Introduction
Human bladder cancer is the one of the most common
neoplasms among urological malignancies, and it is a serious public
health concern worldwide (1).
Chemotherapy, such as Bacillus Calmete-Guerin (BCG) and doxorubicin
therapy, is currently the standard treatment regimen for patients
suffering from bladder cancer (2,3). Bladder
cancer is an aggressive tumor which is always resistant to
chemotherapy, and the current chemotherapeutic drugs are still not
safe enough for patients with bladder cancer because of their
toxicity to normal bladder tissue. Thus, the overall survival rate
remains poor because of recurrence and metastases despite the use
of multimodal therapy (4).
β-Elemene
(β-1-1methyl-1-vinyl-2,4-di-isopropenyl-cyclohexane) is derived
from the essential oil of the Chinese traditional herbal medicine
Curcuma aromatica salisb (5).
β-elemene, which is considered to be a national class II
noncytotoxic antitumor agent in China (6), has been shown to have a variety of
pharmacological effects on multiple types of malignant cells
(7). Moreover, it can enhance the
immunogenicity of tumor cells and improve systematic immunity
(8). β-Elemene has drawn a great deal
of attention because of its small number of side effects and its
bone marrow protection properties (9).
Apoptosis is one of the most important mechanisms
that regulates biological processes, and dysregulation of apoptosis
is associated with a variety of human diseases such as cancer,
infections, and neurodegenerative diseases (10). Apoptosis is the most common and
important mechanism initiated by current chemotherapeutic
compounds. The AKT pathway is implicated to be one of the most
important pathways for cell survival and inhibition of apoptosis.
AKT regulates the process of cell survival by phosphorylating
different substrates that directly or indirectly regulate the
apoptotic program, It has been shown that AKT could regulate
various cellular processes, such as cell apoptosis, survival and
angiogenesis (11). The inactivation
of phosphorylated-AKT (pAKT) has been reported to induce tumor cell
apoptosis (12). Phosphatase and
tensin homolog deleted on chromosome ten (PTEN) is a tumour
suppressor gene that has been shown to be inactivated or to exhibit
some level of functional abnormality in various cancers including
glioma, prostate carcinoma and renal carcinoma (13). PTEN can inhibit the expression of pAKT
which plays an extremely important role in regulating the AKT
pathway (14). and its inactivation
results in the up-regulation of pAKT (15). Moreover, the inactivation of pAKT has
been reported to be one of the key mechanisms leading to apoptosis
induced by a number of antitumor drugs (16).
According to the description given above, we
hypothesized that β-elemene induces its antitumor effect through
the AKT pathway. To investigate the mechanism, we first detected
the expression of PTEN and pAKT in human bladder cancer and normal
tissues and then observed the antitumor effects of β-elemene on
bladder cell proliferation, apoptosis, invasion and migration.
Finally, we verify whether β-elemene has antitumor effects via
regulation of the PTEN-AKT pathway.
Materials and methods
Materials
β-elemene was obtained from Huali JinGang
Pharmaceutical Co., Ltd. (Dalian, China). Human bladder cancer T24
and 5637 cells were purchased from the cell library of the Chinese
Academy of Sciences (Shanghai, China), and normal bladder SV-HUC-1
cells from Saibaikang Biotechnology Co., Ltd. (Shanghai, China).
Antibodies against PTEN, pAKT, AKT, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), and peroxidase-labelled anti-rabbit IgG were
purchased from Abcam (Cambridge, UK). The fetal bovine serum (FBS),
Roswell Park Memorial Institute-1640 (RPMI-1640) and Dulbecco's
modified Eagle's medium (DMEM) were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture
The primary bladder cancer (PBC) cells were derived
from the human bladder cancer tissues of the patients in the
Department of Urology, Affiliated Hospital of Nantong University
(Nantong, China). All of the tissues obtained from patients were
approved by the ethics committee of the Affiliated Hospital of
Nantong University and written informed consent was obtained from
all patients. The human bladder cancer T24 cells, 5637 cells, and
PBC cells were propagated in DMEM nutrient mixture supplemented
with 10% FBS, and SV-HUC-1 cells were propagated in RPMI-1640 with
10% FBS. All of the cell lines were cultured at a 37°C humidified
atmosphere with 5% CO2 and 95% air. We replaced the
culture medium with fresh DMEM or RPMI-1640 that contain 10% FBS
after 24 h. Since then, each cell lines were cultured following
routine laboratory procedure for subsequent experiments.
