Introduction
The prostate gland is an accessory reproductive
organ in males located between the bladder and penis. Cancer of the
prostate is the most common type of cancer in the USA and was the
second leading cause of cancer-associated mortality in 2016
(1). Uncontrolled growth associated
with cellular migration and metastasis is the leading cause of
cancer-associated mortality (2), with
17–34% of patients exhibiting metastatic disease at the time of
initial diagnosis (3). Late-stage
diagnosis remains an important problem, accounting for 37–43% of
all cases, and is a significant risk factor for disease-associated
mortality. Cancer screening tests, including screening for the
prostate-specific antigen, have been useful for early detection,
although the low sensitivity (20.5%) and specificity (93.6%) of
this test highlight its limitations for prostate cancer detection
(4).
Androgens and the androgen receptor (AR) are an
important group of male steroid hormones that regulate prostate
growth. Upon binding to androgen, the AR acts as a transcriptional
factor, serving a major role in the proliferation, invasion and
viability of prostate cancer cells (5). First-line therapies for prostate cancer
directly target this pathway, by blocking androgen production or
through suppression of the AR. However, despite the initial
efficacy of these therapies, acquired resistance remains
unavoidable (6). Subsequent lines of
treatment, including chemotherapy and radiotherapy, may offer
certain benefits, although typically these only extend life for an
additional few months (7,8). The scope for further advances in
prostate cancer treatment remains limited due to mutations in
numerous oncogenes, including phosphatase and tensin homolog,
cellular tumor antigen p53, BRCA2 DNA repair associated, AR and MYC
proto oncogene, which limit the effectiveness of targeted therapies
(9–11). A greater understanding of the genes
and mechanisms underlying prostate cancer progression is therefore
necessary to develop more effective therapies.
Hepatocyte nuclear factor 1b (HNF1B) is an important
transcriptional factor that regulates embryonic survival and
vertebrate development (12).
Recently, it has been demonstrated that increased HNF1B expression
may help protect against prostate cancer (13). By contrast, overexpression of genes
such as enoyl-CoA-(Δ) isomerase 2 (ECI2), an important regulator of
fatty acid metabolism, may promote prostate cancer growth (14), although the complex interaction
between these two genes remains poorly understood. In the present
study, the functional association between HNF1B and ECI2 in
promoting prostate cancer was analyzed.
Materials and methods
Mouse model of prostate cancer
Heterozygous male and female mice at 5 weeks old
[C57BL/6-Tg 8247Ng/J transgenic adenocarcinoma of the mouse
prostate (TRAMP)] were purchased (Jackson Laboratory, Bar Harbor,
ME, USA) and allowed to breed to obtain homozygous males. The males
were bred with female FVB/NJ mice (Jackson Laboratory) to obtain
C57BL/6 TRAMP × FVB mice, as described previously (15). TRAMP mice develop a progressive form
of prostate cancer. Male mice at 6 weeks old (C57BL/6 TRAMP × FVB;
n=18), divided equally into three groups (n=6), were used in all
experiments. The mice were fed an AIN-93G diet and provided water
ad libitum. The housing conditions in which the mice were
maintained were 24–26°C, 55–60% humidity and a light/dark cycle of
14/10 h. Mice without TRAMP overexpression were used as controls.
Mice were sacrificed at 12, 18 and 24 weeks, and the prostate
glands were dissected and subjected to further analysis. All the
animals that were used in the present study, and the protocol
followed, were approved by the institutional ethical committee
(Taihe Hospital, Hubei University of Medicine, Shiyan, China).
Histological imaging
Dissected prostate glands were cut into small pieces
and fixed in 10% formalin solution for 48 h at 40°C. Tissues were
subjected to gradual dehydration by transfer into increasing
concentrations of isopropyl alcohol and embedded in paraffin
blocks. Using a microtome, tissue sections were cut into 5 µm thick
slices and mounted on glass slides. Mounted sections were stained
with hematoxylin (5–7 min) and counterstained with eosin (30 sec)
at room temperature to visualize the cellular nucleus and
cytoplasm. Final mounting was performed using DPX solution, which
hardens to permanently seal the section on the slide. The samples
were visualized under a light microscope at ×20 magnification.
