Introduction
Thyroid cancer (TC) is one of the most common
malignant endocrine and head-neck tumors (1,2), as well
as a malignant tumor with the most rapid increase in the incidence
rate in recent years, with an annual average incidence rate of 6.2%
(3), showing an increasing trend year
by year (4). Research in China has
indicated that the incidence rate of TC in the country is
approximately 1/100,000-3/100,000, accounting for 1.3–1.5% of that
of all the malignant tumors (5). TC
consists of three major pathological types: Differentiated (DTC),
anaplastic (ATC) and medullary thyroid cancer (MTC), of which more
than 90% of TC belongs to DTC. DTC can be further divided into two
types, namely, papillary thyroid cancer (PTC) and follicular
thyroid cancer (FTC) (6). Among the
four pathological types, PTC is the most common, accounting for
nearly 80% of the total cases of TC (7); in addition, it occurs in a wide age
range, from 10 years to 100 years. With the progress of diagnostic
technology and enhancement of prevention awareness, the incidence
rate of papillary thyroid microcarcinoma (PTMC) is increased
significantly, which has been reported by experts and scholars in
the United States, Denmark, India and Germany (8–11). PTMC
refers to the papillary thyroid carcinoma with a diameter ≤10 mm
(12). It was believed previously
that PTMC has a lower invasiveness of primary lesion (13,14).
However, recent studies have shown that PTMC cases, manifested as
local tissue invasion and cervical (central and lateral cervical
areas) lymph node metastasis, are increasing (15). After sorting out and analyzing
clinical data, the experts found that PTMC is not an early cancer
(16).
During the investigation of tumor occurrence and
development, many experts and scholars agree that tumor is a
multi-stage, multi-factor and multi-gene biological phenomenon.
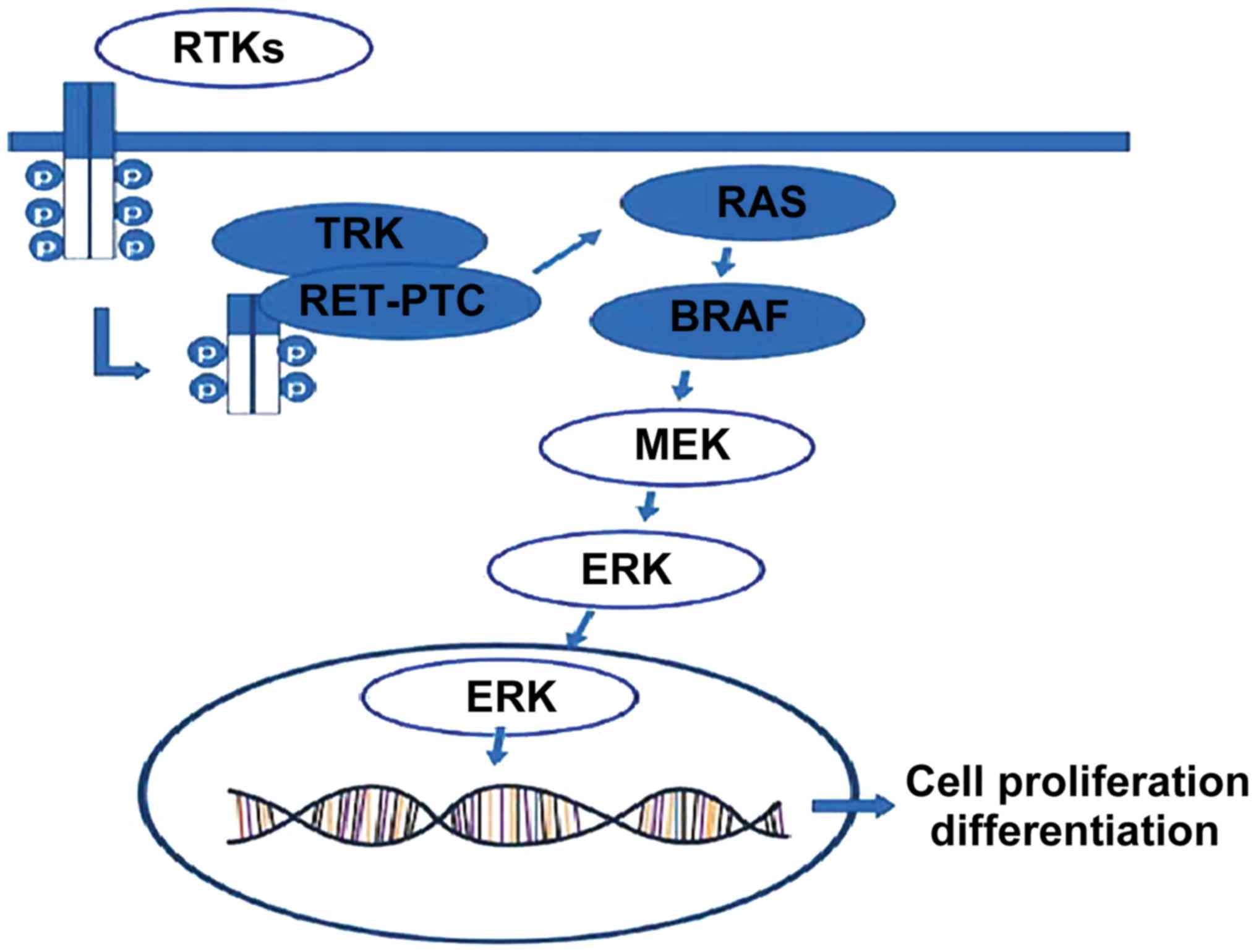
BRAF gene is currently considered as one of the most important
papillary thyroid carcinoma (PTC) driver genes, and a large number
of studies have confirmed that the occurrence of PTC is mainly due
to the activation of RAS-RAF-MEK-MAPK pathway caused by
BRAFV600E mutation, RET/PTC rearrangement and RAS gene
mutation, thus leading to the occurrence of malignant tumors
(17–19) (Fig. 1).
Therefore, the BRAFV600E mutation is closely related to
the invasiveness of PTC, including primary lesion breaking through
the capsule, cervical lymph node metastasis or distant metastasis
and tumor progression (20,21). In recent studies, there have been more
and more studies on BRAF gene mutation and PTC, but few studies on
the association between BRAFV600E mutation and PTMC.
Therefore, the investigation of expression of BRAFV600E
mutation in PTMC and the association analyses combined with its
clinical parameters have important guiding significance in the
clinical diagnosis and treatment of PTMC patients.
