Introduction
Ovarian cancer (OC) is a common gynecological
malignancy (1), ranking third among
the worldwide incidence of gynecological cancers, with its
mortality rate ranking the first (2).
Owing to its subtle onset and atypical early-stage clinical
symptoms, almost 70% of OC patients present with stage III or IV OC
when diagnosed (3). With continued
investigation into OC pathogenesis, the search for early diagnosis
and chemotherapy-sensitive markers has gained increased
attention.
MicroRNAs (miRNAs/miRs) are a class of endogenous
non-coding small RNA molecules which serve roles in cell growth,
differentiation, metabolism and the cell cycle (4,5). The
mutation, deletion and aberrant expression of miRNAs is associated
with the occurrence and development of human tumors. Upregulation
of miRNAs which have tumor suppressing roles is anticipated to be a
novel approach in cancer gene therapy (6,7).
Let-7 was one of the first miRNAs to be identified
in humans, and the 5′ end of human let-7 miRNA family (let-7a-1,
let-7a-2, let-7a-3, let-7b, let-7c, let-7d, let-7e, let-7f-1,
let-7f-2, let-7g, let-7i, miR-202 and miR-98) contains one highly
conserved nucleotide seed sequence which is necessary for miRNA
target binding (8,9). Through the regulation of target genes,
let-7 is involved in multiple biological processes including cell
proliferation, differentiation, apoptosis, hormone secretion,
metabolism, immune regulation and tumorigenesis. Downregulated
expression of let-7 has been observed in several tumor tissues and
cells including breast, lung, prostate and hepatocellular cancer
(10–13). Let-7 is abundant in ovaries, and
serves an important role in reproductive control during distinct
developmental stages, during which the expression levels of let-7
in ovary cells are different (14). A
previous study demonstrated that let-7c is involved with various
ovarian physiological and pathological processes via regulation of
its target genes (15). However, the
underlying molecular mechanisms of let-7c in OC are not well
understood. Prior investigation has revealed that cell division
cycle 25A (CDC25a) is the target gene of let-7c (16). As a cell cycle regulatory protein, the
abnormal expression of CDC25a is associated with the occurrence and
development of multiple tumors (17–21). In
the present study, the biological functions of let-7c in the
development and progression of OC were investigated. The extent by
which let-7c exhibits its cancer suppressive roles through the
regulation of CDC25a expression levels was also investigated.
Materials and methods
Clinical samples
A total of 52 OC tissue samples resected in Henan
Provincial Maternal and Child Health Institute (Zhengzhou, China)
from March 2014 to March 2015 were selected, including 21 poorly
differentiated cases, 18 mid-differentiated cases and 13
well-differentiated cases. The patients were aged between 27 and 68
years, with a mean age of 55.7 years. According to the clinical
staging criteria of OC by the International Federation of
Gynecology and Obstetrics (FIGO) (22), 8 cases were in stage I, 10 cases were
in stage II, 19 cases were in stage III and 15 cases were in stage
IV. The tissue samples included 33 cases of serous adenocarcinoma
and 19 cases of mucinous adenocarcinoma. No chemotherapy,
radiotherapy or immunotherapy was being performed when sampling the
specimens, and all samples were confirmed using histology and
imaging. In the present study, all patients provided written
informed consent, and the Ethics Committee of Third Affiliated
Hospital of Zhengzhou University approved the present study.
Cell culture and transfection
OC cell lines SKOV3 and ES2 were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), cultured in RPMI-1640 cell culture medium
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C with 5%
CO2.
During transfection, the OC cells were seeded in
6-well plates at a concentration of 5×104 cells/well,
and when 80% of the cells had fused, 50 nM let-7c agomir was
transfected into ES2 and SKOV3 cells using a Lipofectamine™ 2000
kit (Invitrogen, Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Let-7c agomir
(5′-UGAGGUAGUAGGGUUGUAUGGUU-3′) and the negative control
(5′-CAGUACUUUUGUGUAGUACAA-3′) were designed and synthesized by
Guangzhou Ribobio Co., Ltd. (Guangzhou, China). The culture medium
was changed 6 h after transfection for a further 24 h in culture,
and the cells were then collected for various assays.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA in the OC and normal ovarian tissues
was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). An RNA extraction kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used according to the manufacturer's protocol
to extract the total RNA from the OC cells. A NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
determine the concentration and purity of the extracted RNA, with
the optical density 260/280 nm value close to 1.8, indicating that
the purity complied with the test requirements.
RT-qPCR was performed to detect the expression of
let-7c in these specimens. An RNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc.) was used according to the manufacturer's
protocol to reverse-transcribe 1.0 µg total RNA into cDNA; ABI
Power SYBR-Green PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) qPCR amplification was performed according to the
manufacturer's protocol to detect the target fragments. An ABI 7500
Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used according to the manufacturer's protocol
to perform PCR, with a Homo sapiens let-7c specific primer
sequence (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
copy number of RNU6B was used as the correction base, to detect the
concentration of let-7c, which was expressed as a relative
expression level using the 2−ΔΔCq method (23). The primer sequences used were as
follows: miRNA-let-7c: 5′-GCGCGTGAGGTAGTAGGTT-3′ (sense) and
5′-GTGCAGGGTCCGAGGT′ (anti-sense); U6 5′-GCGCGTCGAAGCGTTC-3′
(sense) and 5′-GTGAGGGTCCGAGGT-3′ (anti-sense). Hot start PCR
conditions were 10 sec at 95°C, 20 sec at 60°C and 10 sec at 72°C
for 40 cycles.
