Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most fatal malignancies according to cancer statistics of USA
in 2016 (1). Surgical resection, to
the best of our knowledge, is currently the only curative treatment
for PDAC; however, <20% of patients are potential candidates for
pancreatectomy as the majority are diagnosed at the advanced
disease stage (2). Furthermore, even
following successful surgical resection, it has been reported that
the 5-year survival rate following pancreatectomy is only 10–20%
due to a high rate of disease recurrence according to a German
study conducted between 1998–2003 (3), a Korean study conducted between
1983–2011 (4) and a USA study
conducted between 2003–2010 (5).
Recently established chemotherapy regimens, including folinic acid,
fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) and
gemcitabine plus nab-paclitaxel therapy, have improved the
prognosis of patients with PDAC (6–9). However,
the median survival time among patients who underwent the
aforementioned chemotherapy regimens has been reported as <1
year according to literatures published as international
collaborative studies, which included patients from 11 countries
and Japanese domestic studies conducted between 2003–2014 (6–9). Given the
challenges associated with altering the biological behavior of
PDAC, it is important to continue investigating additional ways to
improve patient prognosis.
The length of waiting until medical care has drawn
increasing attention in previous studies as a potential factor that
may affect the prognosis of patients with various types of
malignancies (10–16). Based on previous studies, waiting
times can be classified into four categories: i) Waiting time until
the first medical visit; ii) waiting time until detection of the
disease; iii) waiting time until diagnosis; and iv) waiting time
until treatment (10–16). The impact of waiting time on patients'
prognosis varied in each study, but a number of the patients
revealed reduced outcome with long waiting time, compared with
patients with a reduced waiting time (13,15).
Although a literature search identified 3 reports in which the
authors investigated the prognostic effect of waiting time on the
survival of patients with PDAC, none of the previous studies, to
the best of our knowledge, evaluated the effect of waiting time
between the detection of disease and diagnosis (11–13).
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has
been accepted globally as the gold standard for diagnosing PDAC and
may be used to obtain highly accurate diagnoses (17,18).
However, in contrast to the diagnostic yields of other modalities,
the diagnostic yield of EUS-FNA is highly dependent on the
expertise of the clinicians, cytoscreeners and the pathologists
performing this procedure and assessing the results (19–21).
Consequently, patients who require EUS-FNA are referred to a
limited number of tertiary referral facilities and are have to wait
for a certain length of time prior to undergoing EUS-FNA to obtain
a pathological diagnosis. For example, patients had to wait for
45.2 days until they underwent their second EUS-FNA following the
initial EUS-FNA (20). Additionally,
patients are required to wait prior to commencing treatment, which
may be due to the high demand for pancreatic specialists (11,12). The
aim of the present study was to clarify whether the length of the
waiting times to diagnosis and treatment affected the prognosis of
patients with PDAC.
Materials and methods
Patient sample collection
A retrospective observational study was conducted to
review the data obtained from patients histologically diagnosed
with PDAC using EUS-FNA, who were treated at Fukushima Medical
University Hospital (Fukushima, Japan) between January 2006 and
July 2016. A total of 149 patients were included in the present
study (mean age, 67.2 years; age range, 42.0–86.0; 83 males and 66
females). All patients who underwent scheduled chemotherapy or
surgical resection with known final outcomes were included. To
eliminate the possibility of selection bias in treatment, the
present study included only the patients who were able to undergo
the treatment that was originally recommended at the time of
diagnosis. In the case of a patient who was originally scheduled to
undergo surgical resection, but tumor cell dissemination was
subsequently observed during the surgical laparotomy, the patient
was excluded from the analysis, as it was not clear whether the
prolonged waiting time was the cause of the tumor cell
dissemination or if it was originally present as occult metastasis.