Thyroid-stimulating hormone receptor (TSHR) is a
macromolecule glycoprotein, mainly located in the thyroid
follicular epithelial cell membrane, whose main functions are to
regulate and control the growth and differentiation of thyroid
cells. In recent years, studies in China have found that the
primary papillary thyroid carcinoma (PTC) metastasis is accompanied
with the TSHR expression; some thyroid-related diseases are caused
by abnormal TSHR expression (22). It
has been proven that TSHR is completely expressed in normal thyroid
tissues, and that it is expressed for 86.7% in papillary cancer and
23.8% in ATC. Τhe normal thyroid tissues can be distinguished from
PTC with varied differentiations through different TSHR
expressions, of which the expression level is positively correlated
with the degree of differentiation (23,24). Some
scholars measured the expression of TSHR in paraffin-embedded
specimens collected from primary PTC using immunohistochemical
method (AP method), and the results showed that the positive
expression rate of TSHR in highly differentiated PTC is < that
in typical PTC. Therefore, the TSHR expression has a negative
association with the differentiation and invasiveness of PTC
(25). Chen et al measured the
TSHR in PTC and normal thyroid tissues through AP method (22). It was revealed that the positive
expression rate of TSHR in PTC tissues is similar to that in normal
thyroid tissues; in PTC tissues; the TSHR expression level is
related to tumor-node-metastasis (TNM) staging and lymph node
metastasis of the neck, while it is not correlated with sex, age
and other factors. Μeanwhile, TSHR is highly expressed in PTC
patients with high differentiation, low invasiveness and no lymph
node metastasis (26,27). There have been few studies on the TSHR
expression in PTMC.
How are BRAFV600E mutation and TSHR
expression related to PTMC? What are the BRAF gene mutation rate
and TSHR expression in highly invasive PTMC? Can BRAF gene and TSHR
be used as markers for evaluating the PTMC invasion before
operation? In the present study, the tissue specimens and
clinicopathologic data of 162 PTMC patients in the Department of
Thyroid and Breast Surgery of Liaocheng People's Hospital
(Liaocheng, China) were collected and treated. The
BRAFV600E mutation frequency and TSHR expression were
detected via qPCR and immunohistochemical method, and the
association among parameters were studied to find indexes that can
predict the local tissue invasion and cervical lymph node
metastasis potential of PTMC, evaluate the patients before
operation in a comprehensive and scientific manner, select the
reasonable and accurate surgical methods during operation and
provide individualized diagnosis and treatment support for
patients.
Patients and methods
Inclusion criteria
In the present study, TC patients receiving
operation in the Department of Thyroid Breast Surgery of Liaocheng
People's Hospital from September 1, 2015 to August 31, 2016 were
selected. The serum free triiodothyronine (free T3), free thyroxine
(free T4), TSH, thyroid peroxidase antibody (TPOAB) and
thyroglobulin antibody (TGAb) in all patients were measured via
radio-immunity determination after admission, so as to exclude the
effect of PTMC combined with Hashimoto's thyroiditis on the
research results. Objects enrolled had to meet the following
requirements: i) First visit, no history of thyroid-related
diseases and medications due to thyroid diseases; ii) no history of
Grave's disease, no history of present illness or past medical
history of malignant tumors; iii) complete clinical data;
well-preserved paraffin-embedded specimens of thyroid cancer and
cervical lymph nodes in the Pathology Department; iv) total
resection of affected lobes + total resection of contralateral
lobes + central and (or) cervical lymph node dissection performed
in Thyroid Surgery of hospital; v) TC diagnosed via preoperative
fine-needle aspiration pathocytology, intraoperative rapid
pathology and postoperative routine pathology; and iv) not a large
number of calcifications in primary lesions. The study was approved
by the Ethics Committee of Liaocheng People's Hospital and written
informed consents were signed by the patients or the guardians.
During the above period, a total of 610 cases of PTC
treated in thyroid surgery via operation were selected, among which
357 cases had cervical lymph node metastasis. There were a total of
293 cases of PTMC, including 161 cases with cervical lymph node
metastasis. Those patients who were not treated for the first time,
relapsed after TC operation in the past or underwent operation in
our hospital after irregular operation in other hospital (n=57),
without complete clinical parameters or examinations (early cases,
n=19), with malignant tumors in other sites during treatment (n=7),
without complete primary lesion or cervical lymph node specimens of
PTMC (patients receiving TC operation in another hospital, n=24),
whose data were used for consultation and scientific research and
not returned (n=19), and experimental results that could not be
judged (n=5) were eliminated. As a result, a total of 162 cases
were included.
Collection of pathological specimens
and clinical data
Pathological specimens: A total of 162
paraffin-embedded specimens of primary PTMC cases meeting the
requirements were collected. Thirty paraffin-embedded specimens of
thyroid benign lesions and 30 paraffin-embedded specimens of
para-carcinoma normal tissues in patients receiving operative
treatment during the same period were selected as control group,
respectively. All experimental specimens were provided by the
Pathology Department of Liaocheng People's Hospital.
The following clinical parameters of 162 PTMC
patients were recorded: Sex, age, pathological diagnosis, primary
lesion size, primary lesion and cervical lymph node operation
methods, subsequent treatment, tumor diameter, the number of TC
lesions, the number of central and lateral cervical metastatic
lymph nodes, metastasis rate, and primary tumor breaking through
the capsule.
Treatment methods
Treatment principle of primary
lesion
Patients who were diagnosed with single foci or
unilateral multiple tumor via imaging before operation usually
received the total resection of lobes and isthmus on the affected
side. Those with multiple tumor in bilateral lobes (including the
isthmus) were treated with the total resection of double lobes +
isthmus resection.
Treatment principle of cervical lymph
nodes
Frozen biopsy of primary lesion was performed during
operation, and those diagnosed with PTMC were treated with central
(VI area) lymph node dissection at least. According to the
preoperative imaging examination of the lateral cervical lymph node
formation combined with intraoperative exploration, those diagnosed
with lateral cervical (II–IV area) lymph node cN+ were
treated with lateral cervical lymph node dissection.
Test methods
The qualified primary lesion of PTMC, thyroid benign
mass and para-carcinoma normal tissues were taken from the
Pathology Department and cut into 4 µm-thick sections. The primary
lesion was confirmed to be positive. The BRAFV600E gene
detection and immunohistochemical staining of TSHR protein [the
positive pathological section was used as the positive staining
control, and phosphate buffered saline (PBS), instead of the first
antibody, was used as negative control] were performed in the
primary lesion of PTMC, thyroid benign mass and para-carcinoma
normal tissues by senior physicians in the Pathology
Department.
Main steps of gene detection
DNA extraction of paraffin-embedded
tissues
Six to eight pieces of 5–10 µm paraffin-embedded
tissue specimens were taken and placed into a 1.5 ml Eppendorf
tube, added with 1.0 ml xylene, incubated at 60°C for 10 min, and
centrifuged at 12,000 × g at room temperature for 3 min, and the
supernatant was discarded (cross contamination of specimen should
be avoided). Anhydrous ethanol (1.0 ml) was added into the tube,
mixed upside down evenly at room temperature for 10 sec, and
centrifuged at 12,000 × g for 3 min, and the supernatant was
discarded. The above steps were repeated 2 times. The tube cover
was opened, and the remaining anhydrous ethanol was dried at 55°C
cleavage.