Cell survival assay
The cytotoxic effects of β-elemene on the T24, 5637,
PBC and SV-HUC-1 cells were detected using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In brief, T24 and PBC cells were seeded in 96-well plates
(Corning Incorporated, Corning, NY, USA) at a density of
1×104 cells/well with fresh complete culture medium for
24 h. Then, both groups of cells were treated with β-elemene at a
concentration of 0, 10, 20, 50, 100, 200 or 500 µg/ml and incubated
for 24 and 48 h. The growth of both cell lines was determined by
adding 10 µl MTT to each well. After incubation for 4 h, 150 µl
dimethyl sulphoxide (DMSO) was added to each well and shaken to
dissolve the crystal. The absorbance was read at a wavelength of
490 nm in a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The cell survival percentage was calculated
using the following formula: cell survival (%)=100% × experimental
well/untreated control well.
Assessment of apoptosis
Apoptosis was detected in both cell lines using the
Annexin V assay. After T24, 5637 and PBC cells were treated with 43
µg/ml β-elemene or saline control for 24 h. Both cell lines were
harvested with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA)
and washed with PBS. Then, 5 µl of Annexin V-PE and 7-AAD (Sheng
Nuokang Biotechnology Company, Jiangsu, China) was added to each
well for 15 min at room temperature according to the manufacturer's
instructions. The cell concentration was 1×106 cells/ml.
Apoptotic cell death was measured using Annexin V-PE and 7-AAD
double staining with a FACSCalibur Flow Cytometer and Cellquest
software (BD Biosciences, Franklin Lakes, NJ, USA).
Protein extraction and western blot
analysis
Fresh bladder cancer tissue was washed with ice-cold
phosphate-buffered solution (PBS) and cut into small pieces then 1
ml RIPA Lysis Buffer and 10 µl phenylmethylsulfonyl fluoride (PMSF)
were added. After incubation for 30 min, the cellular extracts were
clarified by centrifugation at 12,000 × g at 4°C for 20 min. T24
and 5637 cells were harvested after β-elemene treatment at a
concentration of 0–43 µg/ml for 48 h and then washed in PBS. Next,
200 µl RIPA Lysis Buffer and 2 µl PMSF were added, and cells were
lysed on ice for 20 min. Supernatant fluids were collected after
centrifugation for 15 min. Both protein concentrations were
measured using the Bradford assay. Protein lysates were separated
by electrophoresis on 10% SDS-polyacrylamide gel and transferred to
a PVDF membrane (1:2,000; Beyotime Institute of Biotechnology,
Haimen, China). After soaking in blocking buffer for 2 h, the
membranes were incubated with PTEN (1:500, 32199; Abcam, Cambridge,
MA, USA) and AKT (1:500, 64148, 38449; Abcam) monoclonal antibody
overnight at 4°C. Next, we incubated with an HRP-conjugated
secondary antibody (1:1,000, 21058; Abcam) at 37°C for 1 h. All
Western bands were quantified using ECL detection (Eastman Kodak,
Rochester, NY, USA), and the results were visualized by Image J
(National Institutes of Health, Bethesda, MD, USA). GAPDH was
detected as a gel loading control. All western bands were
quantified using densitometry and presented in the form of bar
graphs.