In cases involving chemotherapy, first-line chemotherapy regimens
that included monotherapy (gemcitabine or S-1) and combination
therapy (FOLFIRINOX or gemcitabine plus nab-paclitaxel) were
selected based on the Eastern Cooperative Oncology Group
Performance Status Scale (22) and
comorbidity of the patients. Due to the national health insurance
coverage, monotherapy was selected as the first-line chemotherapy
between April 2006 and December 2013. FOLFIRINOX was available in
December 2013 and gemcitabine plus nab-paclitaxel was added to the
list in December 2014. Second-line chemotherapy regimens were
selected based on the physician's discretion, taking into
consideration the patients' general condition. In cases involving
surgical resection, all the patients underwent surgical resection
with curative intent, and those who received preoperative
chemoradiation therapy were excluded from the present study.
Patients were followed up using imaging modalities, including
computed tomography, every 2–3 months following the initiation of
the primary treatment. Median disease-free survival (DFS) and
overall survival (OS) time following surgical resection were
calculated using the Kaplan-Meier method and were each utilized to
determine the cut-off points to divide patients into 2 groups: A
short and a long survival group, with the median value included
within the long survival group. For further analysis, clinical data
on patients in the lower and upper quartiles with respect to
detection-to-diagnosis waiting time (WT1) and
diagnosis-to-treatment waiting time (WT2) were obtained in order to
assess the prognostic effect of immediate and delayed treatment.
Survival analysis was subsequently performed using the Kaplan-Meier
method with log-rank tests to compare the two groups for each type
of treatment.
Variables
Waiting times to diagnosis and treatment were
estimated to be the time from the detection of disease with imaging
(computed tomography) to histological diagnosis using EUS-FNA
(WT1), and the time from histological diagnosis to treatment
initiation (WT2). OS was defined as the time from treatment
initiation to mortality from any cause. In cases involving surgery,
DFS was defined as the survival period during which patients
survived with no signs of recurrence following surgical resection.
Clinical characteristics prior to the initiation of primary
treatment, including age, sex, tumor size, tumor location, Tumor
(T) and Node (N) stages based on the Union for International Cancer
Control classification, ver. 7 (23),
serum levels of tumor markers, including carcinoembryonic antigen
and cancer antigen 19-9, waiting times and the use of adjuvant
chemotherapy, were compared between the short and long survival
groups.
The protocol of the present study was in accordance
to the ethical guidelines of the 1975 Declaration of Helsinki and
was approved by the Institutional Review Committee of Fukushima
Medical University School of Medicine (approval no., 2285;
Fukushima, Japan). The institutional review board waived the
requirement for written informed patient consent due to the
retrospective non-interventional nature of the present study.
Statistical analysis
The demographic and clinical characteristic
distributions for each group were compared using
χ2-tests and Fisher's exact test as appropriate.
Variables that followed a Gaussian distribution were assessed using
parametric tests, one-way analysis of variance (ANOVA) followed by
the post-hoc Tukey's test for differences among all four groups and
unpaired Student's t-tests for differences between two groups.
Variables that did not follow a Gaussian distribution were
log-transformed or assessed using non-parametric tests,
Kruskal-Wallis tests (or for non-parametric, ANOVA) for differences
among all four groups and Mann-Whitney U-tests for differences
between two groups. Survival analysis was conducted using the
Kaplan-Meier method with log-rank tests. All statistical analyses
were performed using GraphPad Prism 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference. Data are presented as the
median ± range.
Results
Patients
During the study period, 451 patients with PDAC were
diagnosed using EUS-FNA. The majority of the patients who were not
considered suitable for surgical resection were referred to other
hospitals for treatment. Consequently, a total of 149 patients,
including 72 patients in the chemotherapy group and 77 patients in
the surgical resection group, were included in the present study
(Table I). Aside from the
aforementioned patients, there were 5 patients who were scheduled
originally for surgical resection, but it was cancelled since liver
metastasis or peritoneal dissemination was indicated during the
waiting time until surgical resection or exploratory laparotomy.