A total of 400 µl supporting GT buffer and 20 µl
proteinase K (10 mg/ml) were added into the treated tissues and
mixed evenly. The mixture was digested at 56°C for 90 min or
overnight, until the specimens were completely cleaved. The
specimens were incubated at 90°C for 30 min, followed by high-speed
centrifugation to collect the liquid on the wall. The supernatant
was transferred into the supporting ultrafiltration tube, followed
by centrifugation at 12,000 × g for 5 min. The clean tissue mixture
after centrifugation was transferred into the matching sample tube.
The sample tube was put in place; in the fourth track of frame
using the program code of 401. After the extraction, DNA was placed
in a refrigerator at −20°C for standby application.
qPCR
The reaction mixture was thawed at room temperature,
and mixed evenly on a vortex vibrator for 15 sec, followed by
high-speed centrifugation at 12,000 × g for 15 sec at 4°C. A total
of 35 µl reaction mixture in each tube was mixed with 0.4 µl Taq
enzyme. Reaction mixture with Taq enzyme (35 µl) was placed into
the PCR tube. DNA sample (5 µl) to be tested (with following
concentration), positive and negative control were added into the
PCR tube. The DNA concentration of paraffin sections was
recommended to be 2–3 ng/µl. The PCR tube was centrifuged to gather
the reagent into the bottom of tube, and then the PCR tube was
placed onto the real-time PCR instrument. The setup window was
opened for setting according to the amplification procedure chart
in the picture below. Stage 1: 95°C for 5 min, 1 cycle. Stage 2:
95°C for 25 sec, 64°C for 20 sec, 72°C for 20 sec, 15 cycles. Stage
3: 93°C for 25 sec, 60°C for 20 sec, 72°C for 20 sec, 31 cycles.
Signal collection: FAM and HEX (or VIC) signals were collected in
Stage 3 at 60°C, the qPCR was performed and the files were saved
(Fig. 2).
Main steps of immunohistochemical
method
Each batch of samples was stained in strict
accordance with reagent instructions.
Preparation
Baking
The sections of positive control tissues and tissues
to be tested were baked in an incubator at 68°C for 120 min.
Dewaxing via xylene was carried out: Thick paraffin sections (4 µm)
were dewaxed using grade I, II and III xylene for 5 min. Hydration:
Sections were hydrated using anhydrous ethanol, 95 and 80% ethanol
for 3 min and washed with PBS 3 times (3 min/time).
Antigen retrieval
High-pressure heat retrieval: The slide was placed
on a heat-resistant staining rack, and added with 0.01 M boiled
sodium citrate buffer solution. Τhe stainless steel pressure cooker
lid and pressurizing valve were covered. Αt 2 min after air
injection, the pressure cooker lid was opened, and the slide was
washed with PBS after natural rewarming.
Immunohistochemical staining
Immunohistochemical PAP pen was used to draw circles
at 0.5 cm away from the tissue border. Hydrogen peroxide solution
(3%) was dropwise added onto each slide for incubation at room
temperature for 10 min to block the endogenous peroxidase. Then the
slice was washed with PBS for 3 min × 3 times. PBS was shaken off,
and appropriate amount of primary antibody rabbit polyclonal to
TSHR (ab 202960; 1:1,000; Abcam, Cambridge, MA, USA) was dropwise
added onto each slide in a refrigerator at 4°C for 12 h. Τhen the
slide was washed with PBS for 5 min × 3 times. Appropriate amount
of biotin-labeled goat anti-rabbit immunoglobulin G (IgG) (cat. no.
sc-2004; 1:3,000; Santa Cruz Biotechnology, Inc. Santa Cruz, CA,
USA) was dropwise added onto each slide, followed by incubation at
room temperature for 20 min. Τhen the slide was washed with PBS for
3 min × 3 times. PBS was shaken off, and horseradish
peroxidase-labeled streptavidin working solution was added,
followed by incubation at room temperature for 30 min. Τhen the
slide was washed with PBS for 3 min × 3 times. PBS was shaken off,
and appropriate amount of freshly-prepared diaminobenzidine (DAB)
was dropwise added onto each slide, the color development time was
controlled under the microscope (Olympus Corporation, Tokyo,
Japan). Νo background staining and strong staining in positive
parts prevailed. Βrown staining indicated positive. The slice was
washed with tap water, re-stained using hematoxylin for 5 min,
differentiated via 0.1 ml hydrochloric acid alcohol, and washed
again with tap water, followed by dehydration using 80, 95 and 100%
anhydrous ethanol, and transparent treatment via grade I, II and
III xylene. The slide was sealed using the automatic sealing
machine (Fig. 3).
Determination of results
Determination of gene detection
results
The curves in the saved files were observed. If the
cycle threshold (Cq) value of carboxy fluorescein (FAM) signal of
specimen was ≥28, the sample was negative (or it was lower than the
lower detection limit of kit). If the Cq value of FAM signal was
<28, the sample was positive.
Determination of immunohistochemical
results
Double-blind method was used to determine the
immunohistochemical results. All the experimental results were
interpreted by senior physicians in the Pathology Department, and
no clinical parameters of patients were not provided for
physicians. In principle, the membrane structure after
cancerization will lead to the dispersion and incorrect positioning
of dyeing agent, so the cell membrane staining may also cause
cytoplasmic staining, but it may be more meaningful for the
expression level on the cell membrane. Under an optical microscope
(Olympus Corporation), the brown yellow-stained cell membrane and
cytoplasm indicated positive cells. TSHR protein was mainly located
in the cell capsule, and some were located in the cytoplasm. Cell
membrane and cytoplasm were stained pale yellow, and gradually
became dark brown with the increase in staining strength.
Determination of cell staining
intensity and percentage of positive cells under an optical
microscope (Olympus Corporation)
Scoring criteria of cell staining intensity: no
staining: 0 point, negative (−); pale yellow: 1 point, weakly
positive (+); brown yellow: 2 points, moderately positive (++);
dark brown: 3 points, strongly positive.
Scoring criteria of positive cell
percentage
The experimental specimens were placed under a
high-power microscope (Olympus Corporation), five non-coincident
fields of view (×400) were randomly selected, and 100 cells were
counted in each field of view. The positive cells were counted and
the percentage of positive cells was calculated. The percentage of
positive cells <5%, 0 point (−); 5–25%, 1 point (+); 25–50%, 2
points (++); ≥ 50%, 3 points (+++).