Western blot analysis
The protein samples of each group were collected.
The cells were digested with 0.25% trypsin and the cell stocks were
centrifuged at 156 × g for 5 min at 4°C. The cells were resuspended
with 1 ml phosphate suffered saline (PBS) and centrifuged at 156 ×
g for 5 min at 4°C. These cells were then lysed with
radioimunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) to lyse the cells, and coomassie
brilliant blue was used to determine the protein concentration.
Following separation using SDS-PAGE (10% gel), the required
proteins (50 µg) were transferred onto a polyvinylidene difluoride
film, followed by 1 h of blocking using 5% skimmed milk in
Tris-buffered saline with Tween-20, and overnight incubation at 4°C
with agitation with diluted primary antibody (1:500; rabbit
anti-human CDC25a antibody; cat. no. SC-97; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following washing using
Tris-buffered saline containing Tween-20 (TBST) for 4×15 min,
diluted secondary antibody (1:1,000; horseradish peroxidase-labeled
IgG; cat. no. SC-2357; Santa Cruz Biotechnology, Inc.) was added
for 1 h to incubate, followed by TBST washing for 4×15 min. An ECL
Western Blotting Detection kit was then used to detect the signals
(GE Healthcare, Chicago, IL, USA) with GAPDH (1:500; cat. no.
sc-47724; Santa Cruz Biotechnology, Inc.) as the reference to
calculate the relative expression of the proteins.
Detection of cellular proliferation
using Cell Counting Kit-8 (CCK-8)
CCK-8 contains WST-8 [chemical name,
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic
acid benzene)-2H-tetrazolium monosodium salt], which is able to be
reduced to a highly water-soluble formazan dye by the dehydrogenase
in the electron carrier 1-methoxy-5-methylphenazinium dimethyl
sulfate in cells. The amount of formazan produced is proportional
to the number of viable cells. Therefore, this trait may be
utilized directly as a cell proliferation assay. A CCK-8 kit
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to
detect the effect of let-7c on cellular proliferation. Cells in the
exponential growth phase were seeded in 96-well plates
(2×103 cells/well), with 10 µl WST-8 solution added into
each well at 24, 48, 72 and 96 h respectively, according to the
manufacturer's protocol to detect the cell proliferation. Results
were expressed as the optical density value of each well at 450
nm.
Plate clone assay
Low melting point agarose solution (0.6%) was mixed
with RPMI-1640 medium containing 10% FBS (1:1), added into 6-well
plates, and allowed to cool and solidify at room temperature. The
cells of each group in the exponential growth phase following
transfection were suspended in the aforementioned culture medium,
and then added into 6-well plates in which the lower gel layer had
already solidified. Following a 12-day incubation at 37°C, the
cells were stained using 1 ml 1% crystal violet to count the cell
clones with the naked eye and light microscopy (×100
magnification). The cell clones with more than 50 cells were
counted as one monoclone, and the mean number of clones on the
plate was used to calculate the cell clone ability.
Detection of apoptosis by flow
cytometry
The cells were digested by 0.2% trypsin at 37°C for
2 min and centrifuged at 156 × g for 5 min at 4°C, and the cells
were counted following suspension in PBS (1×106
cells/ml) 72 h post-transfection. An annexin V-fluoroscein
isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit
(BestBio, Shanghai, China) was used to detect the proportion of
cells undergoing apoptosis and flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) was used to determine the cell apoptosis
within 30 min.
Dual-luciferase reporter gene
assay
Three target gene prediction databases, TargetScan
(http://www.targetscan.org), PicTar
(http://pictar.mdc-berlin.de) and miRanda
(http://microrna.sanger.ac.uk), were used
to predict the target gene of let-7c, with CDC25a identified as the
potential target. The DNA of healthy human subjects was extracted
using a Human blood genomic DNA extraction kit (Promega
Corporation, Madison, WI, USA) following the provision of written
informed consent, and the CDC25a 3′ untranslated region (UTR) that
included the let-7c-binding sites was amplified using PCR. The
reaction protocols were as follows: 95°C pre-degeneration, cycle
95°C for 10 sec degeneration; 1°C annealing from 65°C each cycle,
72°C extension for 2 min, for 10 cycles; 55°C annealing, 15 cycles
of; 72°C for 7 min and 4°C preservation. TaqMan Universal PCR
Master Mix (Applied Biosystems). The primer sequences of CDC25A
used were as follows: 5′-CCGCTCGAGGCGGCAGGACCAGCCAG-3′ (sense) and
5′-GAATGCGGCCGCTCAGAGCTTCCAACAGTTGGTTAG-3′ (anti-sense). The
amplified CDC25a was then recovered by AxyPrep DNA gel recovery kit
(Axygen; Corning Incorporated, Corning, NY, USA) following 1%
agarose electrophoresis, and connected with pmirGLO carrier
(pmirGLO-CDC25a-wt, wild-type CDC25a) using T4 DNA ligase (Takara
Bio, Inc., Otsu, Japan); mutagenic primers targeting the CDC25a
3′UTR seed region were then designed, and amplified using the
overlap method, and inserted into the multi-cloning site of pmirGLO
vector (pmirGLO-CDC25a-mut, mutant CDC25a). Lipofectamine 2000 was
then used to co-transfect the recombinant vectors and let-7c agomir
or negative control into OC cells; 48 h later, a Dual-Luciferase
Reporter assay system (Promega Corporation, Madison, WI, USA) was
used to measure the dual-luciferase signals in each group, with the
Renilla luciferase signal as the reference; each group was
tested in triplicate.