WT1 and WT2 of the aforementioned patients, whose surgical
resection was cancelled, were 20.0 days (15.0–30.0) and 43.0 days
(30.0–55.0), respectively. There were no significant differences in
the lengths of WT1 and WT2 between the 79 patients with surgical
resection and the 5 patients with the originally planned surgical
resection cancelled (P=0.70 and P=0.75, respectively) (Table II).
 | Table I.Clinical characteristics of all
patients. |
Table I.
Clinical characteristics of all
patients.
| Chemotherapy
(n=72) |
|---|
|
|---|
| Characteristics | Median (range) |
|---|
| Age (years) | 66.0 (59.0–68.0) |
| Tumour size (longest
diameter; mm) | 35.0 (25.0–47.5) |
| CEA (ng/ml) | 4.2 (2.4–9.5) |
| CA19-9 (U/ml) | 1,196.0
(157.4–4,899.0) |
| WT1 | 20.0 (11.0–28.0) |
| WT2 | 17.5 (9.0–29.0) |
| Sex |
|
| Male | 39 |
|
Female | 33 |
| Tumour location |
|
| Ph | 37 |
| Pbt | 35 |
| Disease stage (UICC
ver.7) (24) |
|
| Locally
advanced | 22 |
|
Metastatic | 50 |
| Chemotherapy |
|
|
Monotherapy | 53 |
|
Combination | 19 |
| Surgery (n=77) |
|
| Characteristics | Median (range) |
| Age (years) | 71.0 (64.0–76.0) |
| Tumour size (longest
diameter; mm) | 19.0 (15.0–25.0) |
| CEA (ng/ml) | 2.8 (1.9–6.0) |
| CA19-9 (U/ml) | 102.8
(25.8–333.5) |
| WT1 | 21.0 (14.0–36.0) |
| WT2 | 46.0 (29.0–60.0) |
| Sex (n) |
|
| Male | 44 |
|
Female | 33 |
| Tumour location
(n) |
|
| Ph | 52 |
| Pbt | 25 |
| UICC T stage (n)
(24) |
|
| T0-1 | 10 |
| T2 | 12 |
|
T3-T4 | 55 |
| UICC N Stage (n) |
|
| N0 | 49 |
| N1 | 28 |
| Adjuvant
chemotherapy |
|
| Yes | 48 |
| No | 29 |
 | Table II.Comparisons of waiting time between
patients underwent surgery (n=79) and cancelled surgery (n=5). |
Table II.
Comparisons of waiting time between
patients underwent surgery (n=79) and cancelled surgery (n=5).
| Characteristics | Surgery (n=79) | Cancelled surgery
(n=5) | P-value |
|---|
| WT1, days
(range) | 21.0 (14.0–36.0) | 20.0 (15.0–30.0) | 0.70 |
| WT2, days
(range) | 46.0 (29.0–60.0) | 43.0 (30.0–55.0) | 0.75 |
Survival analysis of patients based on
waiting times
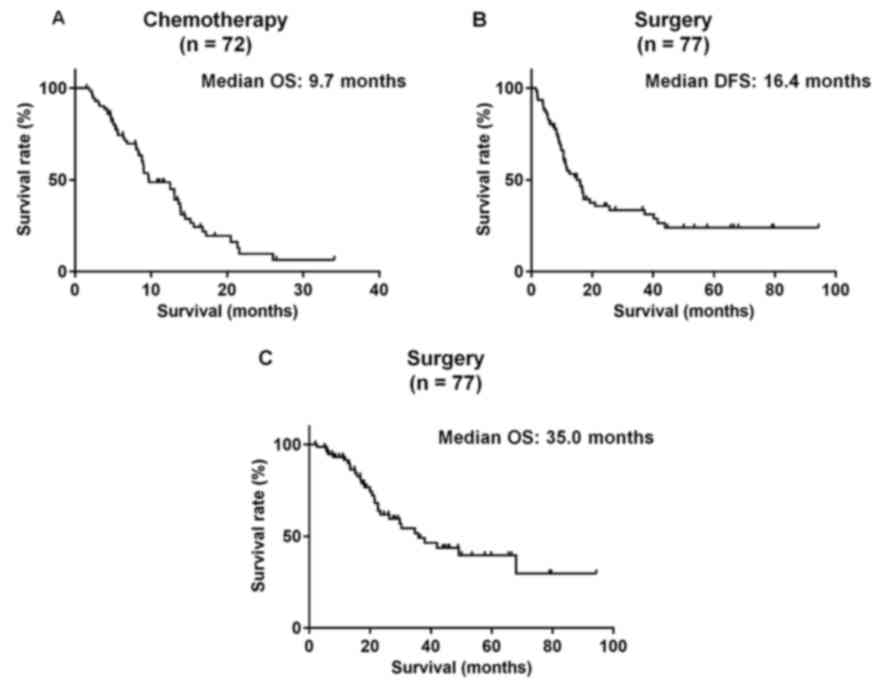
The 72 patients who underwent chemotherapy (median
OS, 9.7 months; Fig. 1A) were divided
into the long survival group (n=42) and the short survival group
(n=30) (Table III). No significant
differences between the long and short survival groups were