Immunohistochemistry
Tissue sections were cut and mounted onto glass
slides, as described above. Cellular endogenous peroxide activity
was blocked by incubating the sections in 3%
H2O2 solution in methanol. Slides were
transferred into antigen retrieval solution (10 mM citrate buffer;
pH 6.0) and incubated at 95°C for 7 min to unmask the antigenic
epitope. Bovine serum albumin (BSA; 4%; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used as a blocking buffer (2 h at room
temperature) to reduce non-specific binding of antibodies. Primary
antibodies against HNF1B (cat. no. CL0374; Abnova, Taipei, Taiwan;
1:200 dilution) or ECI2 (cat. no. 201243; Creative Diagnostics, New
York, NY, USA; 1:250 dilution) were diluted in 1% BSA solution in
1X PBS and poured over the sections. Following treatment, slides
were incubated at 4°C overnight, washed three times with 1X PBS,
and incubated with the anti-mouse or anti-rabbit horseradish
peroxidase (HRP)-conjugated secondary antibody (cat. nos. ab6789 or
ab6721, respectively; 1:4,000 or 1:3,500 dilution, respectively;
both Abcam, Cambridge, MA, USA) at room temperature for 1 h. Slides
were washed again in 1X PBS and developed with
3,3′-diaminobenzidine solution at room temperature for 10 min. The
signals obtained were visualized under a light microscope at ×20
magnification.
Western blotting
Dissected prostate gland tissues were weighed and
cut into small species. Tissue were homogenized with 2X protein
sample buffer [4% SDS, 20% glycerol, 0.004% bromophenol blue,
0.125M Tris/HCl (pH 6.8), 10% 2-mercaptoethanol] and diluted with
water to a final 1X working concentration. The prepared samples
were heated in a boiling water bath for 10 min along with sample
buffer, which helps to preserve protein expression. The protein
concentration was quantified using the Lowry method and 70 µg was
loaded into each well. The protein samples were separated by
SDS-PAGE on a 12% gel, at 50 V for 3 h, transferred to a
polyvinylidene difluoride membrane, and blocked in 3% BSA in TBS
with Tween-20 at room temperature for 1 h. The membrane was probed
with anti-HNF1B antibody (cat. no. CL0374; Abnova; 1:300 dilution),
anti-ECI2 antibody (cat. no. 201243; Creative Diagnostics; 1:500
dilution) or anti-β-actin antibody (cat. no. ab8227; Abcam; 1:1,000
