-enhanced CT (Fig. 3B).
DSA was performed to make a confirmatory diagnosis of recurrence
(Fig. 3C). Postoperative adjuvant
TACE demonstrated a function of enhancing the efficacy of CT to
detect HCCs with a diameter of <1 cm.
Discussion
Frequent postoperative recurrence is the main
contributor to poor long-term survival following resections for
HCC. The presence of non-visible intra-hepatic metastasis and
multicentric occurrence prior to resection are accepted theories
underlying early recurrence (15). A
previous study has demonstrated that tumor capsule invasion,
vascular invasion and multiple nodules occurred in association with
invisible micro-metastasis (6).
Unrecognized intra-hepatic metastasis had an increased likelihood
of occurring in the presence of tumor capsule invasion and vascular
invasion distributed via the hepatic artery and portal vein system,
readily escaping intra-operative detection. Additionally, from the
compression and crushing of tissue during the operative process,
new intra-hepatic metastases may develop. Multiple nodules were a
typical outcome of the growth of previously invisible
micro-metastases. Although the visible metastases were eradicated,
there were still numerous invisible micro-metastases that
preoperative image scans and intraoperative observation failed to
detect. In this situation, liver resection was evidently not an
eradicative treatment. The results of the present study had a high
level of consistency with previous reports (16,17). In
the present retrospective study, tumor capsule invasion, vascular
invasion, and multiple nodules were identified as risk factors of
early recurrence. HBV was considered important for late recurrence.
Preoperative serum AFP level, tumor size, and tumor cell
differentiation were not associated with either early or late
recurrence.
Tiny lesions have the potential to avoid
preoperative or intra-operative detection. This is the primary
mechanism by which micro-metastasis leads to postoperative
recurrence. The normal liver parenchyma receives ~70% of its basic
blood supply from the portal venous system, whereas HCC growth,
including the invisible micro-metastasis, depends primarily on the
blood supply from the hepatic artery (18). Based on this, lipiodol may selectively
accumulate in the invisible metastatic HCC when it is delivered
intra-arterially and acts as a carrier for anticancer drugs.
Additionally, TACE may effectively block the nutrient vessels of
the invisible metastatic HCC, allowing sustainable chemotherapeutic
killing of the microscopic HCC cells (19). Cheng et al (20) and Ren et al (21) demonstrated that postoperative adjuvant
TACE protected against recurrence during the first 6 months
following surgical intervention. The patients selected in these
studies had incompletely encapsulated and vascular invasive tumors.
The results of the present study were consistent with these
reports. However, tumor necrosis in TACE is limited potentially due
to ongoing nutrient transport as the tumor is fed by small nutrient
vessels from the portal venous system (22). In addition, the cytotoxic effects of
chemotherapy drugs that allow them to kill tumor cells follow
log-cell kill kinetics (23).
Presumably, therefore, the preventative efficacy would increase by
repeating cycles of TACE. The present study also demonstrated that
the recurrence rate was significantly lower in patients who
underwent 2–6 cycles of adjuvant TACE compared with patients who
only received one cycle. However, repeated TACE may impair the
remnant liver function following resection, particularly in
patients with chronic liver cirrhosis (8), as well as an increase in the risk of HBV
reactivation (9). Fortunately, the
present study demonstrated that no apparent adverse events occurred
in patients who underwent postoperative TACE. The results of the
present study supported the recommendation of postoperative
adjuvant TACE as an effective and safe treatment to delay or
prevent early recurrence for patients with tumor capsule invasion,
vascular invasion, or multiple nodules. The number of cycles of
adjuvant TACE should depend on the remnant liver function following
resection.
The efficacy of contrast-enhanced CT/MRI to detect
recurrent HCC with diameter <1 cm remains unsatisfactory.
Certain studies (6,24) have demonstrated that due to the
exclusive uptake of lipiodol by HCC lesions, HCC-specific uptake of
lipiodol would be discovered by CT or MRI scan within 3–4 weeks
following lipiodol embolization. Thus, the location and size of
recurrence would be identified by CT/MRI following postoperative
adjuvant TACE. In the present study, in patients experiencing
recurrence, contrast-enhanced CT scans were performed prior to and
following adjuvant TACE and the images between the two phases were
compared in order to evaluate whether this strategy may improve the
efficacy of CT/MRI scans to detect recurrence. DSA is favored over
the CT scan for identification of small and early hepatic
malignancies (6), therefore DSA was
performed to confirm the CT images. The results of the present
study demonstrated that when the diameter of recurrent tumor was
<1 cm, the CT image following lipiodol embolization was superior
to the CT image prior to the procedure in detecting micro recurrent
HCC. The present study revealed that postoperative TACE may serve
an important function in enhancing the efficacy of CT/MRI to
discover the recurrence at an early stage and provide patients with
optimal opportunity to receive secondary eradicative intervention
like secondary resection, RA, or PEI. The clinical benefits of TACE
in improving early detection of HCCs are an addition to its effect
on tumor cell destruction. Postoperative preventative adjuvant TACE
should be recommended in patients with higher risk of early
recurrence.