Comprehensive immunohistochemical
measurement criteria
In order to avoid the experimental errors caused by
ranges of cell staining strength and percentage of positive cells,
semi-quantitative assessment was performed using cell staining
strength and percentage of positive cells. The two scores were
multiplied: A total score of 5 points and above: Strongly positive
(+++); 3–4 points: Moderately positive (++); 1–2 points: Weakly
positive (+); 0 point: Negative. The results with more than 3
points indicated high expression, while those with 0–3 points
indicated low expression.
Statistical analysis
The experimental data were analyzed using
Statistical Product and Service Solutions (SPSS) 20.0 (IBM Corp.,
Armonk, NY, USA) software. Enumeration data were presented as a
case. Kruskal-Wallis test was used for the univariate correlation
analyses and comparisons of mutation rate and expression rate, and
Chi-square test was used for the associations of central lymph node
metastasis with BRAF gene and TSHR. P<0.05 indicates that the
difference was statistically significant.
Results
Clinical and pathological data
The 162 PTMC patients were aged 24–69 years with an
average of 46.37 years. There were 31 male and 131 female patients
(male/female ratio: 1:4.2). The total resection of lobes and
isthmus on the affected side + total resection of contralateral
lobes + central and lateral cervical lymph node dissection were
performed (Table I).
 | Table I.Clinicopathological data of 162 PTMC
patients. |
Table I.
Clinicopathological data of 162 PTMC
patients.
| Clinical
parameter | n (%) |
|---|
| Age (years) |
|
|
≥45 | 101 (62.3) |
|
<45 | 61 (37.7) |
| Sex |
|
|
Male | 31 (19.1) |
|
Female | 131 (81.9) |
| Tumor diameter |
|
| ≤5
mm | 101 (62.3) |
| >5
mm and ≤10 mm | 61 (37.7) |
| Single or multiple
lesion |
|
|
Single | 45 (27.8) |
|
Multiple | 117 (72.2) |
| TNM staging |
|
|
I/II | 110 (67.9) |
|
III/IV | 52 (32.1) |
| Capsular
infiltration |
|
|
Yes | 119 (73.5) |
| No | 43 (26.5) |
| Central lymph node
metastasis |
|
|
Yes |
|
|
>3 | 24 (14.8) |
| ≤3 | 61 (37.7) |
| No | 77 (47.50 |
| Lateral cervical
lymph node metastasis |
|
|
Yes | 44 (51.8) |
| No | 41 (48.2) |
BRAF gene expression
Detection results of
BRAFVE600 mutation
There were 135 cases of BRAFVE600
mutation in 162 cases of primary lesion of PTMC, accounting for
83.3%. Thirty cases of thyroid benign lesions and 30 cases of
para-carcinoma normal tissues had no mutation. The mutant-type and
wild-type BRAF and control group detected via qPCR are shown in
Figs. 4 and 5 and Table
II.
 | Table II.Comparison of mutation rate between
PTMC and control group. |
Table II.
Comparison of mutation rate between
PTMC and control group.
|
| BRAF gene |
|---|
|
|
|
|---|
| Groups | Mutant type | Wild-type | Mutation rate
% |
|---|
| PTMC | 135 | 27 | 83.3 |
| Thyroid benign
lesion | 0 | 30 | 0 |
| Para-carcinoma
normal tissue | 0 | 30 | 0 |
Univariate analyses of BRAF gene
expression and clinical parameters of PTMC
Among 135 cases of BRAFVE600 mutation in
162 cases of primary lesion of PTMC, there were 26 male patients
and 109 female patients. Eighty-five cases of lymph node metastasis
were found in the 162 cases of cervical lymph node dissection, and
the remaining 77 cases had no lymph node metastasis. Eighty-five
cases had the central lymph node metastasis, including 44 cases of
lateral cervical lymph node metastasis and 41 cases without
metastasis. No distant metastasis was found in the patients
(Table III).
 | Table III.Univariate analyses of BRAF gene
expression and clinical parameters in PTMC group. |
Table III.
Univariate analyses of BRAF gene
expression and clinical parameters in PTMC group.
|
|
| BRAF gene
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
parameter | No. | Mutant type | Wild-type | χ2
value | P-value |
|---|
| Age (years) |
|
|
| 0.636 | 0.452b |
|
≥45 | 101 | 86 | 15 |
|
|
|
<45 | 61 | 49 | 12 |
|
|
| Sex |
|
|
| 0.282 | 0.596b |
|
Male | 31 | 26 | 5 |
|
|
|
Female | 131 | 109 | 22 |
|
|
| Tumor diameter |
|
|
| 5.054 | 0.025a |
| ≤5
mm | 101 | 79 | 22 |
|
|
| >5
mm and ≤10 mm | 61 | 56 | 5 |
|
|
| Single or multiple
lesion |
|
|
| 0.055 | 0.814b |
|
Single | 45 | 37 | 8 |
|
|
|
Multiple | 117 | 98 | 19 |
|
|
| TNM staging |
|
|
| 0.566 | 0.452b |
|
I/II | 110 | 90 | 20 |
|
|
|
III/IV | 52 | 45 | 7 |
|
|
| Capsular
infiltration |
|
|
| 3.948 | 0.047a |
|
Yes | 119 | 104 | 15 |
|
|
| No | 43 | 32 | 11 |
|
|
| Central lymph node
metastasis |
|
|
| 6.520 | 0.038a |
|
Yes |
|
>3 | 24 | 23 | 1 |
|
|
| ≤3 | 61 | 53 | 8 |
|
|
| No | 77 | 59 | 18 |
|
|
| Lateral cervical
lymph node metastasis |
|
|
| 5.491 | 0.019a |
|
Yes | 44 | 42 | 2 |
|
|
| No | 41 | 33 | 9 |
|
|
Analysis of association between BRAF
gene mutation and central lymph nodes
Based on the central lymph nodes, Table III, it could be seen that the sample
size was still relatively small after grouping, and it was
difficult to use the rank test in pairwise analyses. The results of
Chi-square test for trend showed that the larger the number of
metastatic central lymph node was, the higher the proportion of
mutant type would be, and the larger the probability of mutation
would also be (trend χ2=5.704, P=0.017).
TSHR expression
TSHR detection results
Among 162 cases of primary lesion of PTMC, there
were 92 cases of low expression TSHR (56.8%) and 70 cases of high
expression TSHR (including the normal expression with 4 points;
43.2%). The normal expression of 30 cases of thyroid benign lesions
and 30 cases of para-carcinoma normal tissues were used as the
control. The high and low expression of TSHR and normal expression
in control group detected via immunohistochemical method are shown
in Figs. 6–9 and Table
IV.
 | Table IV.Comparison of expression level
between PTMC and control group. |
Table IV.
