Introduction
Glioma is the most common primary malignant tumor of
the intracranial tumors, accounting for approximately 45% of all
intracranial tumors. The prognosis of glioma patients is generally
poor, and the higher the malignant degree is, the worse the
prognosis of glioma patients is (1,2). It has
been reported (3) that the median
survival time of patients with grade IV polyglioblastoma is only
one year with less than 5% of 5-year survival rate, which is one of
the malignant tumors with the highest human mortality. The
treatment of glioma is mainly surgery, supplemented by radiotherapy
and chemotherapy (4). Despite the
continuous development of medical technology in recent years, the
improvement of survival rate of glioma patients is still limited.
Many studies have reported that the high mortality rate of glioma
is closely related to the excessive proliferation and invasion of
tumor cells (5,6).
miRNAs is widely expressed in eukaryotic organism,
regulating cell proliferation, differentiation and apoptosis, while
abnormal changes in miRNAs biosynthesis are involved in many
pathophysiological processes (7,8). Many
studies have reported that miRNAs are closely related to biological
behavior such as proliferation and invasion of tumor cells
(9,10). Guo et al (11) reported that the increased expression
level of mir-128a in hepatocellular carcinoma (HCC) could promote
the proliferation of HCC cells. However, in the study of Yamada
et al (12), it was found that
mir-128a could increase the anti-apoptosis ability of Jurkat cells
in leukemia patients. Therefore, we speculate that mir-128a plays
different roles in different tumors, but there are few studies on
the relationship between mir-128a and the biological behavior of
glioma cells.
The purpose of this study is to analyze the effects
of mir-128a on the biological behavior of glioma U251 cells by
regulating the expression level of mir-128a to provide experimental
and theoretical basis for clinical treatment, to prolong the
patient's life span and to improve the patient's quality of
life.
Materials and methods
Cell source
Human glioma U251 cells (cat. no. CC-Y1526),
purchased from Shanghai Enzyme Research Biotechnology Co., Ltd.
(Shanghai, China), were cultured in DMEM (containing 10% fetal
bovine serum) culture medium (Beijing North Tongzheng Biotechnology
Development Co., Ltd., Beijing, China). The culture conditions of
human glioma U251 cells were 37°C and 5% CO2, and the
construction and synthesis of mir-128a expression vector and
scramble shRNA were constructed by Shanghai GenePharma Biology Co.
(Shanghai, China). The constructed mir-128a-shRNA lentivirus vector
(infection group) and scramble shRNA (interference group) were
cultured together with human glioma U251 cells digested with
trypsin in DMEM (containing 10% fetal bovine serum) culture medium,
and then transfected after cultured at culture medium with 37°C 5%
CO2 for 48 h. The specific steps referred to the kit
instructions and other experimental tests were performed after
transfection. The Liposome 2000 transfection kit was purchased from
Shanghai Bayley Biotechnology Co., Ltd. (Shanghai, China). A group
of U251 cells, which was not infected, was used as control
group.
The study was approved by the Ethics Committee of
China-Japan Union Hospital of Jilin University (Changchun,
China).
Extraction of total miRNA from
cells
TRIzol was used to extract and collect
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) the total RNA of
human glioma U251 cells according to the instructions. The
concentration and purity of extracted RNA were analyzed by Micro
ultraviolet spectrophotometer GeneQuant1300/100D [GE Medical System
Trade Development (Shanghai) Co., Ltd., Shanghai, China], and the
RNA specimens of A260/A280 between 1.8 and 2.0 were considered to
meet the test standard. The integrity of RNA was analyzed by 3%
agarose gel electrophoresis (gel electrophoresis set was purchased
from Shanghai Jingke Chemical Technology Co., Ltd., Shanghai,
China).