In vitro cell invasion and migration
assays
24-well Transwell plates with 8-µm polycarbonate
filters were used for the cell invasion assay. T24 and 5637 bladder
cells were seeded in serum-free DMEM with or without β-elemene
(0–43 µg/ml) with a membrane coated with Matrigel basement membrane
matrix (100 µg/cm2). DMEM with 10% serum was added to
the bottom of the plates. To observe the changes of motility
better, after 48 h (17), the cells
that had not invaded through the membrane were removed, and the
invasive cells on the lower surface were fixed in 4%
paraformaldehyde for 20 min before staining with crystal violet
(0.1%) for 10 min. The cells that invaded to the lower side were
counted with an Olympus IX71 microscope (×100). In the wound
healing assay, T24 bladder cells were cultured in 6-well plates and
a scratch was made in the monoculture using a 100-µl pipette tip.
Three reference marks were made in each well. The distances were
measured at 0 and 48 h post-wounding by light-phase photographs
(×40).
Statistical data analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation (SD). The
Student's t-test and one-way ANOVA were used to determine the
statistical significance between the different experimental groups
and the control group. Student-Newman-Keuls test was used as post
hoc test after ANOVA. All data were analysed using SPSS 20.0 (IBM
Corp., Armonk, NY, USA) and GraphPad prism software (v.6; GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
PTEN is downregulated and pAKT is
over-expressed in human bladder cancer
To determine the role of PTEN and AKT in bladder
cancer progression, we used Western blot analysis to examine PTEN
and AKT expression levels in bladder cancer tissues and compared
them to normal tissues. The results shown in Fig. 1A and B indicate results revealed that
PTEN protein levels were down-regulated in bladder cancers relative
to adjacent normal tissues, while the pAKT level was overexpressed.
These results indicate that the decreased PTEN expression and
increased pAKT expression may be related to bladder cancer
progression.
β-elemene suppresses the survival of
bladder cancer cells
The human bladder cancer cells T24, 5637, PBC cell
lines and normal human bladder cell line SV-HUC-1 were used to
investigate the effect of β-elemene on bladder cancer cell growth
and proliferation using the MTT assay. As shown in Fig. 2A and B, β-elemene inhibited the
survival of the T24 and 5637 bladder cancer cell lines, and the
magnitude of inhibition increased in a dose-dependent manner. To
further assess the anti-cancer activity of β-elemene on bladder
cancer, we used primary cancer cells (PBC) derived from patient
with bladder cancer, and the results were same as the former two
bladder cancer cells. β-Elemene exerted dramatic cytotoxicity on
the three bladder cancer cell lines at 10 µg/ml in 48 h, and the
inhibitory rate was >90% at a concentration of 100 µg/ml or
higher in both 24 and 48 h. However, no matter 24 or 48 h, the
results revealed no significant sensitivity to β-elemene in normal
human bladder cells SV-HUC-1. As shown in Fig. 2C and D, the average half-maximal
inhibitory concentration (IC50) of β-elemene was 43±5.9
µg/ml for T24 cells, 47±7.1 µg/ml for 5,367 cells and 33.5±5.9
µg/ml for PBC cells at 24 h, and the magnitude of the
IC50 decreased at 48 h was 21.5±7.6 µg/ml for T24 cells,
23.75±6.3 µg/ml for 5,367 cells and 19.5±5.5 µg/ml for PBC cells.
According to the data from MTT assay, the IC50 values of
β-elemene on SV-HUC-1 were much higher than these three bladder
cancer cells in both 24 and 48 h. These data showed that β-elemene
effectively inhibits the growth and proliferation of bladder cancer
cells without significant cytotoxicity to normal cells.
β-elemene induces apoptosis in human
bladder cancer cells
To investigate the mechanism of β-elemene's
inhibitory activity on the growth of bladder cancer, we first
performed flow cytometry to detect apoptotic cells based on Annexin
V staining on the outer cytoplasmic membrane to identify apoptotic
cells. As shown in Fig. 3A and B, the
ratio of apoptosis was significantly higher in T24, 5637 and PBC
cells treated with β-elemene than in untreated cells in 24 h. In
contrast, no significant changes were observed in SV-HUC-1 cells.
These results indicated that β-elemene induced apoptosis in bladder
cancer cells, but not in normal bladder cells.