Comparison of expression level
between PTMC and control group.
|
| TSHR |
|---|
|
|
|
|---|
| Groups | Low expression | Normal
expression | High
expression |
|---|
| PTMC | 92 | 0 | 70 |
| Thyroid benign
lesion | 0 | 30 | 0 |
| Para-carcinoma
normal tissue | 0 | 30 | 0 |
Univariate analyses of TSHR expression
and clinical parameters of PTMC
The normal expression of TSHR in control group was
used as a reference standard; 0–3 points in 162 cases of primary
lesion of PTMC indicated low expression, while greater than 3
points indicated high expression (Table
V).
 | Table V.Univariate analyses of TSHR
expression and clinical parameters of PTMC in PTMC group. |
Table V.
Univariate analyses of TSHR
expression and clinical parameters of PTMC in PTMC group.
|
|
| TSHR
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
parameter | No. | Low expression | High
expression | χ2 | P-value |
|---|
| Age (years) |
|
|
|
0.044 | 0.834b |
|
≥45 | 101 | 58 | 43 |
|
|
|
<45 | 61 | 34 | 27 |
|
|
| Sex |
|
|
|
0.025 | 0.873b |
|
Male | 31 | 18 | 13 |
|
|
|
Female | 131 | 74 | 57 |
|
|
| Tumor diameter |
|
|
|
4.332 | 0.037a |
| ≤5
mm | 101 | 51 | 50 |
|
|
| >5
mm and ≤10 mm | 61 | 41 | 20 |
|
|
| Single or multiple
lesion |
|
|
|
0.819 | 0.366b |
|
Single | 45 | 23 | 22 |
|
|
|
Multiple | 117 | 69 | 48 |
|
|
| TNM staging |
|
|
|
0.351 | 0.06b |
|
I/II | 110 | 68 | 42 |
|
|
|
III/IV | 52 | 24 | 28 |
|
|
| Capsular
infiltration |
|
|
|
5.371 | 0.021a |
|
Yes | 119 | 74 | 45 |
|
|
| No | 43 | 18 | 25 |
|
|
| Central lymph node
metastasis |
|
|
| 11.157 | 0.004a |
|
Yes |
|
>3 | 24 | 19 | 5 |
|
|
| ≤3 | 61 | 39 | 22 |
|
|
| No | 77 | 34 | 43 |
|
|
| Lateral cervical
lymph node metastasis |
|
|
|
4.158 | 0.041a |
|
Yes | 44 | 29 | 15 |
|
|
| No | 41 | 18 | 23 |
|
|
Analysis of association between TSHR
expression and central lymph node
It could be seen from the data grouping in Fig. 4 that the rank test of pairwise
comparison was less meaningful. The results of Chi-square test for
trend showed that the higher the number of metastatic central lymph
node was, the higher the proportion of low expression would be, and
the larger the probability of low expression would also be (trend
χ2=11.036, P=0.001).
Association between
BRAFVE600 mutation and TSHR expression in PTMC
group
BRAF gene detection and TSHR expression detection
were successfully performed for 162 cases of primary lesion of
PTMC. There were 135 cases of BRAF gene mutation, including 74
cases of low expression TSHR and 61 cases of high expression TSHR.
There were 27 cases of wild-type BRAF gene, including 18 cases of
low expression TSHR and 9 cases of high expression TSHR (Table VI).
 | Table VI.Relationship between
BRAFVE600 mutation and TSHR expression in PTMC
group. |
Table VI.
Relationship between
BRAFVE600 mutation and TSHR expression in PTMC
group.
|
| TSHR |
|
|
|---|
|
|
|
|
|
|---|
| Groups | Low expression | High
expression | Total | P-value | K (coincidence
coefficient) |
|---|
| BRAF gene |
|
|
| 0.256a | 0.06 |
| Mutant
type | 74 | 61 | 135 |
|
|
|
Wild-type | 18 | 9 | 27 |
|
|
|
Total | 70 | 92 | 162 |
|
|
The coincidence coefficient of the two methods was
K=0.06 (P=0.256); the coincidence rate was 51.2%.
Discussion
As one of the most common malignant tumors in the
Head-Neck Surgery Department and Endocrine Department (28), thyroid cancer (TC) is a malignant
tumor with the most rapid increase in the incidence rate in recent
ten years. Τhere are approximately 120,000 cases of TC newly
diagnosed worldwide every year (29),
of which PTMC is especially significant. The reported 10-year
survival rate of PTC is nearly 97% (30). As the most common subtype of PTC, PTMC
with a primary lesion diameter of ≤10 mm is generally difficult to
be discovered by the patients themselves due to its small volume
and occult onset, and the disease can only be discovered in the
patients receiving clinical diagnosis and treatment mainly through
confirmed pathological findings obtained from ultrasound
examination combined with fine-needle aspiration biopsy (FANB).
With the improvement of examination techniques, more and more cases
of PTMC are discovered, whose detection rate is remarkably higher
than that of other types of TC (30,31).
However, it is possible that ultrasound examination is not
popularized among teenagers, so the patients who have been
diagnosed with PTMC so far are generally aged over 18 years.
According to the research findings of American scholars, PTMC has
become the most common malignant tumor among patients aged more
than 45 years in the USA (32). The
clinical biological characteristics of PTMC are moderate, and it
may even have no effect on some patients for the whole life. But
PTMC is still associated with higher central and cervical lymph
node metastases. Studies have confirmed (12,32–34) that
the lymph node metastasis rate of PTMC is 48.1%, the mortality rate
is 0–0.4%, and the recurrence and metastasis rate is 3.3%. Most
scholars have agreed that the microcarcinoma is not the early
cancer. Due to the contradiction of good prognosis and high
transfer rate, there has been no complete consensus on PTMC
surgical treatment in different countries and different scholars.
In particular, the surgical treatment of cervical lymph nodes is
controversial. How to allow PTMC patients to receive more accurate
treatment and determine the lymph node metastasis before operation
is one of the urgent problems to be solved. In addition to
improving the imaging diagnosis and using FANB cytological
pathological results (35,36) to identify the cytological pathological
diagnosis levels for tumor differentiation level, searching the
genes or molecular markers for PTMC invasion is also feasible.
BRAF gene is currently recognized as one of the most
important PTC driver genes, also known as rodent sarcoma viral
pathogen carcinogen homologue B1, which was first discovered and
cloned by Ikawa et al (37) in
1988 in Ewing's sarcoma. Moreover, it is also an important gene in
the RAS-RAF-MEK-MAPK signal transduction process, which mainly
plays an important role in regulating cell growth, division and
proliferation. The BRAF gene is located on human chromosome 7q34
and contains 18 exons. T mutation into A on the nucleotide 1799
replaces the corresponding valine to the 600th codon in protein
translation with glutamic acid, increasing the kinase activity by
approximately 500 times compared with the wild-type (negative),
transmitting the signals to downstream MAPK pathway, and
stimulating cells to produce mitosis and form malignant tumors
(38,39). Bansal et al (40) demonstrated that the mutation of
BRAFV600E, as a proto-oncogene, affects the occurrence
of PTC together with RET/PTC gene rearrangement and RAS gene
mutation. Jung et al (41)
found that in recent classic PTC, the BRAFV600E mutation
rate is increased by 27%, far exceeding the RET/PTC gene
rearrangement and RAS gene mutation. According to research
(42,43), the BRAFV600E mutation rate
is approximately 16–56% in PTMC, and approximately 23–80% in PTC.