When lipiodol selectively accumulates in the
micro-metastasis or recurrent HCC, >90% of tumor blood supply
via the hepatic artery is blocked (15), and sustainably high levels of
chemotherapeutic drugs work to kill the micro-metastatic or the
recurrent HCC cells (19). Adjuvant
TACE was effective in preventing early recurrence in patients with
risk factors for non-visible intra-hepatic metastasis, particularly
within the first 6 months following resection, and restraining the
progression of recurrent HCC. Tanaka et al (25) and Izumi et al (26) reported that postoperative adjuvant
TACE was effective in improving the survival of patients with HCC.
The patients selected in their studies had characteristics of
incomplete capsule, intra-hepatic metastasis or vascular invasion
which were associated with residual tumor and earlier massive
recurrence (27). The results of the
present study were consistent with their reports, revealing that
overall survival was significantly improved by postoperative
adjuvant TACE. In addition, adjuvant TACE may enhance the efficacy
of CT/MRI scans to discover micro (<1 cm) recurrent HCC. Early
discovery of HCCs allows for an earlier opportunity to undergo
eradicative interventions including secondary resection, RFA, or
PEI. Generally, secondary resection, PEI and RFA as eradicative
interventions are widely used to treat recurrent HCC. In contrast,
adjuvant TACE has not been considered as an eradicative
intervention to treat recurrent HCC because tumor necrosis is
limited as stated above (22,23). Although tumor necrosis may be enhanced
by repeated TACE, notably, repeated TACE may damage the remnant
liver parenchyma, particularly in patients with cirrhosis (8), and enhance the risk of HBV reactivation
(9). Nonetheless, when recurrent HCC
was >3 cm, or its location was adjacent to vessels, or occurred
in the presence of other variables unfavorable for secondary
resection, PEI or RFA, then postoperative TACE, may be a viable
option as a palliative intervention to treat recurrent HCC by
restraining tumor progression.
In conclusion, postoperative adjuvant TACE is an
effective and safe therapeutic strategy to suppress early
recurrence in patients with HCC with the characteristics of tumor
capsule invasion, intra-hepatic vascular invasion and multiple
nodules. Additionally, postoperative adjuvant TACE may enhance the
efficacy of CT/MRI scans to discover micro-recurrence at an early
stage. Furthermore, TACE may improve survival in patients with HCC
with acceptable postoperative liver function (Child-Pugh A) by
delaying tumor progression of recurrent HCCs. However,
postoperative adjuvant TACE does not appear to reduce recurrence in
patients without risk factors for early recurrence. Nonetheless,
larger randomized control trials involving patients with more
complicated cases of HCC should examine in more detail the effect
of postoperative adjuvant TACE on HCC prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XK, JY, ZX, GW, FW and HY designed the study. TB,
JC, WG and BX participated in data acquisition. LQ, JZ, LM, NP, CW
and LL performed the data interpretation and statistical analyses.
All authors critically revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Affiliated Minzu Hospital of Guangxi Medical
University (Guangxi, China).
Patient consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qin A, Zhu J, Liu X, Zeng D, Gu M and Lv
C: MicroRNA-1271 inhibits cellular proliferation of hepatocellular
carcinoma. Oncol Lett. 14:67832017.PubMed/NCBI
|
|
2
|
Hasegawa K, Kokudo N, Makuuchi M, Izumi N,
Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O and
Matsuyama Y: Comparison of resection and ablation for
hepatocellular carcinoma: A cohort study based on a Japanese
nationwide survey. J Hepatol. 58:724–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnaoutakis DJ, Mavros MN, Shen F,
Alexandrescu S, Firoozmand A, Popescu I, Weiss M, Wolfgang CL,
Choti MA and Pawlik TM: Recurrence patterns and prognostic factors
in patients with hepatocellular carcinoma in noncirrhotic liver: A
multiinstitutional analysis. Ann Surg Oncol. 21:147–154. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang JH, Guo Z, Lu HF, Wang XB, Yang HJ,
Yang FQ, Bao SY, Zhong JH, Li LQ, Yang RR and Xiang BD: Adjuvant
transarterial chemoembolization after curative resection of
hepatocellular carcinoma: Propensity score analysis. World J
Gastroenterol. 21:4627–4634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong JH and Li LQ: Postoperative adjuvant
transarterial chemoembolization for participants with
hepatocellular carcinoma: A meta-analysis. Hepatol Res. 40:943–953.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu X, Zhao H, Yang H, Mao Y, Sang X, Miao
R, Xu Y, Du S, Xu H, Chi T, et al: A prospective clinical study on
early recurrence of hepatocellular carcinoma after hepatectomy. J
Surg Oncol. 100:488–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Zhang B, Yin X, Ren Z, Qiu S and
Zhou J: Lipiodolized transarterial chemoembolization in
hepatocellular carcinoma patients after curative resection. J