Reaction of mir-128a to RT-qPCR
The total RNA extracted above was synthesized of
cDNA by reverse transcription according to the instructions in the
TaqMan MicroRNA reverse transcription kit [Symevier Technology
(China) Co., Ltd., Shenzhen, China]. The cDNA amplification
reaction system was 10 µl, 1.0 µl for oligo DT primer, 1.0 µl for
dNTP mixture, 2 µg for total RNA, 1 µl for Taq DNA polymerase, and
non-ribonuclease distilled water added to 10 µl. Reverse
transcription reaction: 37°C for 45 min and 65°C for 5 min. The
reaction system was 50 µl, 2 µl for cDNA template, 32.5 µl for
SYBR-Green Mix (Guangzhou Dongsheng Biotechnology Co., Ltd.), 0.5
µl for upstream primer and 0.5 µl for downstream primer, and double
distilled water added to 50 µl. PCR amplification: 3 min after
pre-denaturation at 95°C, 30 sec for denaturation at 95°C, 30 sec
for annealing at 55°C, 60 sec for extension at 72°C, 30 cycles, and
5 min for extension at 72°C after the completion of cycle. GAPDH
was used as the internal parameter of the reaction. All the samples
were repeated three times and the result was analyzed by
2−ΔΔCq (13) method.
Primer sequences are shown in Table
I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Sequence |
|---|
| mir-128a |
5′-ACACTCCAGCTGGGTCACAGTGAACCG-3′ |
5′-CCCAAGCTTATGAAGCCAAATGATGCAAAAT-3′ |
| GAPDH |
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
Proliferation in vitro of human glioma
U251 cells detected by MTT assay
The human glioma U251 cells were prepared into
single cell suspension and 96-well cell culture plate was used for
cell routine inoculation culture. Some of the cultured cells were
taken out at 6 h, and 20 µl MTT (5 mg/ml) were added. The
supernatant containing impurity was sucked out at 37°C for 4 h,
then the dimethylsulfoxide was added and placed on a horizontal
shaking bed for 15 min. Finally, the absorbance at 570 nm
wavelength was determined by enzyme linked immunosorbent assay
(ELISA). The above steps were repeated in the experiment at 12, 24,
48 and 72 h, respectively. MTT test kit was purchased from Shanghai
Lianmai Bioengineering Co., Ltd., Shanghai, China.
Transwell invasion in vitro
The prepared U251 cell suspension was inoculated
into the Transwell chamber and the number of cells passed through
was detected after two weeks. Three parallel trials were conducted
simultaneously. The Transwell chamber was purchased from Shanghai
Yuanzi Biotechnology Co., Ltd., Shanghai, China.
TUNEL cell apoptosis assay
U251 cells were cultured for 48 h, approximately
5×107/ml, were fixed at room temperature with 4% neutral
formaldehyde for 10 min and the excess liquid was removed and
washed twice with PBS for 5 min each time. PBSs containing 2%
hydrogen peroxide were treated at room temperature for 5 min and
the excess liquid was removed and washed twice with PBS for 5 min
each time. l TUNEL assay solution (50 μl) was added to each
well (the ratio of TdT enzyme to fluorescent labeling solution was
1:24), and incubated at 37°C for 60 min. The cells were dyed
according to the instructions provided by TUNEL kit (Shanghai Rong
Wei Da Industrial Co., Ltd., Shanghai, China). The cell was sealed
with antifade mounting medium and stored at 2–8°C. The number of
TUNEL positive cells in 5 fields of vision under the 400-fold
microscope was counted by image analysis software (Image-Pro Plus
5.0), and the cumulative optical density value was used to indicate
the total number of TUNEL-positive cells. The asssay was repeat
three times.
Cell migration ability detected by
wound scratch assay
The cells transfected with each group for 48 h were
inoculated to a six-well plate, and three groups of repeat wells
were set up. When the cell fusion reached approximately 90%, 20 µl
was used perpendicular to the 6-well culture plate and drawn
according to the pre-prepared horizontal line. PBS was used to wash
the plate three times, and 1% FBS DMEM medium was used for
continuous cultivation. This was repeated three times with imaging
measurement. Cell imaging system Phase-contrast microscope
(EVOS® FL Cell Imaging System), Thermo Fisher
Scientific, Inc., Waltham, MA, USA.
Statistical analysis
Using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA). The
comparison of ratio was tested with χ2. The measurement
data were expressed as mean ± standard deviation, the comparison of
groups was analyzed by ANOVA and the differences between two groups
were detected and analyzed by LSD. The differences were
statistically significant (P<0.05).