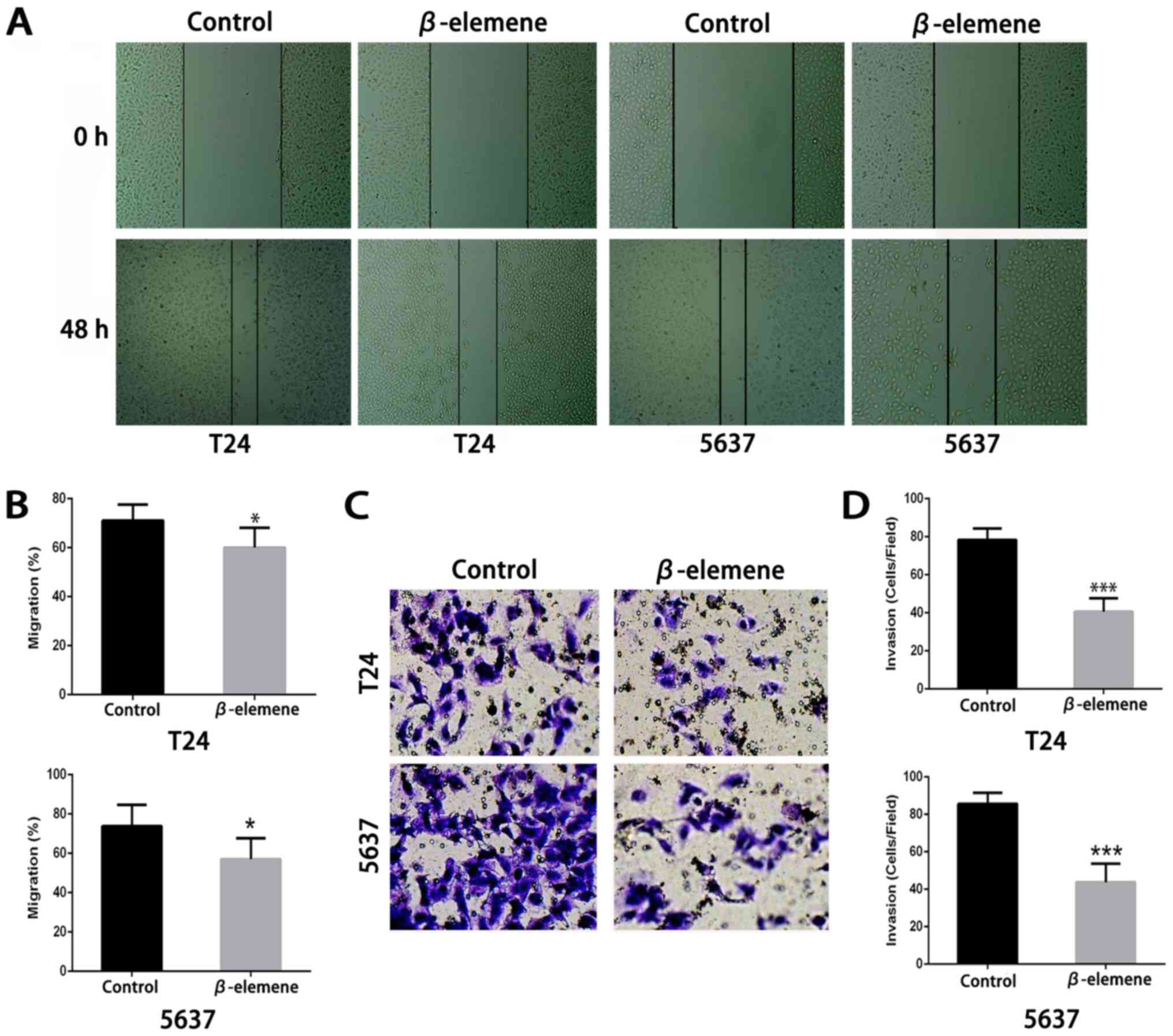
β-elemene changes the motility of
bladder cancer cells
To further study the changes of mobility in bladder
cancer cells after β-elemene treatment, we used the wound healing
assay and transwell assay to detect cell migration and changes in
invasion. As shown in Fig. 4A and B,
in both types of bladder cancer cells, β-elemene (approximately 10
µg/ml) could significantly restrain bladder cancer cells migration
compared with the controls. In the Matrigel invasion assay, the
percentage of invading T24 and 5637 cells significantly decreased
after β-elemene treatment (Fig. 4C and
D). These results suggested that β-elemene could negatively
regulate bladder cancer cell motility by weakening bladder cancer
cell migration and invasion.