dilution) overnight at 4°C. Following washing three times with 1X
PBS with Tween 20, the membrane was incubated with a specific
HRP-conjugated secondary antibody (anti-mouse IgG HRP; Abcam; cat.
no. ab6789; 1:5,000 dilution) or (anti-rabbit IgG HRP; Abcam; cat.
no. ab6721; 1:4,000 dilution) at room temperature for 2 h.
Following incubation, the membrane was washed with 1X PBST and
developed using a diaminobenzidine substrate kit (Abcam; cat. no.
ab64238). HNF1B and EC12 expression levels were normalized to
β-actin expression levels. The band intensities were measured using
ImageJ software (version 1.5; http://imagej.net/ImageJ).
Statistical analysis
Statistical analyses for the obtained data were
performed using SPSS for Windows (version 11.0; SPSS, Inc.,
Chicago, IL, USA). All the experiments were performed independently
in triplicate and the results are expressed as the mean ± standard
error of the mean. The statistical significance was assessed using
analysis of variance followed by Tukey's post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Mice that express TRAMP show
progressive prostate cancer
Prostate cancer progression was assessed in C57BL/6
TRAMP × FVB, a transgenic mouse line with enhanced susceptibility
to prostate cancer due to TRAMP overexpression. Pathological
outcomes were assessed at 12, 18 and 24 weeks, and were determined
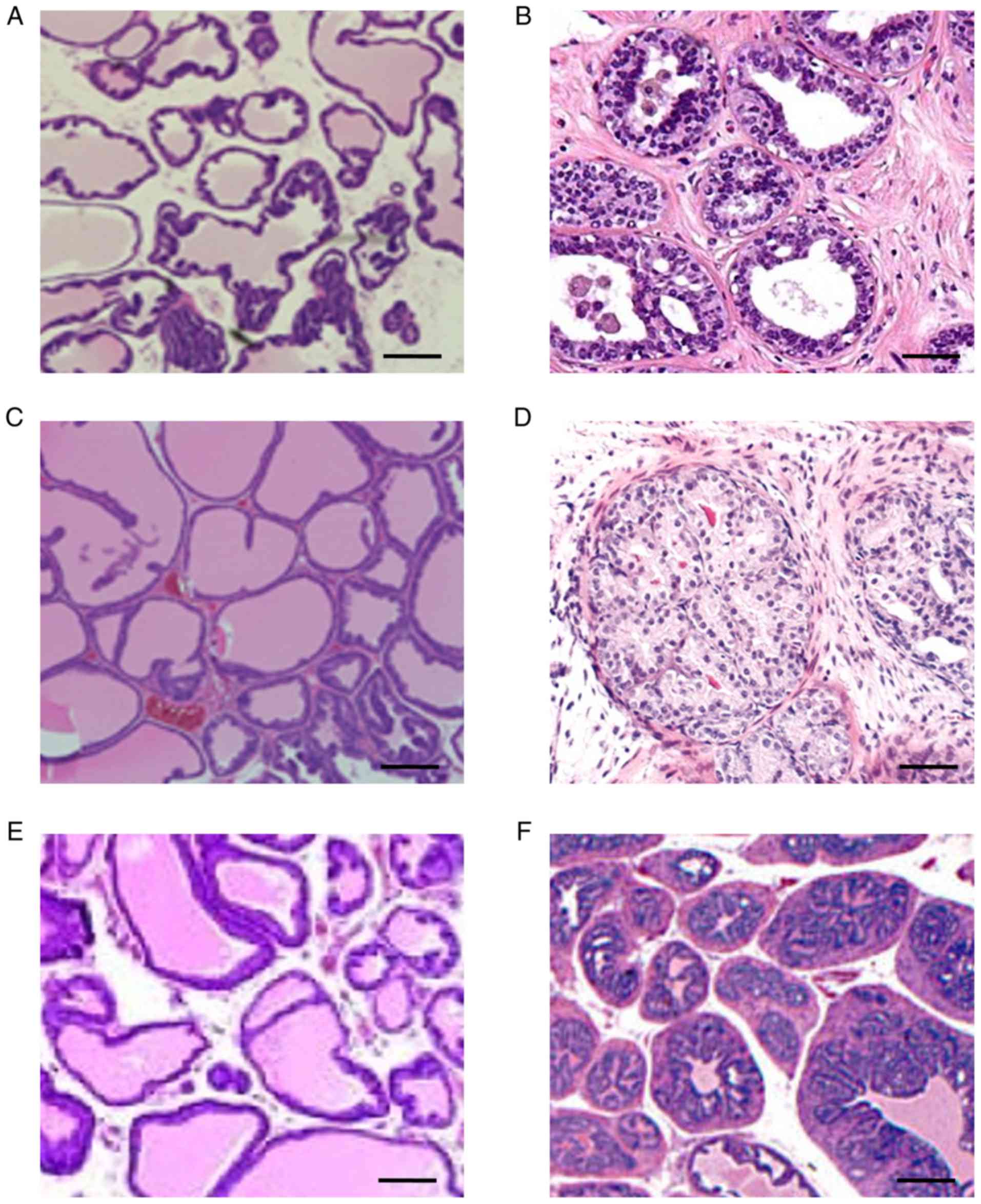
based on histological analysis of the prostate gland (Fig. 1). Prostates from 12-week-old control
mice revealed a multi-lobed structure with a minimal glandular
epithelium, and regular spacing between lobes (Fig. 1A). By contrast, 12-week-old
TRAMP+ mice exhibited an initial thickening of the
glandular epithelium with marked proliferation between the lobes
(Fig. 1B). Development of fatty
tissue between prostate lobes was evident by week 18 in control
mice (Fig. 1C), compared with
increased cellular infiltration in TRAMP+ mice with
migration towards the interior of the lobes (Fig. 1D). By week 24, control mice displayed
a gradual thickening of epithelial cells with minimal hyperplasia
(Fig. 1E), whereas TRAMP+
mice exhibited a progressive hardening of the lobes with extensive
thickening of glandular cells and increased hyperplasia (Fig. 1F).