Recovery assay
The CDC25a fragment free of 3′UTR was amplified and
inserted into the eukaryotic expression vector pcDNA3.1
(pcDNA3.1-CDC25a). pcDNA3.1-CDC25a was then transfected into SKOV3
cells; meanwhile, let-7c agomir or negative control was also
transfected using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The cells of each group were collected 48 h later,
expression levels of CDC25a protein were investigated using western
blotting; and apoptosis was investigated using flow cytometry.
Statistical analysis
SPSS (version 18.0; SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Data are expressed as the mean ±
standard deviation. The differences between groups was analyzed
using Student's t-test or analysis of variance with
Student-Newman-Keuls method as a post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
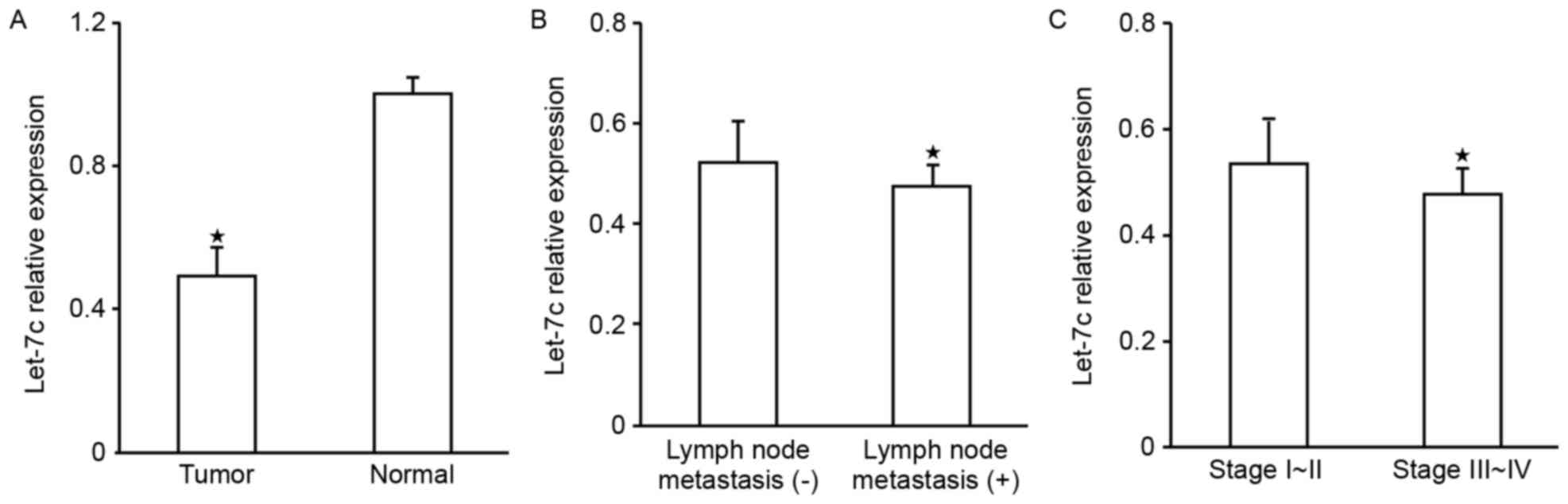
Expression of let-7c in OC tissue
The expression levels of let-7c in 52 OC cases
determined using RT-qPCR demonstrated that, compared with normal
tissue, let-7c was significantly downregulated in the OC tissue
(P<0.05; Fig. 1A); further
analysis of the association of let-7c expression with the
pathological factors of patients revealed that let-7c was
associated with lymph node metastasis (Fig. 1B) and clinical stage (P<0.05;
Fig. 1C and Table I), the expression levels of let-7c in
samples from patients with lymph node metastasis were decreased
compared with those with no lymph node metastasis, and were
decreased in the higher clinical grading group; however, there was
no observable association with age, pathological type or
differentiation degree (P>0.05; Table
I).
 | Table I.Association between let-7c expression
and clinical pathological factors in OC. |
Table I.
Association between let-7c expression
and clinical pathological factors in OC.