Results
Analysis of mir-128a detection in U251
cells by RT-qPCR
The result of mir-128a detection in U251 cells by
RT-qPCR showed that the expression level of mir-128a in U251 cells
of infection group was 1.123±0.012, which was significantly higher
than that in U251 cells of interference group, 0.203±0.001
(P<0.05). The expression level of mir-128a in U251 cells of
control group was 0.573±0.004, which was significantly lower than
that in U251 cells of infection group (P<0.05), but higher than
that in U251 cells of interference group (P<0.05) (Fig. 1).
Cell proliferation of U251 cells
detected by MTT assay
The result of proliferation in vitro of U251
cells detected by MTT assay showed that the OD values of infection
group and control group were lower than that of interference group
at 6, 12, 24, 48 and 72 h, and the OD values of infection group
were lower than that of control group at 6, 12, 24, 48 and 72 h.
The cell proliferation ability of infection group and control group
was lower than that of interference group and the cell
proliferation ability of U251 cells of infection group was lower
than that of U251 cells of control group. The differences were
statistically significant (P<0.05) (Fig. 2).
 | Figure 2.Proliferation in vitro of U251
and HA1800 cells detected by MTT assay. The result of MTT assay
showed that the OD values of infection and normal control group
were lower than that of interference group at 6, 12, 24, 48 and 72
h, and the OD values of infection were lower than that of normal
control group at 6, 12, 24, 48 and 72 h. The cell proliferation
ability of infection and normal control group was lower than that
of interference group, and the cell proliferation ability of U251