Expression of HNF1B with prostate
cancer progress
HNF1B is a transcriptional factor that regulates a
variety of important cellular processes (16,17),
including responses to hormones and growth factors. In the present
study, the expression levels of HNF1B in different grades of
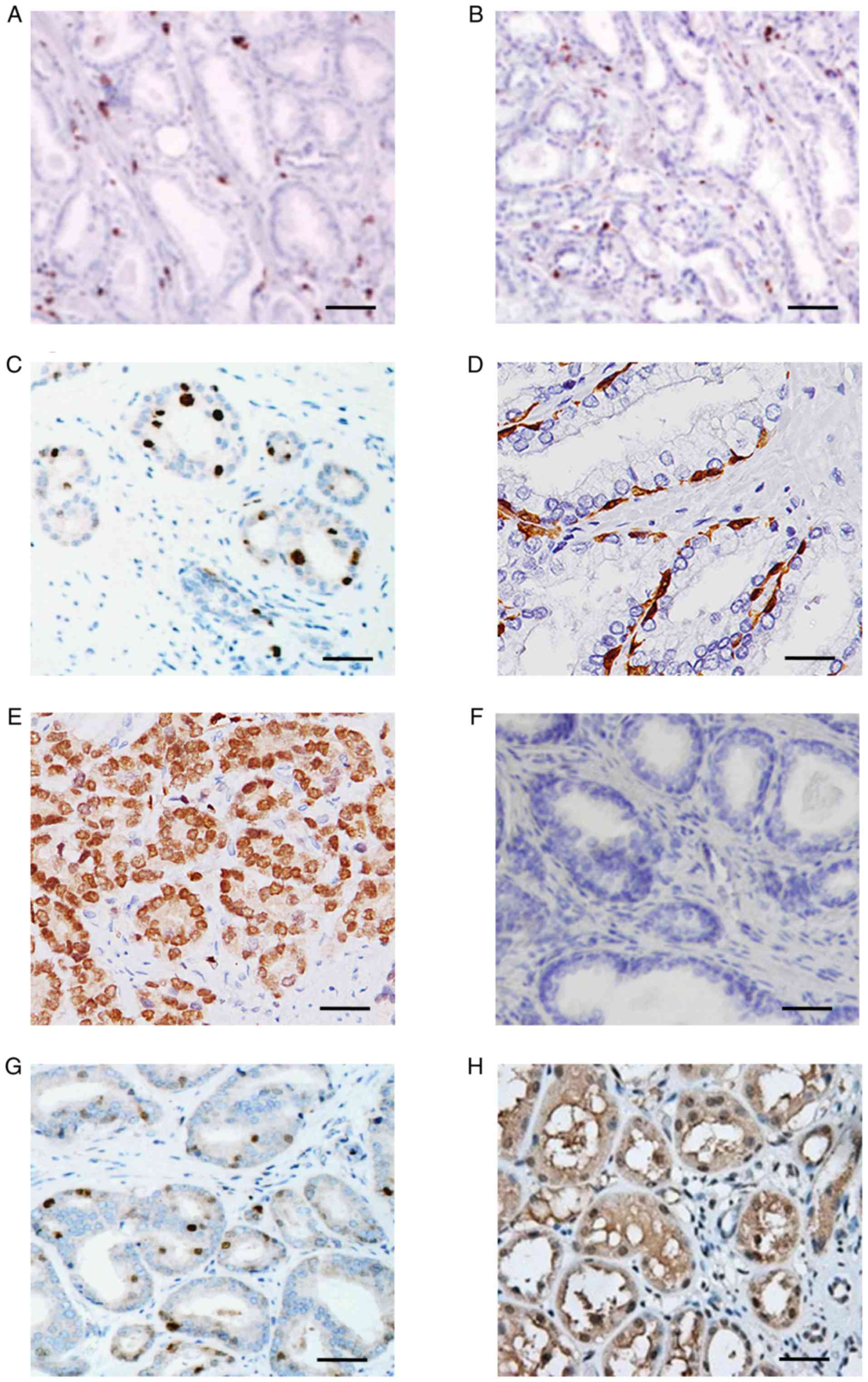
prostate cancer histological sections were assessed (Fig. 2). HNF1B displayed minimal expression
levels in control and TRAMP+ tissues (Fig. 2A and C) at 12 weeks. However, at week
18, TRAMP+ mice exhibited HNF1B expression in ~90% of
cells (Fig. 2E), consistent with the
greater degree of cellular proliferation observed in these mice. By
contrast, the more advanced tumors observed in 24-week-old
TRAMP+ mice were characterized by decreases in HNF1B
expression levels, indicative of important alterations in gene
regulation in vivo (Fig.
2G).
Altered ECI2 expression in response to
HNF1B
Given the importance of transcription factors,
including HNF1B, the role of HNF1B gene expression on other
downstream targets, including ECI2, was assessed. ECI2 exhibited
minimal alterations in gene expression levels in control mice at 12
weeks (Fig. 2B), compared with mild
overexpression in TRAMP+ mice (Fig. 2D). Notably, 18-week-old
TRAMP+ mice exhibited marked downregulation of ECI2
expression levels (Fig. 2F) compared
with 12-week-old TRAMP+ mice (Fig. 2B); however, this trend did not
persist, with prominent overexpression of ECI2 evident as the tumor
progressed further (Fig. 2H).