Therefore, BRAF gene has become the genetic mutation with the
greatest significance in the diagnosis and treatment of PTMC, which
is closely related to the occurrence and development of PTMC. BRAF
gene mutation in PTMC may enhance the cell proliferation and
transformation capacities. Studies have shown that (44) the detection rate of
BRAFV600E mutation in the lymph node infiltration and
metastatic malignant tumors (clinical stage III/IV) is high, and it
is related to the tumor size, capsular infiltration and other
clinical factors, so the BRAFV600E gene mutation is an
index of predicting PTMC invasion.
In this experiment, it was found that 135 out of 162
cases of primary lesion of PTMC had BRAFV600E mutation,
accounting for 83.3%. The Chi-square test of BRAF gene showed that
the BRAFV600E mutation was associated with the size of
primary lesion of PTMC, capsular infiltration and lymph node
metastasis, but not related to the age, sex, tumor lymph nodes
metastasis (TNM) staging and the number of primary lesions. In
principle, the larger the primary lesion is, the higher the
possibility of capsular infiltration or even breaking, lymph node
metastasis and mutation will be, and the stronger the invasion will
also be. Notably, this experiment showed that among 162 cases of
cervical lymph node dissection, there were 85 cases of lymph node
metastasis and 77 cases of no metastasis; furthermore, there were
85 cases of central lymph node metastasis, including 44 cases of
lateral cervical lymph node metastasis and 41 cases of no
metastasis. All patients with lymph node metastasis had cervical
lymph node metastasis. The Chi-square test for trend showed that
the larger the number of metastatic central lymph node was, the
higher the proportion of mutant type would be, and the larger the
probability of mutation would also be (trend χ2=5.704,
P=0.017). The above experimental results were consistent with those
reported by Xing et al (21).
Therefore, BRAFV600E mutation can not only help
distinguish between benign and malignant tumors, but also suggest
the intensity of local invasion and lymph node metastasis of
malignant tumors, providing biological significance for the high
invasiveness of primary lesion of PTMC.
In recent years, studies have found that the
metastasis of primary lesion of PTC is often accompanied by the
change in TSHR expression. TSHR, as a macromolecule glycoprotein,
is the main antigen of toxic diffuse goiter in the
G-protein-coupled receptor superfamily, whose gene is located on
14q31 with a length of 60 kb, containing 10 exons and 9 introns,
encoding 764 amino acids. TSHR is mainly located on the thyroid
follicular epithelial cell membrane, while some are located in the
cytoplasm and outside the cells, and its main mechanism is to
regulate and control the thyroid cell growth and differentiation.
In principle, TSH binds to extracellular TSHR to regulate Tg, TPOAB
and sodium iodide transporters, and promote the thyroid hormone
secretion and thyroid cell growth through the cyclic adenosine
monophosphate (cAMP) pathway (45);
moreover, it also indirectly promotes the synthesis of thyroid
hormones through activating the phospholipid- CA2+
pathway and stimulating the TPO activity (46–48). The
expression of TSHR are high in PTC (86.7%) and low in ATC (23.8%).
Whether the expression of TSHR in cells can be used to show the
biological changes in cells due to thyroid malignant
transformation, and to assess the invasion of its primary lesion
combined with other clinical parameters need clarification.
In this study, it was confirmed that the cell
membrane and cytoplasm were stained after immunohistochemistry. The
expression on cell membrane was theoretically more accurate, but
the expression in the cytoplasm may be affected by the incorrect
TSHR protein positioning or the protein may enter the cytoplasm due
to the lack of TSHR in the cytoplasm, aggravating staining and
resulting in experimental errors, so the expression on cell
membrane was used as an experimental standard. The 30 cases of
thyroid benign tumors and 30 cases of para-carcinoma normal tissues
selected showed the normal staining expression; the
semi-quantitative calculation was performed using the positive cell
percentage and cell staining intensity; cases with the score of
approximately 4 points could be set as the control group. At the
same time, 92 out of 162 cases of PTMC had a score less than 4
points, indicating low expression (56.8%), and the remaining 70
cases indicated high expression (43.2%). The univariate analysis of
expression results and clinical parameters showed that the results
were consistent with the BRAF gene detection. The TSHR protein was
also associated with the diameter, capsular infiltration and lymph
node metastasis of primary lesion of PTMC, but not related to age,
gender, TNM staging and number of primary lesions. After the
introduction of invasion, it was found that the low-expression TSHR
indicates high invasion of primary lesion. The Chi-square test for
trend was used for comparison between groups with greater than 3
central metastatic lymph nodes and less than or equal to 3 central
metastatic lymph nodes, and the results revealed that the higher
the number of metastatic lymph nodes was, the higher the proportion
of low expression would be, and the larger the probability of
invasion would also be (trend χ2=5.704, P=0.017).
Therefore, it is concluded that the larger diameter of primary
lesion of PTMC leads to capsular infiltration, more lymph node
metastasis and low expression of TSHR protein, and the TSHR
expression is negatively correlated with invasion.
Through this study, it was found that although the
single factor detection can identify and diagnose benign and
malignant tumors, but it cannot independently determine and then
conclude the invasion of malignant tumors, so it is feasible to
apply BRAF gene combined with TSHR in the diagnosis of high
invasiveness of PTMC. The univariate analysis was performed for
them with clinical parameters collected, and the associations with
risk factors were obtained. The results showed that there were 135
cases of BRAF gene mutations, including 74 cases of low expression
TSHR and 61 cases of high expression TSHR. There were 27 cases of
wild-type BRAF gene, including 18 cases of low expression TSHR and
9 cases of high expression TSHR. The coincidence coefficient
obtained via chi-square test was K=0.06, P=0.256, and the
coincidence rate was 51.2%. Although the BRAFV600E
mutation was not correlated with the expression of TSHR protein,
the coincidence coefficient indicated that they had different
diagnostic significance in PTMC in clinical application;
BRAFV600E mutation and TSHR protein can be combined and
applied in the prediction of PTMC invasion and lymph node
metastasis, which may be more meaningful for clinical guidance.