cells of infection group was lower than that of HA1800 cells of
normal control group (P<0.05). The differences were
statistically significant (P<0.05). aP<0.05, more
infection group; bP<0.05, more normal control
group. |
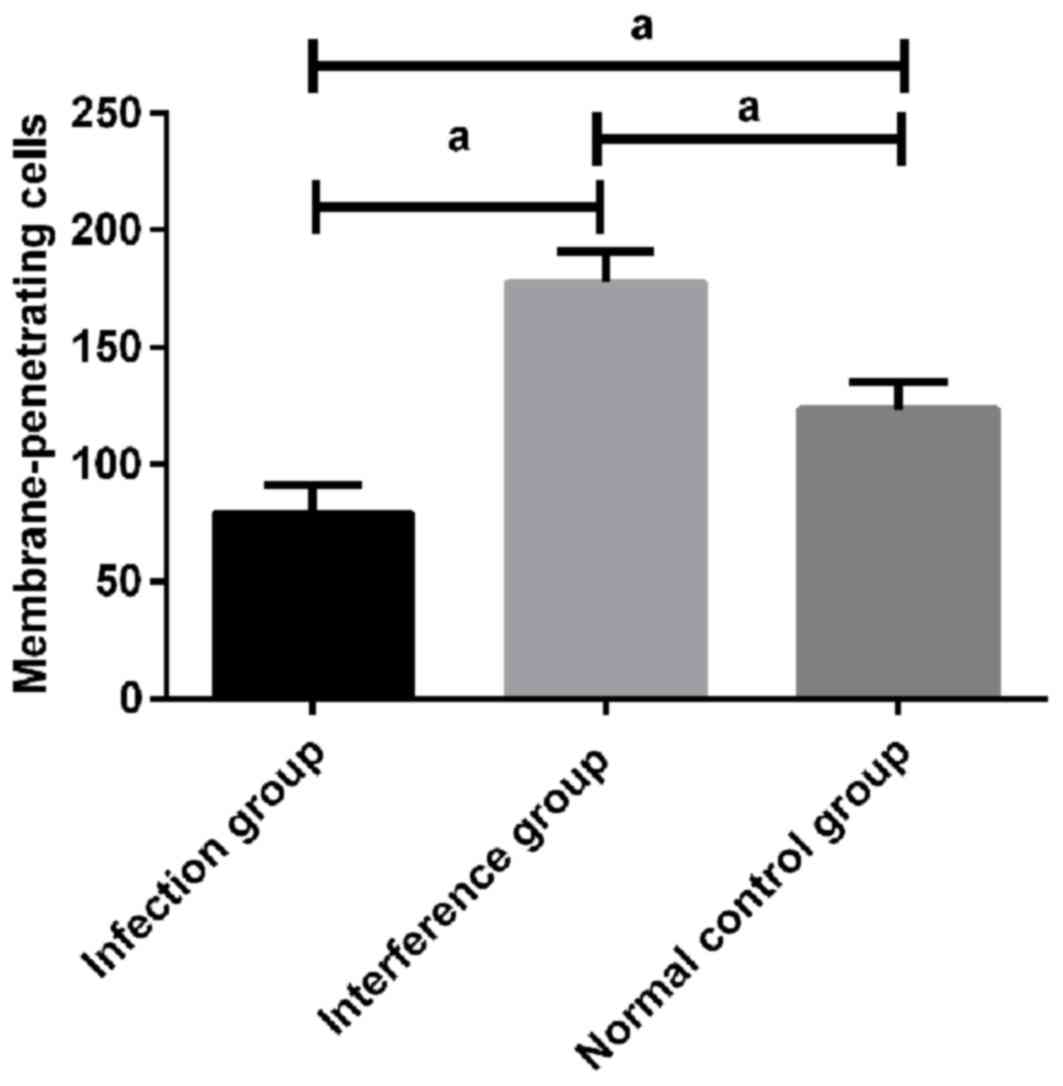
Invasion of U251 cells detected by
Transwell migration in vitro
The result of invasion of U251 cells detected by
Transwell migration in vitro showed that compared with
infection group (79.13±12.04), the number of membrane-penetrating
cells (177.58±13.49) in U251 cells of interference group increased
significantly (P<0.05). The number of membrane-penetrating cells
in U251 cells of control group was 123.72±11.45, which was higher
than that of infection group (P<0.05), but lower than that of
interference group (P<0.05) (Fig.
3).
Apoptosis of U251 cells detected by
TUNEL cell apoptosis assay
The result of apoptosis of U251 cells detected by
TUNEL cell apoptosis assay showed that the apoptosis rate of
infection and control group (38.47±1.26%, 28.32±1.23%) was
significantly higher than that of interference group (11.88±1.11%).
The apoptosis rate of infection group was significantly higher than
that of control group, and the differences were statistically
significant (P<0.05) (Fig. 4).
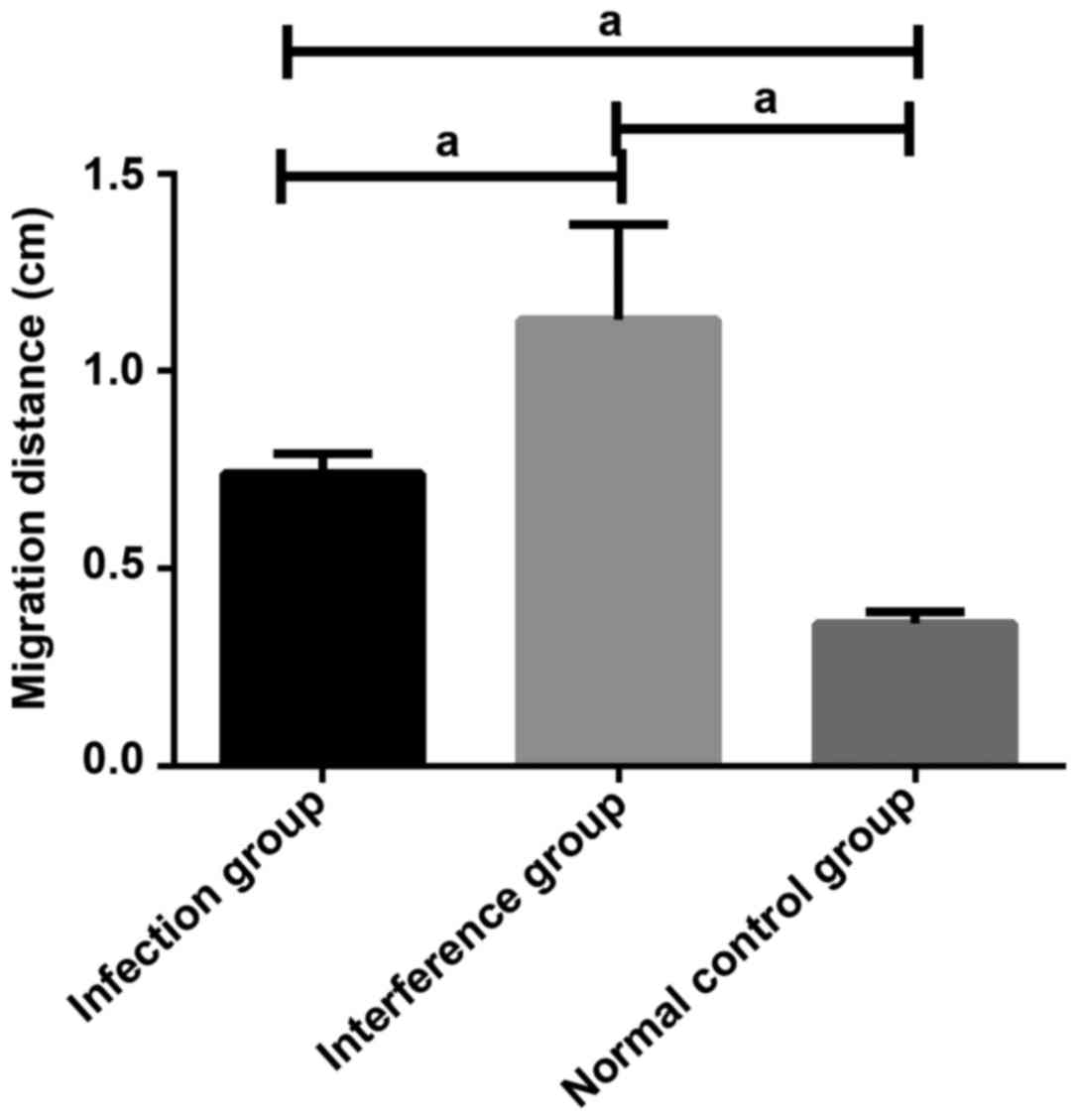
Migration ability of U251 cells in
cell wound scratch assay
Through observing the width of the wound scratch
under an inverted optical microscope, it could be found that the
migration distance of U251 cells of infection group and
interference group was significantly larger than that of U251 cells
of control group, and the difference was statistically significant
(P<0.05). The migration distance of U251 cells of interference