Protective effect of HNF1B through
regulation of ECI2 in the initial proliferative phase of prostate
cancer
Given the differential expression levels observed
between HNF1B and ECI2, western blotting was used to confirm the
alterations in protein expression levels in TRAMP+ mice
over time. The patterns of protein expression observed for HNF1B
and ECI2, as revealed by western blotting (Figs. 3 and 4),
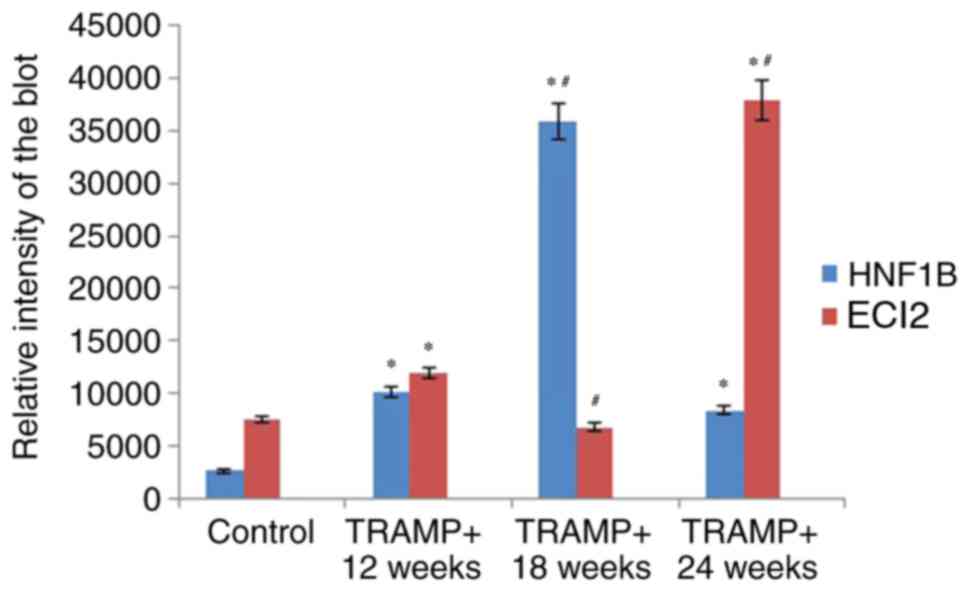
were similar to those revealed by immunohistochemistry (Fig. 2). The level of HNF1B expression in
prostate tissue was reported to be 3.55-fold increased in
18-week-old TRAMP+ mice compared with 12-week-old
TRAMP+ mice, and it subsequently became downregulated
(by a factor of 0.17) in 24-week-old TRAMP+ mice
compared with 12-week-old TRAMP+ mice (Fig. 4). The results for ECI2 demonstrate
that its expression was 0.44-fold downregulated in 18-week-old
TRAMP+ mice compared with 12-week-old TRAMP+
mice, and its expression pattern indicated 3.17-fold overexpression
in 24-week-old TRAMP+ mice compared with 12-week-old
TRAMP+ mice (Fig. 4).
These results suggested that ECI2 may be regulated by HNF1B in
TRAMP+ mice at 18 and 24 weeks in the TRAMP model of
prostate cancer (Figs. 3 and 4).
Discussion
Prostate cancer is the most common form of cancer in
men, with the incidence being higher among older men (18,19). In
the present study, the TRAMP mouse model of prostate cancer was
used in order to better assess various aspects of disease
progression in vivo (20).
TRAMP mice were demonstrated to develop various pathological
features with increasing age, characterized by potential neoplasia
in the early stages of disease (12 weeks), followed by remodeling
of epithelial structures at week 18 (21). As the tumor progressed, lobes present
in the inner tissues of the prostate began to harden, although the
mechanisms underlying these alterations remain poorly understood
(22).
HNF1B is a transcriptional factor that serves an
important role in organelle development and tumorigenesis (23) and has direct connections to at least
12 cancer-associated genes, including nuclear receptor subfamily 4
group A member 1, BCL2-associated athanogene 1 and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-γ.