Many studies have suggested that (49,50) BRAF
gene mutation is the most closely related to lymph node metastasis,
followed by thyroid capsular infiltration. Russo et al
(51) reported that the BRAF gene
mutant type in PTMC is associated with cervical lymph node
metastasis and TNM staging (III/IV). Wang et al (52) enrolled more than 300 cases of PTMC
(clinical TNM staging of I/II) in the study, and found that
BRAFV600E mutation is related to the capsular
infiltration and multiple lesions. Combined with our experiments,
most conclusions were consistent with the views of experts and
scholars, and some small differences might be caused by the impact
of experimental environment, experimental techniques, uneven sample
size and other clinical parameters not included. But in general,
predicting the invasiveness (small cancer lesion and great
metastasis) of PTMC should be combined with various factors. It is
believed that the poorly differentiated and mutant primary lesion
of PTMC has larger possibility of invasion, and the lesion is more
likely to break through the capsule, leading to lymph node
metastasis. However, is it feasible to combine preoperative FNAB
pathological results (53) and blood
marker examination for better prediction? We consider it is
possible. Under reasonable indications, the application of BRAF
gene and TSHR protein in the cytological pathological examination
and preoperative serum detection of PTMC may have more reasonable
guiding significance than the postoperative paraffin-embedded
specimens. There have been few studies on the combined application
of BRAF gene and TSHR protein, so subsequent in-depth exploration
is still needed to confirm the experimental results.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant
from funding agencies in the public, commercial, or not-for-profit
sectors.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ and JL contributed to the conception of the
study. YW designed the study and performed the research. SX
performed the data analyses and wrote the manuscript. YZ helped
perform the analysis with constructive discussions. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Liaocheng People's Hospital (Liaocheng, China) and written informed
consents were signed by the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cox AE and LeBeau SO: Diagnosis and
treatment of differentiated thyroid carcinoma. Radiol Clin North
Am. 49:453–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao Y, Xia J, Li Y, Wang L and Jiang Z:
Study on the effect of salt plus iodine on the hospitalization of
thyroid diseases in a hospital. Chin J Health Stat. 29:236–242.
2012.(In Chinese).
|
|
5
|
Zhao H, Jiang W, Guan F, Hou F and Cheng
H: Application of 131I in the study of residual thyroid
tissue dose after differentiated thyroid cancer. J Jilin Univ (Med
Ed). 35:191–194. 2009.(In Chinese).
|
|
6
|
Sciuto R, Romano L, Rea S, Marandino F,
Sperduti I and Maini CL: Natural history and clinical outcome of
differentiated thyroid carcinoma: A retrospective analysis of 1503
patients treated at a single institution. Ann Oncol. 20:1728–1735.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi YM, Kim TY, Song DE, Hong SJ, Jang
EK, Jeon MJ, Han JM, Kim WG, Shong YK and Kim WB: Papillary thyroid
carcinoma arising from a thyroglossal duct cyst: A single
institution experience. Endocr J. 60:665–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vergamini LB, Frazier AL, Abrantes FL,
Ribeiro KB and Rodriguez-Galindo C: Increase in the incidence of
differentiated thyroid carcinoma in children, adolescents, and
young adults: A population-based study. J Pediatr. 164:1481–1485.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Londero SC, Krogdahl A, Bastholt L,
Overgaard J, Pedersen HB, Frisch T, Bentzen J, Pedersen PU,
Christiansen P and Godballe C: Papillary thyroid carcinoma in
Denmark 1996-2008: An investigation of changes in incidence. Cancer
Epidemiol. 37:e1–e6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radespiel-Tröger M, Batzler WU, Holleczek
B, Luttmann S, Pritzkuleit R, Stabenow R, Urbschat I, Zeissig SR
and Meyer M; Im Namen der Gesellschaft der epidemiologischen
Krebsregister in Deutschland e.V, . Rising incidence of papillary
thyroid carcinoma in Germany. Bundesgesundheitsblatt
Gesundheitsforschung Gesundheitsschutz. 57:84–92. 2014.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joshi P, Nair S, Nair D and Chaturvedi P:
Incidence of occult papillary carcinoma of thyroid in Indian
population: Case series and review of literature. J Cancer Res
Ther. 10:693–695. 2014.PubMed/NCBI
|
|
12
|
Wu LS and Milan SA: Management of
microcarcinomas (papillary and medullary) of the thyroid. Curr Opin
Oncol. 25:27–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moosa M and Mazzaferri EL: Occult thyroid
carcinoma. Cancer. 10:180–188. 1997.
|
|
14
|
Pacini F: Thyroid microcarcinoma. Best
Pract Res Clin Endocrinol Metab. 26:421–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim NH, Beak SK, Baik SH, Choi DS and Kim
SG: A patient with micropapillary thyroid carcinoma and
macronodular lung metastasis: Stable disease for eight years
without treatment. Thyroid. 19:309–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Cui Z, Sun S, Ren Y, Xu J, Yao Y,
Chen Q, Zhang W, Li R, Guan Z, et al: Study on the significance and
method of cervical lymph node dissection in differentiated thyroid
carcinoma. Chin J Pract Surg. 31:414–416. 2011.(In Chinese).
|
|
17
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacCorkle RA and Tan TH: Mitogen-activated
protein kinases in cell-cycle control. Cell Biochem Biophys.
43:451–461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang BH and Guan KL: Activation of B-Raf
kinase requires phosphorylation of the conserved residues Thr598
and Ser601. EMBO J. 19:5429–5439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tufano RP, Teixeira GV, Bishop J, Carson
KA and Xing M: BRAF mutation in papillary thyroid cancer and its
value in tailoring initial treatment: A systematic review and
meta-analysis. Medicine (Baltimore). 91:274–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Yin D, Lu X and Qiu X: Expression
and significance of TSHR ER and CyclinD1 in differentiated thyroid
carcinoma. J Zhengzhou Univ (Med Sci). 5:3542005.(In Chinese).
|
|
23
|
Shi Y, Zou M and Farid NR: Expression of
thyrotrophin receptor gene in thyroid carcinoma is associated with
a good prognosis. Clin Endocrinol (Oxf). 39:269–274. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka K, Inoue H, Miki H, Masuda E,
Kitaichi M, Komaki K, Uyama T and Monden Y: Relationship between
prognostic score and thyrotropin receptor (TSH-R) in papillary
thyroid carcinoma: Immunohistochemical detection of TSH-R. Br J
Cancer. 76:594–599. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X and Gao M: The relationship between
the anti-oxidative conduction pathway of thyroxine and the invasion
force of thyroid papillary carcinoma. Chin J Otorhinolaryngol Head
Neck Surg. 44:287–291. 2009.(In Chinese).
|
|
26
|
Shi C, Qin H, Ding C, Sun Y, Lyu Y and Shi
T: Association between BRAF V600E mutation and central lymph node
metastasis in patients with papillary thyroid carcinoma. Zhonghua
Zhong Liu Za Zhi. 37:123–127. 2015.(In Chinese). PubMed/NCBI
|
|
27
|
Shi X, Liu R, Basolo F, Giannini R, Shen
X, Teng D, Guan H, Shan Z, Teng W, Musholt TJ, et al: Differential
clinicopathological risk and prognosis of major papillary thyroid
cancer variants. J Clin Endocrinol Metab. 101:264–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stewart BW and Kleihues P: World Cancer
Report. IARC Press; Lyon: 2003
|
|
29
|
Kim HJ, Kim NK, Choi JH and Kim SW, Jin
SM, Suh S, Bae JC, Min YK, Chung JH and Kim SW: Radioactive iodine
ablation does not prevent recurrences in patients with papillary
thyroid microcarcinoma. Clin Endocrinol (Oxf). 78:614–620. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee KJ, Cho YJ, Kim JG and Lee DH: How
many contralateral papillary thyroid carcinomas can be missed?