group was significantly larger than that of U251 cells of infection
group, and the difference was statistically significant (P<0.05)
(Fig. 5).
Discussion
The occurrence and development of tumor is a
pathological process where multi-step and multi-molecular are
involved, and its most important biological features are the
ability of almost infinite proliferation and extremely high ability
of invasion and metastasis of malignant tumor cells (14,15). The
main reason for the high recurrence rate and low survival rate of
glioma patients is the invasiveness and metastasis of glioma cells
(16,17). Therefore, it is of great significance
to explore the related molecular mechanism of glioma cell
proliferation and invasion, to provide experimental and theoretical
reference for inhibiting the excessive proliferation and invasion
of glioma cells, to increase the survival rate of patients and to
improve their quality of life.
In this study, mir-128a-shRNA lentivirus vector was
constructed to upregulate the expression level of mir-128a in human
glioma cell U251, and scramble shRNA was constructed to interfere
with the expression of mir-128a in human glioma cell U251. The
result of comparison with the expression level of mir-128a in
uninfected U251 cells showed that the expression level of mir-128a
in U251 cells of infection group was significantly higher than that
in U251 cells of interference group, and the expression level of
mir-128a in U251 cells of control group was significantly lower
than that in U251 cells of infection group, but higher than that in
U251 cells of interference group. The expression vector was
successfully transfected into glioma U251 cells and expressed
successfully, which was consistent with the previous expectation.