| Clinicopathological
characteristic | n | let-7c
expression | P-value |
|---|
| Age, years |
|
| 0.087 |
|
<50 | 23 | 0.4741±0.0770 |
|
|
≥50 | 29 | 0.5132±0.0828 |
|
| Tissue type |
|
| 0.844 |
| Serous
adenocarcinoma | 33 | 0.4976±0.0853 |
|
|
Mucinous adenocarcinoma | 19 | 0.4929±0.7768 |
|
|
Differentiation |
|
| 0.263 |
|
High | 21 | 0.4819±0.0744 |
|
|
Medium | 18 | 0.4892±0.0924 |
|
|
Poor | 13 | 0.5278±0.0752 |
|
| Clinical stage |
|
| 0.015a |
| I and
II | 18 | 0.5333±0.0831 |
|
| III and
IV | 34 | 0.4761±0.0750 |
|
| Lymph node
metastasis |
|
| 0.033a |
| No
(−) | 24 | 0.5218±0.0833 |
|
| Yes
(+) | 28 | 0.4737±0.0751 |
|
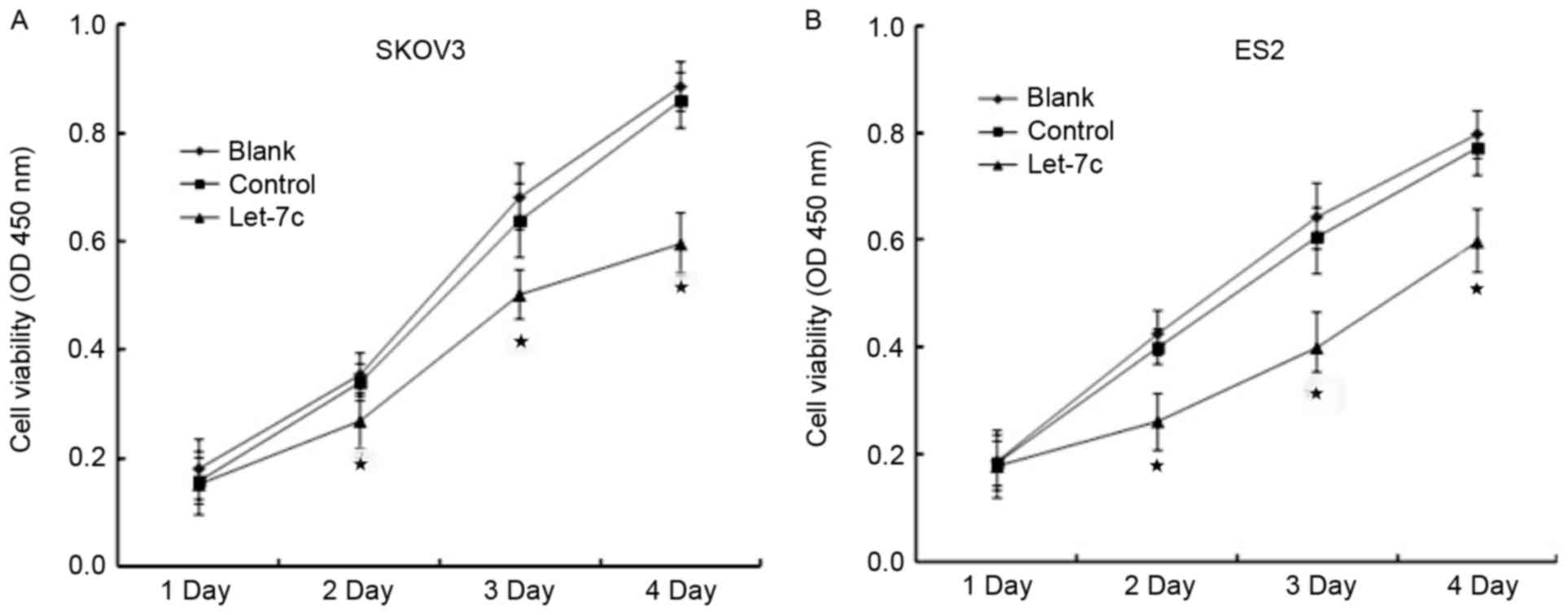
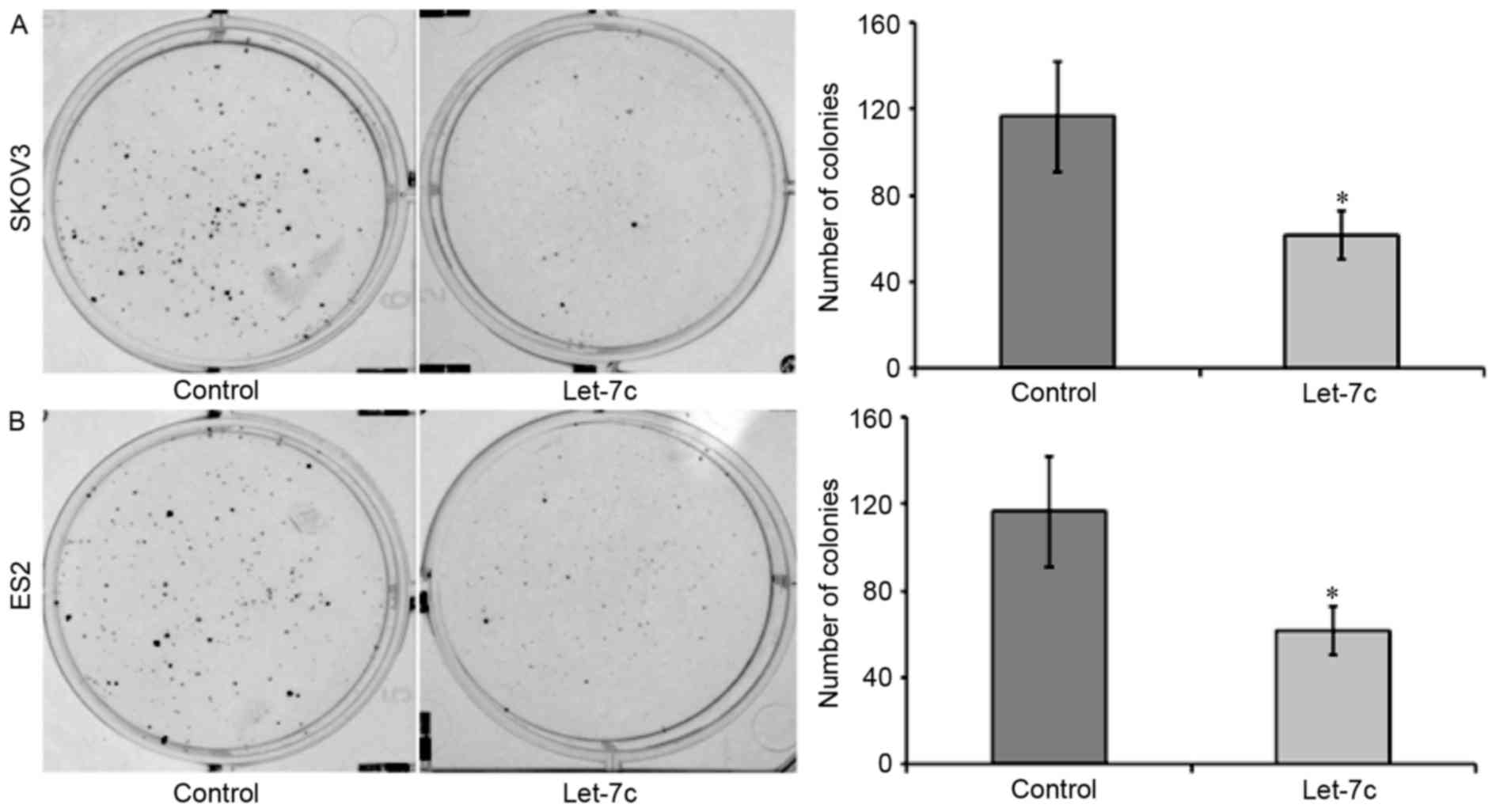
Effects of let-7c on the proliferation
of OC cells
CCK-8 and plate clone assays were performed to
analyze the effects of let-7c on the proliferation of OC cells. A
CCK-8 assay revealed that, after 48 h of transfection of the let-7c
agomir into the OC cell lines, the relative proliferation rates of
SKOV3 and ES2 were significantly decreased compared with the blank
group and the control group (P<0.05), and no statistically
significant difference was identified between the blank group and
the control group at any time point (P>0.05; Fig. 2). The plate clone assay demonstrated
that the let-7c agomir significantly decreased the number of OC
cell colonies (P<0.05; Fig. 3),
suggesting that the overexpression of let-7c inhibited the
proliferative abilities of the OC cell lines SKOV3 and ES2.
Effects of let-7c on apoptosis of OC
cells
Flow cytometry revealed that, following transfection
with let-7c agomir, the apoptotic rates of SKOV3 and ES2 cells were
significantly increased compared with the control group (P<0.05;
Fig. 4), suggesting that the
overexpression of let-7c leads to the apoptosis of SKOV3 and ES2
cells.
Expression inhibition of CDC25a mRNA
3′UTR by let-7c
The miRNA bioinformatics database analysis
identified that CDC25a was the target gene of let-7c (Fig. 5A). The present study inserted human
CDC25a wild-type and mut-type 3′UTRs into the dual-luciferase
reporter vector pmirGLO, and co-transfected this recombinant vector
and let-7c agomir or negative control into OC cells to detect the
fluorescent signal changes in each group. The results demonstrate