World J Surg. 37:780–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pedrazzini L, Baroli A, Marzoli L,
Guglielmi R and Papini E: Cancer recurrence in papillary thyroid
microcarcinoma: a multivariate analysis on 231 patients with a
12-year follow-up. Minerva Endocrinol. 38:269–279. 2013.PubMed/NCBI
|
|
33
|
Liu T and Qi J: Diagnosis and surgical
treatment of differentiated thyroid carcinoma. Int J Endocrinol.
26:344–346. 2006.
|
|
34
|
Chung YJ, Lee JS, Park SY, Park HJ, Cho
BY, Park SJ, Lee SY, Kang KH and Ryu HS: Histomorphological factors
in the risk prediction of lymph node metastasis in papillary
thyroid carcinoma. Histopathology. 62:578–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bruno R, Giannasio P, Chiarella R, Capula
C, Russo D, Filetti S and Costante G: Identification of a neck lump
as a lymph node metastasis from an occult contralateral papillary
microcarcinoma of the thyroid: Key role of thyroglobulin assay in
the fine-needle aspirate. Thyroid. 19:531–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dohán O, De la Vieja A, Paroder V, Riedel
C, Artani M, Reed M, Ginter CS and Carrasco N: The sodium/iodide
symporter: Characterization, regulation, and medical significance.
Endorcrine Rev. 24:48–77. 2003. View Article : Google Scholar
|
|
37
|
Ikawa S, Fukui M, Ueyama Y, Tamaoki N,
Yamamoto T and Toyoshima K: B-raf, a new member of the raf family,
is activated by DNA rearrangement. Mol Cell Biol. 8:2651–2654.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ji Y and Xie N: Clinical study of BRAF
gene mutation in thyroid papillary carcinoma. Guangdong Med J.
30:1183–1185. 2009.(In Chinese).
|
|
39
|
Kumar R, Angelini S, Czene K, Sauroja I,
Hahka-Kemppinen M, Pyrhönen S and Hemminki K: BRAF mutations in
metastatic melanoma: A possible association with clinical outcome.
Clin Cancer Res. 9:3362–3368. 2003.PubMed/NCBI
|
|
40
|
Bansal M, Gandhi M, Ferris RL, Nikiforova
MN, Yip L, Carty SE and Nikiforov YE: Molecular and histopathologic
characteristics of multifocal papillary thyroid carcinoma. Am J
Surg Pathol. 37:1586–1591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jung CK, Little MP, Lubin JH, Brenner AV,
Wells SA Jr, Sigurdson AJ and Nikiforov YE: The increase in thyroid
cancer incidence during the last four decades is accompanied by a
high frequency of BRAF mutations and a sharp increase in RAS
mutations. J Clin Endocrinol Metab. 99:E276–E285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ
and Qu JM: The BRAF mutation is predictive of aggressive
clinicopathological characteristics in papillary thyroid
microcarcinoma. Ann Surg Oncol. 17:3294–3300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SJ, Lee KE, Myong JP, Park JH, Jeon
YK, Min HS, Park SY, Jung KC, Koo H and Youn YK: BRAF V600E
mutation is associated with tumor aggressiveness in papillary
thyroid cancer. World J Surg. 36:310–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kwak JY, Jeong JJ, Kang SW, Park S, Choi
JR, Park SJ, Kim EK and Chung WY: Study of peripheral BRAF(V600E)
mutation as a possible novel marker for papillary thyroid
carcinomas. Head Neck. 35:1630–1633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guan H, Guan H, Lu J, Rong S, Guo X and Li
X: Meta-analysis of thyroid hormone level and thyroid cancer in
Chinese population. J Environ Health. 30:1115–1116. 2013.(In
Chinese).
|
|
46
|
Lu X, Ge M, Ling Z, Hu S, Xu Z, Zheng C,
Tan Z and Chen C: Correlation and clinical significance of abnormal
methylation of hMLH, NIS and TSHR in thyroid papillary carcinoma.
Tumor. 33:445–453. 2013.(In Chinese).
|
|
47
|
Tonacchera M, Viacava P, Fanelli G,
Agretti P, De Marco G, De Servi M, Di Cosmo C, Chiovato L, Pinchera
A and Vitti P: The sodium-iodide symporter protein is always
present at a low expression and confined to the cell membrane in
nonfunctioning nonadenomatous nodules of toxic nodular goitre. Clin
Endocrinol (Oxf). 61:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Smith JA, Fan CY, Zou C, Bodenner D and
Kokoska MS: Methylation status of genes in papillary thyroid
carcinoma. Arch Otolaryngol Head Neck Surg. 133:1006–1011. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li C, Lee KC, Schneider EB and Zeiger MA:
BRAF V600E mutation and its association with clinicopathological
features of papillary thyroid cancer: Meta-analysis A. J Clin
Endocrinol Metab. 97:4559–4570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang J, Wu G, Ma X, Liu Y, Chen H and
Huang W: BRAF gene mutation and clinical significance in thyroid
tumor tissue. Chin J Exp Surg. 28:1102–1104. 2011.(In Chinese).
|
|
51
|
Russo M, Malandrino P, Nicolosi ML,
Manusia M, Marturano I, Trovato MA, Pellegriti G, Frasca F and
Vigneri R: The BRAF(V600E) mutation influences the short- and
medium-term outcomes of classic papillary thyroid cancer, but is
not an independent predictor of unfavorable outcome. Thyroid.
24:1267–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang W, Lu R, Dong Y and Liang J: V600E
BRAF gene mutation and I-II papillary thyroid cancer clinical
pathological characteristics and prognosis of correlation analysis.
Linchuang Zhongliuxue Zazhi. 18:1100–1103. 2013.(In Chinese).
|
|
53
|
Min JJ, Chung JK, Lee Y, Jeong J, Lee D,
Jang J, Lee M and Cho B: Relationship between expression of the
sodium/iodide symporter and (l31)I uptake in recurrent lesions of
differentiated thyroid carcinoma. Eur J Nucl Med. 28:639–645. 2001.
View Article : Google Scholar
|