In this study, the result of biological behavior detection of
glioma U251 cells showed that upregulation the expression level of
mir-128a could inhibit the ability of proliferation, invasion and
migration of glioma U251 cells, and promote apoptosis level of
glioma U251 cells. Therefore, we conclude that mir-128a could
inhibit the malignant biological behavior of glioma cells.
Nie et al (18)
reported that upregulation of mir-128a could inhibit the
proliferation ability of glioma U87 cells. Venkataraman et
al (19) found that mir-128a
target regulated Bmi-1, increased the level of intracellular
reactive oxygen species, and promoted the senescence of neural
tubular tumor cells, thereby inhibiting tumor proliferation. From
these results we can infer that mir-128a may play a role as
anti-oncogene in central nervous system tumors, but the mechanism
of action of mir-128a in glioma cells needs to be further verified.
The Bmi-1 and reactive oxygen species level is a very good
direction and Bmi-1 gene is one of the core members of the PcG
family. Bmi-1 is highly expressed in tumor cells, which makes tumor
cells regenerate into cancer stem cells (19). Nevertheless, De Luca et al
(20) reported that lowering the
level of mir-128a could induce the increase of Lin28a expression
and LIN28 could regulate the self-renewal of stem cells and improve
bone marrow differentiation disorder in patients with acute myeloid
leukemia. Guo et al (11)
found that miR-128a was upregulated in hepatocellular carcinoma and
promoted the proliferation of hepatocellular carcinoma cells by
targeting RND3 and RND3 was a member of the Rnd subgroup of the Rho
family of GTP enzymes and regulated the tissue of actin
cytoskeleton through the response of extracellular growth factors.
From their results we can find that miR-128a plays a role similar
to oncogene in hepatocellular carcinoma and acute myeloid leukemia.
Therefore, we speculate that miR-128a may inhibit the biological
behavior of tumors in the central nervous system, such as glioma,
thus promoting the biological behavior of hepatocellular carcinoma,
leukemia and other organic cancers as well as blood cancer.
Ye et al (21)
found that U-87MG glioblastoma cells exposed to X-ray radiation
could decrease the expression level of mir-128a, induce the
upregulation of the expression level of Bmi-1, and then lead to the
decrease of reactive oxygen species in cells, which would cause the
cells to escape from aging and death. This may also be one of the
reasons why the radiotherapy effects of glioma patients is not
satisfactory, and we will further verify the effects of mir-128a on
the radiotherapy effects of glioma cells in the future studies.
This study also investigated cell lines, so more clinical studies
are needed to prove the results of this study.
Collectively, mir-128a may play a role similar to
anti-oncogene in glioma, inhibiting the ability of proliferation,
invasion and migration of glioma cells, and promoting apoptosis of
glioma cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GH drafted the manuscript. GH and WF were mainly
devoted to extraction of total miRNA from cells. GH and NL were
responsible for transwell invasion in vitro. CL performed
RT-qPCR. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
China-Japan Union Hospital of Jilin University (Changchun,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eckel-Passow JE, Lachance DH, Molinaro AM,
Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML,
Smirnov IV, et al: Glioma groups based on 1p/19q, IDH, and TERT
promoter mutations in tumors. N Engl J Med. 372:2499–2508. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogelstein B, Kinzler KW, Parsons DW,
Zhang X, Lin CH, Leary RJ, Angenendt P, Papadopoulos N, Velculescu
V, Parmigiani G, et al: Genetic alterations in isocitrate
dehydrogenase and other genes in malignant glioma. US Patent
2017/0081730 A1. Filed November 16, 2016; issued. March
23–2017.
|
|
3
|
Ceccarelli M, Barthel FP, Malta TM,
Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh
A, Pagnotta SM, et al; TCGA Research network, . Molecular profiling
reveals biologically discrete subsets and pathways of progression
in diffuse glioma. Cell. 164:550–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noch EK, Ramakrishana R and Magge R:
Challenges in the treatment of glioblastoma: Multisystem mechanisms
of therapeutic resistance. World Neurosurg. 116:505–517. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Venugopal C, McFarlane NM, Nolte S,
Manoranjan B and Singh SK: Processing of primary brain tumor tissue
for stem cell assays and flow sorting. J Vis Exp. 67(pii):
41112012.