identified with respect to WT1 (20.0 vs. 19.0 days; P=0.98) or WT2
(17.0 vs. 18.5 days; P=0.93); however, the proportion of patients
with locally advanced disease (P=0.02) and the administration of
combination chemotherapy (P=0.003) were significantly associated
with long-term survival, compared with short-term survival
(Table III). Among the 77 patients
who underwent surgical resection, the median DFS following
pancreatic resection was 16.4 months, and the median OS was 35.0
months (Fig. 1B and C). The patients
were further divided into short (n=47) vs. long (n=30) DFS groups,
and short (n=57) vs. long (n=20) OS groups. Additionally, no
significant between-group differences were observed for WT1 in the
short vs. long DFS group (21.0 vs. 21.0 days; P=0.65) and the short
vs. long OS group (21.0 vs. 23.0 days; P=0.45) or WT2 in the short
vs. long DFS group (46.0 vs. 44.5 days; P=0.45) and the short vs.
long OS group (46.0 vs. 41.0 days; P=0.36) (Table III). Advanced T and N stages were
significantly associated with short DFS (P=0.049 and P=0.03,
respectively), compared with long DFS; however, only advanced T
stage was significantly associated with short OS (P=0.02; Table IV).
 | Table III.Comparisons between patients receiving
chemotherapy in short survival and long survival groups (n=72). |
Table III.
Comparisons between patients receiving
chemotherapy in short survival and long survival groups (n=72).
| Characteristics | Short survival
(n=42) | Long survival
(n=30) | P-value |
|---|
| WT1 | 20.0
(13.0–28.0) | 19.0
(11.0–28.0) | 0.98 |
| WT2 | 17.0
(9.0–30.0) | 18.5
(12.0–27.0) | 0.93 |
| Age (years) | 64.5
(58.0–67.0) | 66.0
(59.0–70.0) | 0.31 |
| Tumour size
(longest diameter; mm) | 34.0
(26.0–45.0) | 30.0
(23.0–50.0) | 0.40 |
| CEA (ng/ml) | 4.1 (2.0–13.4) | 4.2 (2.5–7.5) | 0.97 |
| CA19-9 (U/ml) | 1,957.0
(116.7–11,025.0) | 628.8
(99.0–3,197.0) | 0.17 |
| Sex (n) |
|
|
|
|
Male | 23 | 16 | 1.00 |
|
Female | 19 | 14 |
|
| Tumour location
(n) |
|
|
|
| Ph | 23 | 14 | 0.63 |
|
Pbt | 19 | 16 |
|
| Disease stage (UICC
ver.7) (n) (24) |
|
|
|
| Locally
advanced | 8 | 14 | 0.02 |
|
Metastatic | 34 | 16 |
|
| Chemotherapy
(n) |
|
|
|
|
Monotherapy | 35 | 18 | 0.003 |
|
Combination therapy | 7 | 12 |
|
 | Table IV.Comparisons between
surgically-treated patients in the short and long survival groups
(n=77). |
Table IV.