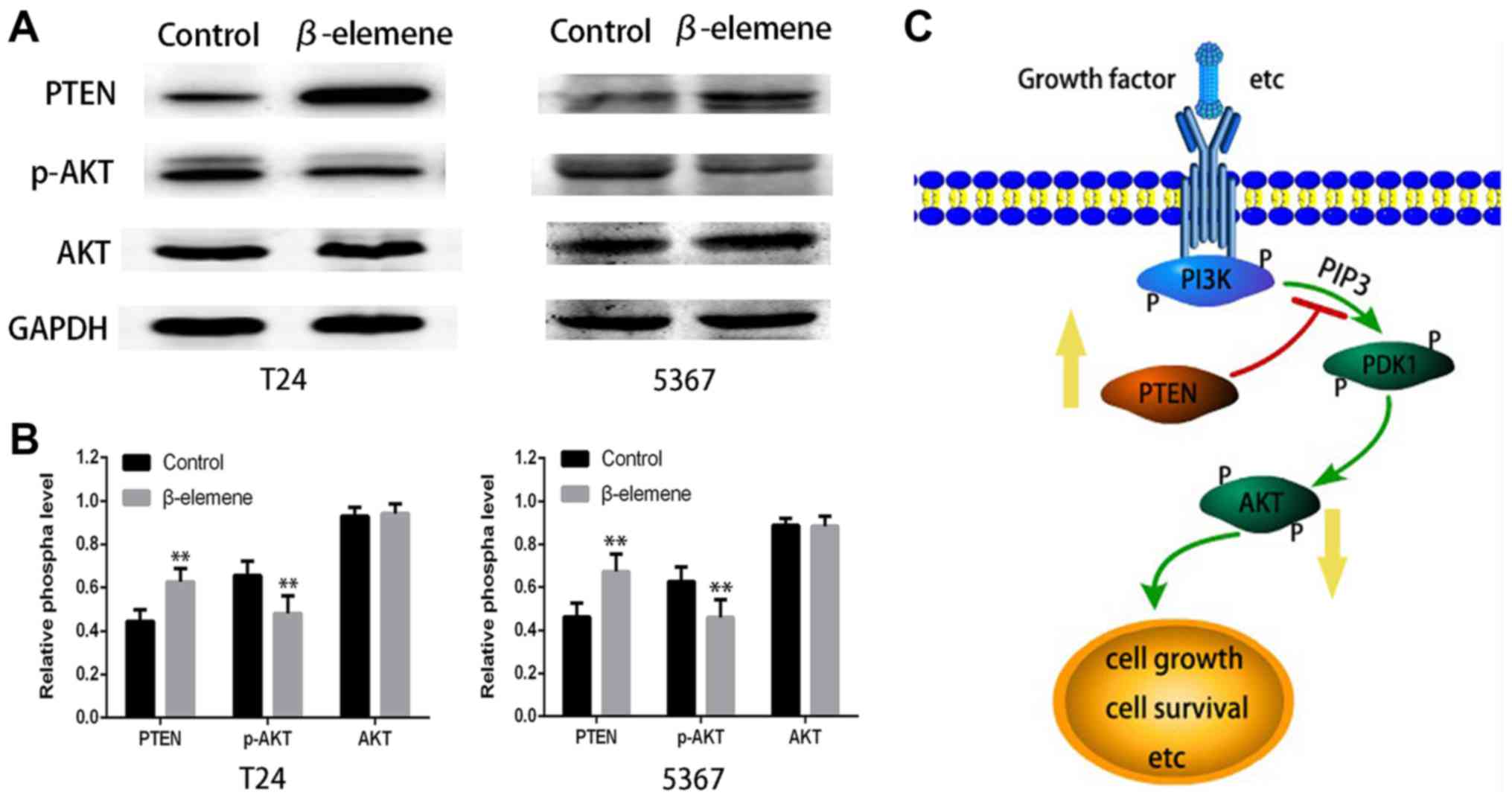
β-elemene enhances PTEN and inhibits
pAKT activation
To further investigate the mechanism underlying
β-elemene's anticancer effect, we performed Western blot analysis
to detect changes in AKT pathway activation, which plays an
important role in regulating cell survival and apoptosis. As shown
in Fig. 5A and B, AKT phosphorylation
was greatly decreased in β-elemene-treated T24 and 5637 cells
compared with both control groups in 24 h. Moreover, there was
significantly more PTEN expression than in controls. However, there
was no effect on the expression of total AKT in both bladder cancer
cells. The schematic of the study design is shown in Fig. 5C. These results indicated that
β-elemene induced an anticancer effect in bladder cancer through
upregulation of PTEN and suppression of AKT phosphorylation.