that following co-transfection with let-7c agomir and
pmirGLO-CDC25a-wt, luciferase activity was significantly inhibited
(P<0.05; Fig. 5B); however, the
co-transfection of let-7c agomir and pmirGLO-CDC25a-mut
demonstrated no significant change in luciferase activity
(P>0.05; Fig. 5B), suggesting that
let-7c may combine with the CDC25a 3′UTR seed region, thus
resulting in the downregulation of the CDC25a gene. Western
blotting results also revealed that let-7c agomir was able to
decrease the expression of CDC25a protein in OC cells (Fig. 5C).
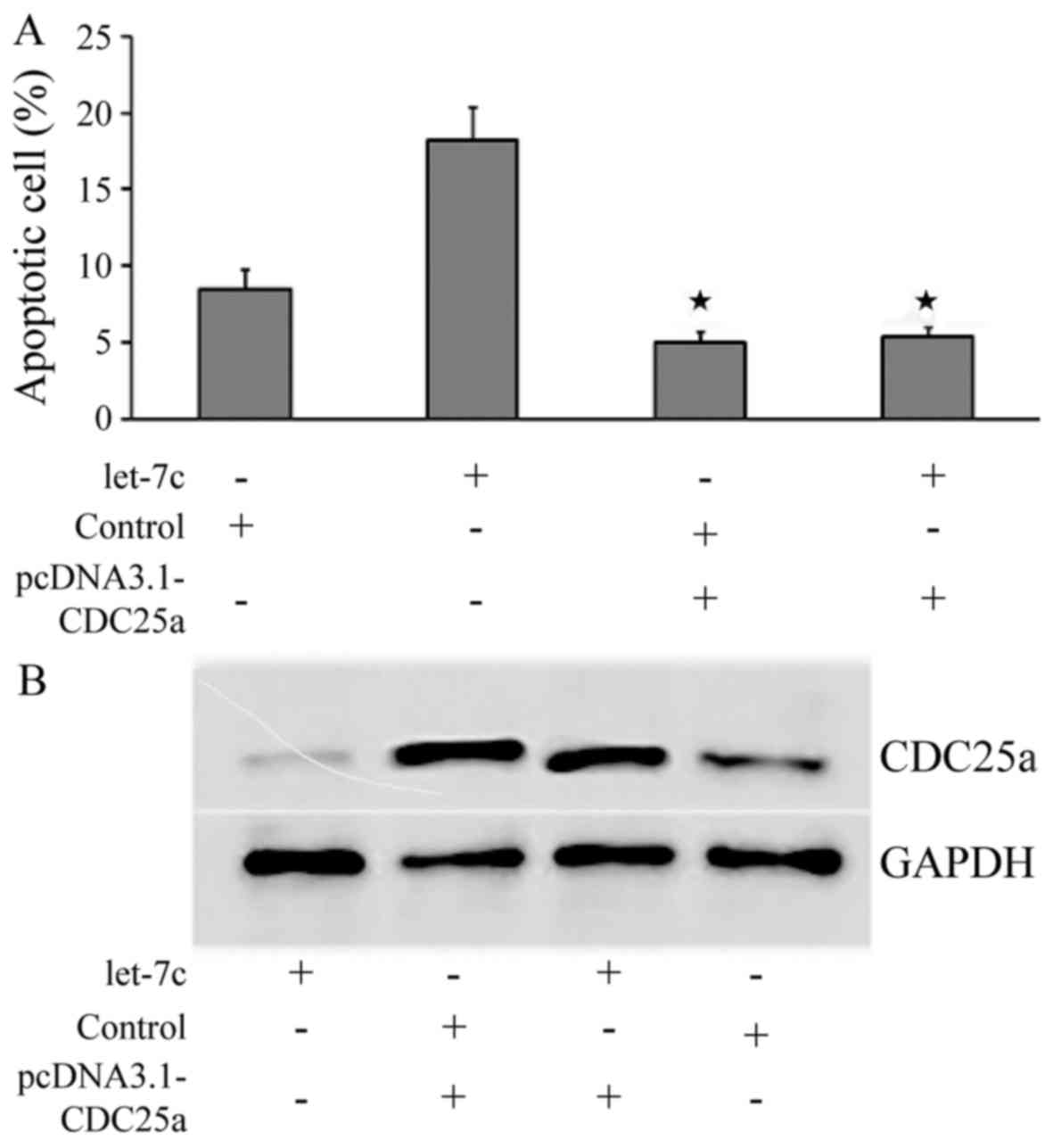
Overexpression of CDC25a is able to
recover the pro-apoptotic effect of let-7c towards OC cells
In order to further explore the targets of let-7c,
pcDNA3.1-CDC25a was constructed and co-transfected alongside let-7c
agomir or a negative control into SKOV3 cells; furthermore, let-7c
agomir or a negative control were separately transfected into SKOV3
cells. An apoptosis assay demonstrated that when only let-7c agomir
was transfected, was the apoptotic rate significantly increased;
however, the apoptotic rate was significantly decreased when
pcDNA3.1-CDC25a was transfected or pcDNA3.1-CDC25a and let-7c
agomir were co-transfected into the cells (Fig. 6A). Western blot analysis revealed
that, following the co-transfection of pcDNA3.1-CDC25a and let-7c
agomir, CDC25a protein was increased, indicating that
pcDNA3.1-CDC25a is able to recover the inhibition of let-7c towards
the expression of CDC25a protein (P<0.05; Fig. 6B). This further illustrates that
let-7c may act on the CDC25a 3′UTR seed region, thus regulating the
CDC25a expression exhibiting pro-apoptotic effects towards OC.
Discussion
miRNAs are able to regulate transcription by binding
to the 3′UTR of target genes, thus serving a role in a variety of
physiological processes (24). A
previous study revealed that the expression profiles of miRNAs in
tumor tissue and normal tissue exhibit significant differences, and
the expression levels of miRNAs are associated with the
development, differentiation, metastasis and prognosis of tumor
cells (25). Previous studies have
analyzed miRNA expression profiles in order to investigate miRNAs
in a variety of malignant tumors, including OC and OC cell lines,