Comparisons between
surgically-treated patients in the short and long survival groups
(n=77).
|
Characteristics | Short DFS
(n=48) | Long DFS
(n=29) | P-value | Short OS
(n=57) | Long OS (n=20) | P-value |
|---|
| WT1 (days) | 21.0
(15.0–38.0) | 21.0
(6.0–100.0) | 0.65 | 21.0
(14.0–36.0) | 23.0
(17.0–40.0) | 0.45 |
| WT2 (days) | 46.0
(29.0–62.0) | 44.5
(13.0–33.7) | 0.45 | 46.0
(29.0–62.0) | 41.0
(28.0–55.0) | 0.36 |
| Age (years) | 69.0
(62.0–77.0) | 73.0
(69.0–76.0) | 0.21 | 69.0
(62.0–77.0) | 73.0
(68.0–75.0) | 0.31 |
| Tumour size
(longest diameter; mm) | 19.0
(15.0–25.0) | 20.0
(15.0–20.0) | 0.69 | 34.0 (8–45.0) | 30.0
(10.0–30.0) | 0.40 |
| CEA (ng/ml) | 3.0 (1.9–6.7) | 2.45 (1.7–3.8) | 0.78 | 2.9 (1.0–6.2) | 2.4 (1.8–5.6) | 0.97 |
| CA19-9 (U/ml) | 174.6
(28.4–518.0) | 73.2
(16.0–263.0) | 0.53 | 109.0
(22.5–447.0) | 79.7
(26.7–270.0) | 0.17 |
| Sex |
|
|
|
|
|
|
|
Male | 29 | 15 | 0.48 | 33 | 11 | 1.00 |
|
Female | 19 | 14 |
| 24 | 9 |
|
| Tumour
location |
|
|
|
|
|
|
| Ph | 35 | 17 | 0.21 | 31 | 11 | 1.00 |
|
Pbt | 13 | 12 |
| 26 | 9 |
|
| T stage (UICC
ver.7) (24) |
|
|
|
|
|
|
|
T0-1 | 4 | 6 | 0.049 | 8 | 2 | 0.02 |
| T2 | 5 | 7 |
| 5 | 7 |
|
|
T3-4 | 39 | 16 |
| 44 | 11 |
|
| N stage (UICC
ver.7) (24) |
|
|
|
|
|
|
| N0 | 26 | 23 | 0.03 | 38 | 14 | 1.00 |
| N1 | 22 | 6 |
| 19 | 6 |
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
Yes | 30 | 18 | 1.00 | 36 | 12 | 0.79 |
| No | 18 | 11 |
| 21 | 8 |
|
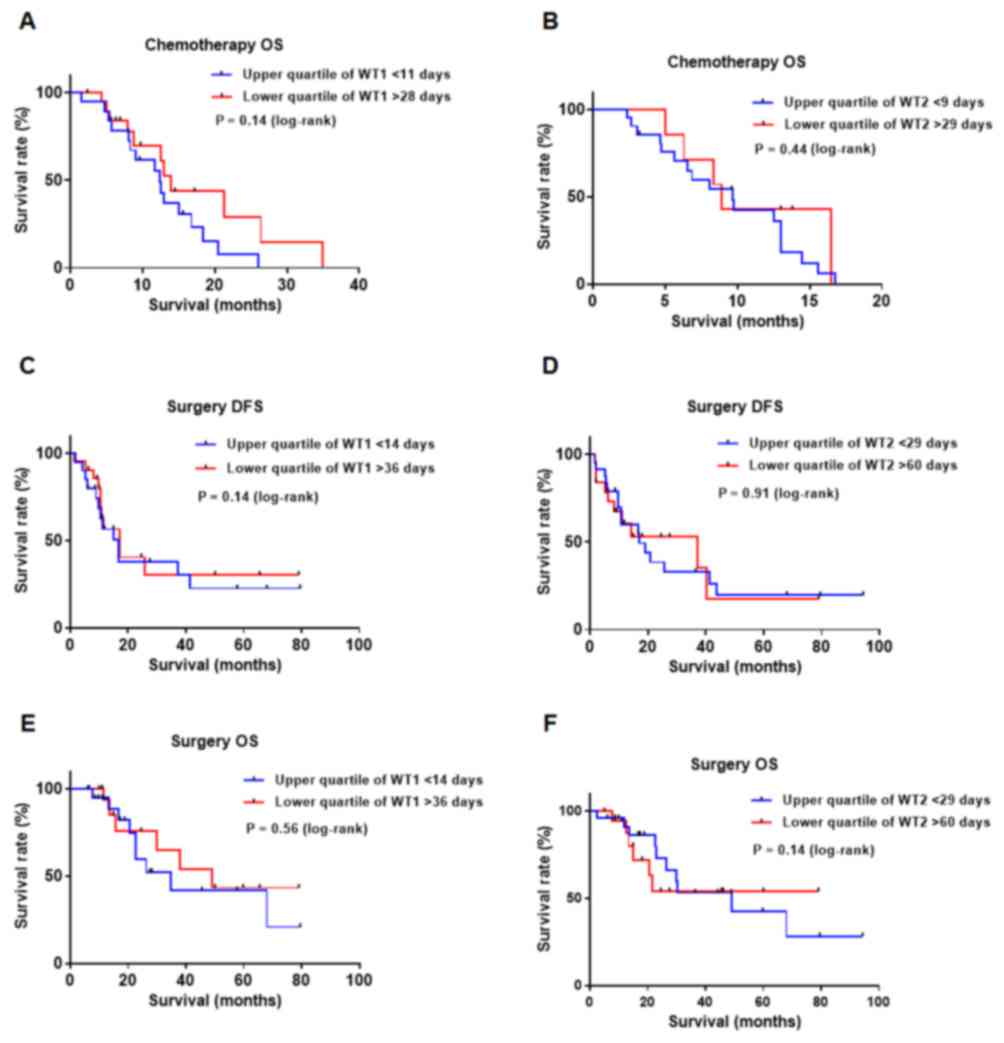
As indicated in Fig.
2, survival analysis for DFS and OS did not reveal any
significant survival differences between patients in the upper and
lower quartiles for WT1 or WT2 (Fig.
2A-F).
Discussion
The present retrospective study was conducted in
order to elucidate whether prolonged waiting times are associated
with the prognosis of unresectable and resectable PDAC. To the best