Discussion
The development of bladder cancer is complicated,
involving multiple genes, processes and stages. Abnormality in
apoptosis correlates is closely correlated with bladder cancer
progression. Chemotherapeutic agents often kill tumor cells by
promoting apoptosis, and the AKT signalling pathway has been shown
to be one of the most important pathways for tumor cell
proliferation and apoptosis (18).
PTEN appears to regulate the AKT signalling pathway by inhibiting
the activation of AKT, leading to the inhibition of bladder cancer
cell growth in vitro (15). In
this study, we found PTEN protein levels to be down-regulated and
pAKT to be over-expressed in bladder cancers relative to adjacent
normal tissues, which indicate that the decreased PTEN expression
and increased pAKT expression may be related to bladder cancer
progression.
β-elemene, a natural active element extracted from
Curcuma aromatica salisb, has many diverse functions (19). An emulsion form of β-elemene has been
applied as a class II noncytotoxic antitumor agent in China
(20). The major advantages of
β-elemene as an anticancer agent are that it has antitumor activity
toward a broad spectrum of cancer types, including lung cancer,
brain tumors and malignancies of the alimentary tract, and it is
associated with a low level of toxicity which is well-tolerated by
patients with cancer (21–23). Consistent with other reports, this
study showed that β-elemene exhibits high levels of anticancer
activity on T24, 5637 and PBC bladder cancer cells by suppressing
survival and promoting apoptosis compared with SV-HUC-1 normal
bladder cells. In addition, we also found β-elemene could weaken
the bladder cancer cells migration and invasion.
However, the mechanism by which β-elemene inhibits
tumors is not fully clear although it has been studied for years.
The ability of β-elemene to regulate signaling pathways has
attracted much attention recently (24). The TGF-β pathway and AKT pathway have
drawn special attention (18). Lu
et al reported that β-elemene could inhibit the
proliferation of T24 bladder cancer cells through up-regulation of
the expression of Smad4 (25). It has
also been demonstrated that the inhibition of pAKT can induce tumor
cell apoptosis, pAKT and its downstream targets are thought to be
associated with the development of drug resistance (14). The AKT pathway is frequently activated
in human bladder cancer cells, and activation of AKT is associated
with anti-apoptosis, cell proliferation and cellular energy
metabolism effects. Tanaka and Grossman found that PTEN gene
therapy can suppress bladder cancer cell growth by down-regulating
pAKT (26). For this reason,
inhibition of the AKT pathway may be as a promising strategy for
cancer treatment, because apoptosis induction is one of the major
mechanisms by which natural compounds, such as curcumin and
chorophyllin, exert their anti-tumor activity on bladder cancer
cells. Lin et al demonstrated that baicalin induced
apoptotic cell death through inhibition of the AKT signal pathway
in human bladder cancer cells (27).
And Zhao et al observed that altholactone down-regulates the
expression of the anti-apoptotic protein pAKT in T24 cells
(28).