expecting to identify miRNAs with diagnostic potential (26,27). Zhang
et al (28) detected the
expression of 173 mature miRNAs in OC cell lines and human ovarian
surface epithelial cell lines, and identified 35 miRNAs with
significant expression differences, among which 31 were
downregulated and four were upregulated. Iorio et al
(29) analyzed the miRNA expression
profiles in OC tissue, and revealed that miRNA was not only
differentially expressed between tumor and normal tissues, but also
differentially expressed in different histological subtypes.
miRNAs themselves may become the target of cancer
therapies which alter the expression of miRNA so as to regulate the
target genes and treat cancer. Mature let-7 is able to bind to the
3′UTR of its target gene and degrade the target mRNA or inhibit its
translation. Han et al (30)
overexpressed let −7c, which inhibited the invasion and metastasis
of colorectal cancer cells. Zhao et al (31) demonstrated that let-7c acts directly
on downstream genes mitogen-activated protein kinase kinase kinase
kinase 3 and integrin subunit β3, thus inhibiting the invasion and
metastasis of non-small cell lung cancer. Wang et al
(32) demonstrated that let-7c
inhibits the proliferation of lung cancer cells by acting on
tribbles homolog 2. Therefore, during the development of OC, the
similar role of let-7c as a cancer suppressing gene may also result
from its regulation of target genes. The present study detected the
let-7c expression levels in OC tissue and normal ovarian tissue,
and demonstrated that let-7c was downregulated in OC, with its
level associated with lymph node metastasis and clinical stage,
suggesting that miR-26a has certain associations with the
occurrence of OC. At the same time, following transfection of
let-7c agomir into SKOV3 and ES2 cells, it was revealed that the OC
cell proliferation was decreased; however, the apoptotic rate was
increased, suggesting that let-7ca is able to inhibit the
proliferation of OC, consistent with its biological effects in
other tumors.
Bioinformatics analysis revealed that CDC25a may be
a potential target site of let-7c. The CDC25 gene can express CDC25
phosphatase protein, a cell cycle regulatory protein which serves a
role in the normal cell cycle (33).