of our knowledge, no prior studies investigated the prognostic
effect of waiting time-to-diagnosis in patients with PDAC. Contrary
to our hypothesis, the results demonstrated that neither diagnostic
nor treatment waiting times significantly affected OS.
Additionally, the survival analysis did not reveal any notable
differences in survival time between patients who received
immediate treatment (patients in the lower quartile of waiting
times) and patients whose treatment was delayed (patients in the
upper quartile of waiting times).
A total of 3 previous studies that addressed the
waiting times for patients with PDAC were identified: Raptis et
al (12) combined diagnostic and
treatment waiting times and investigated the prognostic impact of
overall waiting time. Consistent with the results of the present
study, their findings indicated that the length of waiting time
from initial referral to diagnosis or treatment did not affect
operability, resectability or survival. Jooste et al
(11) conducted a population-based
study that included 450 patients from two French population-based
cancer registries. Factors associated with patient delay (time
between the first onset of symptoms and first consultation) and
treatment delay (time between first consultation and treatment)
were evaluated. Once the results were adjusted for a number of
clinical factors, including symptoms and treatments, it was
revealed that neither patient nor treatment delay were associated
with patient outcomes. Similarly, Sanjeevi et al (13) demonstrated that a short interval
between detection of the disease and surgical resection did not
affect OS among patients who underwent successful resection of
PDAC; however, it may have been associated with a decreased risk of
unresectability. Collectively, the aforementioned results did not
clearly establish whether timely medical care improves the
prognosis of patients with PDAC.