|
|
7
|
Leidinger P, Backes C, Meder B, Meese E
and Keller A: The human miRNA repertoire of different blood
compounds. BMC Genomics. 15:4742014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brabants E, Pyfferoen L, Everaert C,
Tavernier SJ, Heyns K, De Cabooter N, Wagemans G, Deswarte K,
Hammad H, de wever O, et al: Specific myelomonocytic cells heavily
infiltrate orthotopic lung tumors and display a hypoxia-driven
miRNA expression signature that directs tumor-supporting functions
and negatively impacts on clinical outcome. Cancer Immunol Res. 4
Suppl 11:Abst A091. 2016. View Article : Google Scholar
|
|
9
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bleckmann A, Leha A, Artmann S, Menck K,
Salinas-Riester G, Binder C, Pukrop T, Beissbarth T and Klemm F:
Integrated miRNA and mRNA profiling of tumor-educated macrophages
identifies prognostic subgroups in estrogen receptor-positive
breast cancer. Mol Oncol. 9:155–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo X, Cao C, Sun J, Zhang D, Liu L and Wu
D: miR-128a is up-regulated in hepatocellular carcinoma and
promotes tumor cell proliferation by targeting RND3. Nan Fang Yi Ke
Da Xue Xue Bao. 34:1408–1413. 2014.(In Chinese). PubMed/NCBI
|
|
12
|
Yamada N, Noguchi S, Kumazaki M, Shinohara
H, Miki K, Naoe T and Akao Y: Epigenetic regulation of
microRNA-128a expression contributes to the apoptosis-resistance of
human T-cell leukaemia jurkat cells by modulating expression of
fas-associated protein with death domain (FADD). Biochim Biophys
Acta. 1843:590–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Topalian SL, Sznol M, McDermott DF, Kluger
HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB,
Powderly JD, et al: Survival, durable tumor remission, and
long-term safety in patients with advanced melanoma receiving
nivolumab. J Clin Oncol. 32:1020–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra242014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buckner JC, Shaw EG, Pugh SL, Chakravarti
A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, et
al: Radiation plus procarbazine, CCNU, and vincristine in low-grade
glioma. N Engl J Med. 374:1344–1355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mellinghoff IK, Touat M, Maher E,
DeLaFuente M, Cloughesy TF, Holdhoff M, Cote G, Burris H, Janku F,
Huang R, et al; ACTR-46. AG120, a first-in-class mutant IDH1
inhibitor in patients with recurrent or progressive IDH1 mutant
glioma, . Results from the phase 1 glioma expansion cohorts. Neuro
Oncol. 18 Suppl 6:vi122016. View Article : Google Scholar
|
|
18
|
Nie QM, Lin YY, Yang X, Shen L, Guo LM,
Que SL, Li XX, Ge JW, Wang GS, Xiong WH, et al:
IDH1R¹32H decreases the proliferation of U87 glioma
cells through upregulation of microRNA-128a. Mol Med Rep.
12:6695–6701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Venkataraman S, Alimova I, Fan R, Harris
P, Foreman N and Vibhakar R: MicroRNA 128a increases intracellular
ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer
cell growth by promoting senescence. PLoS One. 5:e107482010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Luca L, Trino S, Laurenzana I,
Tagliaferri D, Falco G, Grieco V, Bianchino G, Nozza F, Campia V,
D'Alessio F, et al: Knockdown of miR-128a induces Lin28a expression
and reverts myeloid differentiation blockage in acute myeloid
leukemia. Cell Death Dis. 8:e28492017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye L, Yu G, Wang C, Du B, Sun D, Liu J, Qi
T, Yu X, Wei W, Cheng J, et al: MicroRNA-128a, BMI1 polycomb ring
finger oncogene, and reactive oxygen species inhibit the growth of
U-87 MG glioblastoma cells following exposure to X-ray radiation.
Mol Med Rep. 12:6247–6254. 2015. View Article : Google Scholar : PubMed/NCBI
|