In a series of human malignancies including hepatocellular
carcinoma, ovarian cancer, colorectal cancer, esophageal cancer and
non-Hodgkin's lymphoma, CDC25a exhibits increased expression levels
(17–21). CDC25a exhibits pro-cancer traits in
two distinct manners. First, it is able to promote cell
proliferation (34). When cells enter
S phase, CDC25a activates the cyclin E-cyclin dependent kinase-2
(CDK2) and cyclin A-CDK2 complexes by dephosphorylation, thus
promoting cells to enter S phase (35). If the CDC25a protein is overexpressed,
it may cause cells to rapidly enter S phase from G phase, followed
by an increase in DNA synthesis, malignant cell proliferation or
even development of cancer (36).
Secondly, CCD25a is considered to be a checkpoint gene, and acts
simultaneously on two checkpoints, the G1/S phase and
the G2/M phase, thus serving roles in DNA damage and
repair (37). Therefore, the
overexpression of CDC25a may lead to disorders of cell cycle
regulation and decreased response to DNA damage, so abnormal cell
proliferation or even tumors may be observed as a result of CDC25a
overexpression.
Western blotting revealed that the overexpression of
let-7c may lead to the downregulation of CDC25a protein in OC
cells. CDC25a 3′UTR was then cloned into the dual-luciferase
reporter vector pmirGLO, and the results revealed that let-7c is
able to bind to the CDC25a 3′UTR seed region, thus negatively
regulating its expression. The overexpression of CDC25a revealed by
the recovery assay may restore the pro-apoptotic effects of let-7c
towards OC cells. This further demonstrates that let-7c may bind to
CDC25a mRNA 3′UTR, thus regulating its expression, and inhibiting
the proliferation and inducing the apoptosis of OC cells.
Therefore, CDC25a may possibly act as a target gene of let-7c, thus
regulating the malignant proliferation of OC cells.
The development of OC is regulated by a complex
network of numerous cytokines, enzymes and genes, among which
let-7c serves a role as a tumor suppressor, thus affecting the
development, as well as staging and treatment, of tumors. Previous
research has also demonstrated that let-7c may bind to several
oncoproteins and several key regulatory factors involved in the
mitotic pathway and regulate their expression (38). CDC25a is only one target gene of
let-7c (16,39). The activities of CDC25a are regulated
by multiple mechanisms, including the ubiquitin ligase
anaphase-promoting complex/cyclosome (40) and transforming growth factor β
(41). Therefore, the present study
demonstrated only that let-7c may target CDC25a, thus inhibiting
the proliferation of OC cells; further research into other
biological functions of let-7c is required.
The abnormal expression of let-7c in OC tissue is
associated with the proliferation of OC; let-7c may act on CDC25a
3′UTR and inhibit its expression, thus serving a similar role to a
tumor suppressor gene. Further in-depth studies into the target
genes and mechanisms of let-7c may reveal its potential as a novel
diagnostic and therapeutic target in OC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ designed the research. WG, WZ, QZ, ZB and JC
conducted the experiments. TJ TC, DL and CL analyzed the data. The
manuscript was drafted by WG and WZ. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Third Affiliated Hospital of Zhengzhou University and written
informed consent was obtained from all participants.
Patient consent for publication
All participants provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salani R and Bristow RE: Surgical
management of epithelial ovarian cancer. Clin Obstet Gynecol.
55:75–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sokol NS: Small temporal RNAs in animal
development. Curr Opin Genet Dev. 22:368–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Contreras J and Rao DS: MicroRNAs in
inflammation and immune responses. Leukemia. 26:404–413. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruan K, Fang X and Ouyang G: MicroRNAs:
Novel regulators in the hallmarks of human cancer. Cancer Lett.
285:116–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roush S and Slack FJ: The let-7 family of
microRNAs. Trends Cell Biol. 18:505–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan Y, Zhang F, Fan Q, Li X and Zhou K:
Breast cancer-specific TRAIL expression mediated by miRNA response
elements of let-7 and miR-122. Neoplasma. 61:672–679. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai CH, Lin LT, Wang CY, Chiu YW, Chou
YT, Chiu SJ, Wang HE, Liu RS, Wu CY, Chan PC, et al:
Over-expression of cofilin-1 suppressed growth and invasion of
cancer cells is associated with up-regulation of let-7 microRNA.
Biochim Biophys Acta. 1852:851–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Kelnar K, Vlassov AV, Brown D, Wang
J and Tang DG: Distinct microRNA expression profiles in prostate
cancer stem/progenitor cells and tumor-suppressive functions of
let-7. Cancer Res. 72:3393–3404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie K, Liu J, Zhu L, Liu Y, Pan Y, Wen J,
Ma H, Zhai X and Hu Z: A potentially functional polymorphism in the
promoter region of let-7 family is associated with survival of
hepatocellular carcinoma. Cancer Epidemiol. 37:998–1002. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang L, Cui X, Zhang Y, Yang C and Jiang
Y: Identification of miRNAs associated with sexual maturity in
chicken ovary by Illumina small RNA deep sequencing. BMC Genomics.
14:3522013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Helland Å, Anglesio MS, George J, Cowin
PA, Johnstone CN, House CM, Sheppard KE, Etemadmoghadam D, Melnyk
N, Rustgi AK, et al: Deregulation of MYCN, LIN28B and LET7 in a
molecular subtype of aggressive high-grade serous ovarian cancers.