In this work, further experiments showed that the
inactivation of the AKT pathway is closely associated with the
effect of β-elemene on bladder cancer cells. The data gathered from
T24 and 5637 bladder cancer cells with treated β-elemene revealed
that the expression of PTEN was increased, and AKT phosphorylation
was suppressed. Taken together, Theses promising results suggested
that β-elemene induced anticancer effects in bladder cancer
occurred through up-regulation of PTEN and suppression of AKT
phosphorylation. However further investigation is needed to
validate how β-elemene leads to inactivation of AKT.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81141056).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
BC conceived and designed the experiments, and
contributed to the cell culture, cell survival assay, assessment of
apoptosis and manuscript preparation. LM contributed to the
collection data and statistical analysis in this work. SN, YW and
XG contributed to purchase reagents and materials, protein
extraction, Western blot analysis, cell invasion and migration
assays. JP contributed to the conception, design and suggestion of
the study, approved the final version and other routine work.
Ethics approval and consent to
participate
This work was approved by the Ethics Committee of
the Affiliated Hospital of Nantong University. Written informed
consent was obtained from patients.
Patient consent for publication
Patients consented to the publication of this
work.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaprin AD, Kostin AA, Rapoport LM,
Tsarichenko DG, Bernikov AN, Vorob'ev NV and Golovashchenko MP:
Cancer of urinary bladder. Urologiia. 6 Suppl:S64–S88. 2016.(In
Russian).
|
|
2
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmstrom PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lan Y, Liu D and Lin M: Comparison of the
combination therapy of bacillus Calmette-Guerin and mitomycin C
with the monotherapy for non-muscle-invasive bladder cancer: A
meta-analysis. Neoplasma. 63:967–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krasnow RE, Drumm M, Roberts HJ, Niemierko
A, Wu CL, Wu S, Zhang J, Heney NM, Wszolek MF, Blute ML, et al:
Clinical outcomes of patients with histologic variants of
urothelial cancer treated with trimodality bladder-sparing therapy.
Eur Urol. 72:54–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding XF, Shen M, Xu LY, Dong JH and Chen
G: 13,14-bis(cis-3,5-dimethyl-1-piperazinyl)-β-elemene, a novel
β-elemene derivative, shows potent antitumor activities via
inhibition of mTOR in human breast cancer cells. Oncol Lett.
5:1554–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu Z, Wu H, Li Y, Hou Q, Wang Y, Li S, Xia
B and Wu S: β-Elemene inhibits the proliferation of esophageal
squamous cell carcinoma by regulating long noncoding RNA-mediated
inhibition of hTERT expression. Anticancer Drugs. 26:531–539. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou S, Wang C, Cui Z, Guo P, Meng Q, Shi
X, Gao Y, Yang G and Han Z: β-Elemene induces apoptosis of human
rheumatoid arthritis fibroblast-like synoviocytes via reactive
oxygen species-dependent activation of p38 mitogen-activated
protein kinase. Pharmacol Rep. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen JC, Duan WL, Bai RR, Yao HQ, Wu XM,
Shang J and Xu JY: Synthesis of 13-β-elemene ester derivatives and
evaluation of their antioxidant activity in human umbilical vein
endothelial cells. Chin J Nat Med. 13:618–627. 2015.PubMed/NCBI
|
|
9
|
Hong L, Zeng Y and Yang D: Inhibitory
effect of β-elemene on human airway granulation tissue in vivo and
in vitro. Respiration. 92:329–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai H, Li H, Li W, Gui T, Yang J, Cao D
and Shen K: The PI3K/AKT/mTOR pathway is a potential predictor of
distinct invasive and migratory capacities in human ovarian cancer
cell lines. Oncotarget. 6:25520–25532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olasz J, Doleschall Z, Dunai Z, Pazsitka A
and Csuka O: PI3K/AKT pathway activation and therapeutic
consequences in breast cancer. Magy Onkol. 59:346–351. 2015.(In