Cancer Res Clin Oncol. 139:773–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ono T, Yamanoi A, El Assal Nazmy O, Kohno
H and Nagasue N: Adjuvant chemotherapy after resection of
hepatocellular carcinoma causes deterioration of long-term
prognosis in cirrhotic patients: Metaanalysis of three randomized
controlled trials. Cancer. 91:2378–2385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng JW, Lin GN and Jiang XM: Hepatitis B
virus reactivation in hepatocellular carcinoma patients underg oing
transcatheterarterial chemoembolization therapy. Asia Pac J Clin
Onco. 8:356–361. 2012. View Article : Google Scholar
|
|
10
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin ZZ, Hsu C, Hu FC, Shao YY, Chang DY,
Yang CH, Hong RL, Hsu CH and Cheng AL: Factors impacting prognosis
prediction in BCLC stage C and Child-Pugh class A hepatocellular
carcinoma patients in prospective clinical trials of systemic
therapy. Oncologist. 17:970–977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shah SA, Greig PD, Gallinger S, Cattral
MS, Dixon E, Kim RD, Taylor BR, Grant DR and Vollmer CM: Factors
associated with early recurrence after resection for hepatocellular
carcinoma and outcomes. J Am Coll Surg. 202:275–283. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L,
Ye XP, Peng T, Xie GS and Li LQ: Hepatic resection associated with
good survival for selected patients with intermediate and
advanced-stage hepatocellular carcinoma. Ann Surg. 260:329–340.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu SL, Zhong JH, Ke Y, Xiao HM, Ma L,
Chen J, You XM and Li LQ: Comparative efficacy of postoperative
transarterial chemoembolization with or without antiviral therapy
for hepatitis B virus-related hepatocellular carcinoma. Tumour
Biol. 36:6277–6284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li DH and Sun B: Research progress in
intrahepatic metastasis and multiple centre carcinogenesis of
hepatocellular carcinoma. Int J Surg. 33:28–31. 2006.
|
|
16
|
Sutcliffe R, Maguire D, Murphy P, Portmann
B, Rela M, O'Sullivan G, Mufti G and Heaton N: Detection and
clinical significance of bone marrow micrometastases in patients
undergoing liver transplantation for hepatocellular carcinoma.
Transplantation. 80:88–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding T, Xu J, Zhang Y, Guo RP, Wu WC,
Zhang SD, Qian CN and Zheng L: Endothelium-coated tumor clusters
are associated with poor prognosis and micrometastasis of
hepatocellular carcinoma after resection. Cancer. 117:4878–4889.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee DS and Seong J: Radiotherapeutic
options for hepatocellular carcinoma with portal vein tumor
thrombosis. Liver Cancer. 3:18–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roayaie S, Frischer JS, Emre SH, Fishbein
TM, Sheiner PA, Sung M, Miller CM and Schwartz ME: Long-term
results with multimodal adjuvant therapy and liver transplantation
for the treatment of hepatocellular carcinomas larger than 5
centimeters. Ann Surg. 235:533–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng HY, Wang X, Chen D, Xu AM and Jia
YC: The value and limitation of transcatheter arterial
chemoembolization in preventing recurrence of resected
hepatocellular carcinoma. World J Gastroenterol. 11:3644–3646.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren ZG, Lin ZY, Xia JL, Ye SL, Ma ZC and
Ye QH: Postoperative adjuvant arterial chemoembolization improves
survival of hepatocellular carcinoma patients with risk factors for
residual tumor: A retrospective control study. World J
Gastroenterol. 10:2791–2794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY,
Chen MS and Shi M: Transarterial chemoembolization for unresectable
hepatocellular carcinoma with portal vein tumor thrombosis: A
prospective comparative study. Ann Surg Oncol. 18:413–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Norton L: Adjuvant breast cancer therapy:
Current status and future strategies-growth kinetics and the
improved drug therapy of breast cancer. Semin Oncol. 26 1 Suppl
3:S1–S4. 1999.
|
|
24
|
Dai D, Xu W, Liu J, Zhu L, Zhu X and Ma X:
Safety and efficacy of a peripheral intravenous bolus of Licartin
for the treatment of advanced hepatocellular carcinoma. Exp Ther
Med. 6:1417–1422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka K, Shimada H, Togo S, Takahashi T,
Endo I and Sekido H: Use of transcatheter arterial infusion of
anticancer agents with lipiodol to prevent recurrence of
hepatocellular carcinoma after hepatic resection.
Hepatogastroenterology. 46:1083–1088. 1999.PubMed/NCBI
|
|
26
|
Izumi R, Shimizu K and Miyazaki I:
Postoperative adjuvant locoregional chemotherapy in patients with
hepatocellular carcinoma. Hepatogastroenterology. 43:1415–1420.
1996.PubMed/NCBI
|
|
27
|
Yamanaka J, Yamanaka N, Nakasho K, Tanaka
T, Ando T, Yasui C, Kuroda N, Takata M, Maeda S, Matsushita K, et
al: Clinicopathologic analysis of stage II–III hepatocellular
carcinoma showing early massive recurrence after liver resection. J
Gastroenterol Hepatol. 15:1192–1198. 2000. View Article : Google Scholar : PubMed/NCBI
|