The present study was originally conducted based on
the hypothesis that prolonged waiting times from detection of
disease to diagnosis or to the initiation of treatment may
negatively affect patient prognosis. If that hypothesis had been
validated, the next step would have been to establish rapid
clinical processing of patients to facilitate prompt diagnosis and
treatment. However, the results of the present study did not
support this hypothesis; nevertheless, healthcare teams should aim
to provide medical care to all patients in a timely manner. The
results also demonstrated that in accordance with common knowledge,
early-stage disease at the initiation of treatment, and the choice
of treatment (combination chemotherapy) were significantly
associated with good outcomes in PDAC (3,6,7).
However, the present study did have certain
limitations, including the fact that it was conducted in a single
center with a limited number of patients. Additionally, there may
have been selection bias, particularly in patients who were not
referred for surgical resection, as the majority of these patients
were not treated in Fukushima Medical University Hospital.
Furthermore, there could be a possibility that the length of
waiting time may affect the choice of treatment, which may
subsequently influence prognosis. In fact, 5 patients had their
planned surgical resection cancelled due to metastasis detected
during waiting time or exploratory laparotomy. However, in terms of
the length of waiting time of these patients, the delay in
management was not considered a direct cause of this consequence.
Patients could have occult metastasis at the time of diagnosis.
Additionally, the patients who underwent chemotherapy in Fukushima
Medical University Hospital may have had additional reasoning,
including comorbidities or good performance status. However, the
present study included almost all the surgical candidates who met
the inclusion criteria. Therefore, the results of the present study
should be validated by assessing an increased number of patients
from multiple clinical sites. In conclusion, the present study did
not provide any evidence that waiting time is associated with the
prognosis in patients with PDAC.
Acknowledgements
The abstract was published as abstract no. e15726 in
J Clin Oncol 35 (Suppl): 2017.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study were included in this published article.
Authors' contribution
RS designed the experiment. RS, TT, TH, MS, NK, HA,
KW, JN, SM and HO performed the experiments. RS wrote the
manuscript. RS, TT and HA analyzed the data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Committee of Fukushima Medical University School of Medicine
(approval no., 2285; Fukushima, Japan). The institutional review
board waived the requirement for written informed patient consent,
due to the retrospective non-interventional nature of the present
study.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geer RJ and Brennan MF: Prognostic
indicators for survival after resection of pancreatic
adenocarcinoma. Am J Surg. 165:68–72. 1995. View Article : Google Scholar
|
|
3
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang MJ, Jang JY, Chang YR, Kwon W, Jung W
and Kim SW: Revisiting the concept of lymph node metastases of
pancreatic head cancer: Number of metastatic lymph nodes and lymph
node ratio according to N stage. Ann Surg Oncol. 21:1545–1551.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Picozzi VJ, Oh SY, Edwards A, Mandelson
MT, Dorer R, Rocha FG, Alseidi A, Biehl T, Traverso LW, Helton WS
and Kozarek RA: Five-year actual overall survival in resected
pancreatic cancer: A contemporary single-institution experience
from a multidisciplinary perspective. Ann Surg Oncol. 24:1722–1730.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okusaka T, Ikeda M, Fukutomi A, Ioka T,
Furuse J, Ohkawa S, Isayama H and Boku N: Phase II study of
FOLFIRINOX for chemotherapy-naive Japanese patients with metastatic
pancreatic cancer. Cancer Sci. 105:1321–1326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dedey F, Wu L, Ayettey H, Sanuade OA,
Akingbola TS, Hewlett SA, Tayo BO, Cole HV, de-Graft Aikins A,
Ogedegbe G and Adanu R: Factors associated with waiting time for
breast cancer treatment in a teaching hospital in Ghana. Health
Educ Behav. 43:420–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jooste V, Dejardin O, Bouvier V, Arveux P,
Maynadie M, Launoy G and Bouvier AM: Pancreatic cancer: Wait times
from presentation to treatment and survival in a population-based
study. Int J Cancer. 139:1073–1080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raptis DA, Fessas C, Belasyse-Smith P and
Kurzawinski TR: Clinical presentation and waiting time targets do
not affect prognosis in patients with pancreatic cancer. Surgeon.