Hungarian). PubMed/NCBI
|
|
12
|
Brotelle T and Bay JO: PI3K-AKT-mTOR
pathway: Description, therapeutic development, resistance,
predictive/prognostic biomarkers and therapeutic applications for
cancer. Bull Cancer. 103:18–29. 2016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rana C, Piplani H, Vaish V, Nehru B and
Sanyal SN: Downregulation of PI3-K/Akt/PTEN pathway and activation
of mitochondrial intrinsic apoptosis by Diclofenac and Curcumin in
colon cancer. Mol Cell Biochem. 402:225–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chun SH, Jung CK, Won HS, Kang JH, Kim YS
and Kim MS: Divergence of P53, PTEN, PI3K, Akt and mTOR expression
in tonsillar cancer. Head Neck. 37:636–643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim HJ, Crowe P and Yang JL: Current
clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment
of human cancer. J Cancer Res Clin Oncol. 141:671–689. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chin CC, Li JM, Lee KF, Huang YC, Wang KC,
Lai HC, Cheng CC, Kuo YH and Shi CS: Selective β2-AR blockage
suppresses colorectal cancer growth through regulation of
EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J Cell
Physiol. 231:459–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Dai B, Zhang H, Shi G, Shen Y and
Ye D: Long non-coding RNA LOC572558 inhibits bladder cancer cell
proliferation and tumor growth by regulating the AKT-MDM2-p53
signaling axis. Cancer Lett. 380:369–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Wang T, Xu S, Zhang P, Lin A, Wu
L, Yao H, Xie W, Zhu Z and Xu J: Discovery of novel antitumor
nitric oxide-donating β-elemene hybrids through inhibiting the
PI3K/Akt pathway. Eur J Med Chem. 135:414–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu X, Xu M, Li N, Li Z, Li H, Shao S, Zou
K and Zou L: β-elemene inhibits tumor-promoting effect of M2
macrophages in lung cancer. Biochem Biophys Res Commun.
490:514–520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mu L, Wang T, Chen Y, Tang X, Yuan Y and
Zhao Y: β-Elemene enhances the efficacy of gefitinib on
glioblastoma multiforme cells through the inhibition of the EGFR
signaling pathway. Int J Oncol. 49:1427–1436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Zhang HD, Yao YF, Zhong SL, Zhao
JH and Tang JH: β-elemene reverses chemoresistance of breast cancer
cells by reducing resistance transmission via exosomes. Cell
Physiol Biochem. 36:2274–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Wang R, Wang T, Ding Q, Khalil A,
Xu S, Lin A, Yao H, Xie W, Zhu Z and Xu J: Antioxidant properties
of novel dimers derived from natural β-elemene through inhibiting
H2O2-induced apoptosis. ACS Med Chem Lett.
8:443–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma C, Zhou W, Yan Z, Qu M and Bu X:
β-Elemene treatment of glioblastoma: A single-center retrospective
study. Onco Targets Ther. 9:7521–7526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Wang Y, Luo H, Luo Z, Zhang T,
Yang N, Long X, Xie H, Qiu W, Zhang B, Ding J and Yang L: β-elemene
acts as an antitumor factor and downregulates the expression of
survivin, Bcl-xL and Mta-1. Mol Med Rep. 6:989–995. 2012.PubMed/NCBI
|
|
25
|
Lu X, Wang Y, Luo H, Qiu W, Han H and Chen
X: β-elemene inhibits the proliferation of T24 bladder carcinoma
cells through upregulation of the expression of Smad4. Mol Med Rep.
7:513–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka M and Grossman HB: In vivo gene
therapy of human bladder cancer with PTEN suppresses tumor growth,
downregulates phosphorylated Akt, and increases sensitivity to
doxorubicin. Gene Ther. 10:1636–1642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC,
Lin MW, Hsu YM, Lee MR, Amagaya S, Huang WW, et al: AKT
serine/threonine protein kinase modulates baicalin-triggered
autophagy in human bladder cancer T24 cells. Int J Oncol.
42:993–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao B and Li X: Altholactone induces
reactive oxygen species-mediated apoptosis in bladder cancer T24
cells through mitochondrial dysfunction, MAPK-p38 activation and
Akt suppression. Oncol Rep. 31:2769–2775. 2014. View Article : Google Scholar : PubMed/NCBI
|