PLoS One. 6:e180642011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu X, Wu L, Yao J, Jiang H, Wang Q, Yang
Z and Wu F: MicroRNA let-7c inhibits cell proliferation and induces
cell cycle arrest by targeting CDC25A in human hepatocellular
carcinoma. PLoS One. 10:e01242662015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Broggini M, Buraggi G, Brenna A, Riva L,
Codegoni AM, Torri V, Lissoni AA, Mangioni C and D'Incalci M: Cell
cycle-related phosphatases CDC25A and B expression correlates with
survival in ovarian cancer patients. Anticancer Res. 20:4835–4840.
2000.PubMed/NCBI
|
|
18
|
Rodrigues S, Rodrigue CM, Attoub S, Fléjou
JF, Bruyneel E, Bracke M, Emami S and Gespach C: Induction of the
adenoma-carcinoma progression and Cdc25A-B phosphatases by the
trefoil factor TFF1 in human colon epithelial cells. Oncogene.
25:6628–6636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishioka K, Doki Y, Shiozaki H, Yamamoto
H, Tamura S, Yasuda T, Fujiwara Y, Yano M, Miyata H, Kishi K, et
al: Clinical significance of CDC25A and CDC25B expression in
squamous cell carcinomas of the oesophagus. Br J Cancer.
85:412–421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aref S, Fouda M, El-Dosoky E, Menessy A,
Mabed M, Saleeb M and Zalata K: c-Myc oncogene and Cdc25A cell
activating phosphatase expression in non-Hodgkin's lymphoma.
Hematology. 8:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Molinari M, Mercurio C, Dominguez J,
Goubin F and Draetta GF: Human Cdc25A inactivation in response to S
phase inhibition and its role in preventing premature mitosis. EMBO
Rep. 1:71–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Resnick KE, Alder H, Hagan JP, Richardson
DL, Croce CM and Cohn DE: The detection of differentially expressed
microRNAs from the serum of ovarian cancer patients using a novel
real-time PCR platform. Gynecol Oncol. 112:55–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katz B, Tropé CG, Reich R and Davidson B:
MicroRNAs in ovarian cancer. Hum Pathol. 46:1245–1256. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langhe R: microRNA and ovarian cancer. Adv
Exp Med Biol. 889:119–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Volinia S, Bonome T, Calin GA,
Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K,
et al: Genomic and epigenetic alterations deregulate microRNA
expression in human epithelial ovarian cancer. Proc Natl Acad Sci
USA. 105:7004–7009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han HB, Gu J, Zuo HJ, Chen ZG, Zhao W, Li
M, Ji DB, Lu YY and Zhang ZQ: Let-7c functions as a metastasis
suppressor by targeting MMP11 and PBX3 in colorectal cancer. J
Pathol. 226:544–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao B, Han H, Chen J, Zhang Z, Li S, Fang
F, Zheng Q, Ma Y, Zhang J, Wu N and Yang Y: MicroRNA let-7c
inhibits migration and invasion of human non-small cell lung cancer
by targeting ITGB3 and MAP4K3. Cancer Lett. 342:43–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang PY, Sun YX, Zhang S, Pang M, Zhang
HH, Gao SY, Zhang C, Lv CJ and Xie SY: Let-7c inhibits A549 cell
proliferation through oncogenic TRIB2 related factors. FEBS Lett.
587:2675–2681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kristjánsdóttir K and Rudolph J: Cdc25
phosphatases and cancer. Chem Biol. 11:1043–1051. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu X, Yamamoto H, Sakon M, Yasui M, Ngan
CY, Fukunaga H, Morita T, Ogawa M, Nagano H, Nakamori S, et al:
Overexpression of CDC25Aphosphatase is associated with hypergrowth
activity and poor prognosis of human hepatocellular carcinomas.
Clin Cancer Res. 9:1764–1772. 2003.PubMed/NCBI
|
|
35
|
Blomberg I and Hoffmann I: Ectopic
expression of Cdc25A accelerates the G(1)/S transition and leads to
premature activation of cyclin E- and cyclin A-dependent kinases.
Mol Cell Biol. 19:6183–6194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao Z, Chen Z, Gunasekera AH, Sowin TJ,
Rosenberg SH, Fesik S and Zhang H: Chk1 mediates S and G2 arrests
through Cdc25A degradation in response to DNA-damaging agents. J
Biol Chem. 278:21767–21773. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ray D and Kiyokawa H: CDC25A levels
determine the balance of proliferation and checkpoint response.
Cell Cycle. 6:3039–3042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo R, Abdelmohsen K, Morin PJ and Gorospe
M: Novel MicroRNA reporter uncovers repression of Let-7 by GSK-3β.
PLoS One. 8:e663302013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhan M, Qu Q, Wang G, Liu YZ, Tan SL, Lou
XY, Yu J and Zhou HH: Let-7c inhibits NSCLC cell proliferation by
targeting HOXA1. Asian Pac J Cancer Prev. 14:387–392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Donzelli M, Squatrito M, Ganoth D, Hershko
A, Pagano M and Draetta GF: Dual mode of degradation of Cdc25 A
phosphatase. EMBO J. 21:4875–4884. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ray D, Terao Y, Nimbalkar D, Chu LH,
Donzelli M, Tsutsui T, Zou X, Ghosh AK, Varga J, Draetta GF and
Kiyokawa H: Transforming growth factor beta facilitates
beta-TrCP-mediated degradation of Cdc25A in a Smad3-dependent
manner. Mol Cell Biol. 25:3338–3347. 2005. View Article : Google Scholar : PubMed/NCBI
|