8:239–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanjeevi S, Ivanics T, Lundell L, Kartalis
N, Andrén-Sandberg Å, Blomberg J, Del Chiaro M and Ansorge C:
Impact of delay between imaging and treatment in patients with
potentially curable pancreatic cancer. Br J Surg. 103:267–275.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song H, Fang F, Valdimarsdóttir U, Lu D,
Andersson TM, Hultman C, Ye W, Lundell L, Johansson J, Nilsson M
and Lindblad M: Waiting time for cancer treatment and mental health
among patients with newly diagnosed esophageal or gastric cancer: A
nationwide cohort study. BMC Cancer. 17:22017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neal RD, Tharmanathan P, France B, Din NU,
Cotton S, Fallon-Ferguson J, Hamilton W, Hendry A, Hendry M, Lewis
R, et al: Is increased time to diagnosis and treatment in
symptomatic cancer associated with poorer outcomes? Systematic
review. Br J Cancer. 112 (Suppl 1):S92–S107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perri T, Issakov G, Ben-Baruch G, Felder
S, Beiner ME, Helpman L, Hogen L, Jakobson-Setton A and Korach J:
Effect of treatment delay on survival in patients with cervical
cancer: A historical cohort study. Int J Gynecol Cancer.
24:1326–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Yang R, Lu Y, Xia Y and Zhou H:
Diagnostic accuracy of endoscopic ultrasound-guided fine-needle
aspiration for solid pancreatic lesion: A systematic review. J
Cancer Res Clin Oncol. 138:1433–1441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hewitt MJ, McPhail MJ, Possamai L, Dhar A,
Vlavianos P and Monahan KJ: EUS-guided FNA for diagnosis of solid
pancreatic neoplasms: A meta-analysis. Gastrointest Endosc.
75:319–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hikichi T, Irisawa A, Bhutani MS, Takagi
T, Shibukawa G, Yamamoto G, Wakatsuki T, Imamura H, Takahashi Y,
Sato A, et al: Endoscopic ultrasound-guided fine-needle aspiration
of solid pancreatic masses with rapid on-site cytological
evaluation by endosonographers without attendance of
cytopathologists. J Gastroenterol. 44:322–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki R, Lee JH, Krishna SG, Ramireddy S,
Qiao W, Weston B, Ross WA and Bhutani MS: Repeat endoscopic
ultrasound-guided fine needle aspiration for solid pancreatic
lesions at a tertiary referral center will alter the initial
inconclusive result. J Gastrointestin Liver Dis. 22:183–187.
2013.PubMed/NCBI
|
|
21
|
Hébert-Magee S, Bae S, Varadarajulu S,
Ramesh J, Frost AR, Eloubeidi MA and Eltoum IA: The presence of a
cytopathologist increases the diagnostic accuracy of endoscopic
ultrasound-guided fine needle aspiration cytology for pancreatic
adenocarcinoma: A meta-analysis. Cytopathology. 24:159–171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eastern Cooperative Oncology Group. ECOG
performance status. http://ecog-acrin.org/resources/ecog-performance-statusJune
3–2015
|
|
23
|
International Union against Cancer, .
Sobin LH, Gospodrowicz MK and Wittekind CH: TNM Classification of
Malignant Tumours. 7th. New York, NY: Wiley-Liss; 2009
|