Furthermore, this gene has been reported to serve a role in
prostate cancer by modulating androgenic hormone signaling
(24), making it a good candidate for
further study. It was demonstrated that HNF1B expression levels
were associated with ECI2 expression levels, which has important
implications for future studies. However, in the present study, the
expression levels of HNF1B and ECI2 exhibited opposing trends in
18- and 24-week-old TRAMP+ mice. Previous studies have
suggested mechanisms by which ECI2 may be regulated by AR (25); however, the precise mechanisms
regulating this gene, and its association with HNF1B, remain
elusive. In the present study it was demonstrated that the initial
proliferative time point for prostate cancer (18 weeks) was
characterized by increased expression levels of HNF1B, conferring a
potential protective effect against tumor development.
Taken together, the data presented demonstrate that
the TRAMP model is optimal for studying prostate cancer
progression. The analyses revealed baseline alterations between
initial proliferative cells, thickening of epithelial cells and
hardening of tissues. It was also reported that the increased
expression levels of HNF1B in initial stages of the disease serve a
tumor-protective role, although upon tumor progression its
expression is downregulated and expression levels of ECI2 are
upregulated.
Acknowledgements
Not applicable.
Funding
The present study was funded by Taihe Hospital
(Shiyan, China), the Scientific and Technological Project of Shiyan
City of Hubei Province (grant no. 17Y13) and the Natural Science
Foundation of Hubei Province of China (grant no. 2013CFC070).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CD and HZ conducted a literature search, designed
experiments, analyzed data and wrote the manuscript. CD, HZ and WZ
performed experiments. LH and XG interpreted the data and
contributed to the experimental work. HL, EY and LW performed minor
experiments and critically revised the manuscript. QY designed
experiments, provided critical feedback and obtained funding.
Ethical approval and consent to
participate
The animals used in the present study, and the
protocols followed, were approved by the institutional ethical
committee at Taihe Hospital, Hubei University of Medicine (Shiyan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Munkley J, Vodak D, Livermore KE, James K,
Wilson BT, Knight B, Mccullagh P, Mcgrath J, Crundwell M, Harries
LW, et al: Glycosylation is an androgen-regulated process essential
for prostate cancer cell viability. EBioMedicine. 8:103–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munkley J, McClurg UL, Livermore KE,
Ehrmann I, Knight B, Mccullagh P, Mcgrath J, Crundwell M, Harries
LW, Leung HY, et al: The cancer-associated cell migration protein
TSPAN1 is under control of androgens and its upregulation increases
prostate cancer cell migration. Sci Rep. 7:52492017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ankerst DP and Thompson IM: Sensitivity
and specificity of prostate-specific antigen for prostate cancer
detection with high rates of biopsy verification. Arch Ital Urol
Androl. 78:125–129. 2006.PubMed/NCBI
|
|
5
|
Snoek R, Cheng H, Margiotti K, Wafa LA,
Wong CA, Wong EC, Fazli L, Nelson CC, Gleave ME and Rennie PS: In
vivo knockdown of the androgen receptor results in growth
inhibition and regression of well-established, castration-resistant
prostate tumors. Clin Cancer Res. 15:39–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson WG, De Marzo AM and Isaacs WB:
Prostate cancer. N Engl J Med. 349:366–381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grasso CS, Wu YM, Robinson DR, Cao X,
Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC,
et al: The mutational landscape of lethal castrate resistant
prostate cancer. Nature. 487:239–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robinson D, Van Allen EM, Wu YM, Schultz
N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC,
Attard G, et al: Integrative clinical genomics of advanced prostate
cancer. Cell. 161:1215–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar A, White TA, MacKenzie AP, Clegg N,
Lee C, Dumpit RF, Coleman I, Ng SB, Salipante SJ, Rieder MJ, et al:
Exome sequencing identifies a spectrum of mutation frequencies in
advanced and lethal prostate cancers. Proc Natl Acad Sci USA.
108:17087–17092. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiesberger T, Shao X, Gourley E, Reimann
A, Pontoglio M and Igarashi P: Role of the hepatocyte nuclear
factor-1beta (HNF-1beta) C-terminal domain in Pkhd1 (ARPKD) gene
transcription and renal cystogenesis. J Biol Chem. 280:10578–10586.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ross-Adams H, Ball S, Lawrenson K, Halim
S, Russell R, Wells C, Strand SH, Ørntoft TF, Larson M, Armasu S,
et al: HNF1B variants associate with promoter methylation and
regulate gene networks activated in prostate and ovarian cancer.
Oncotarget. 7:74734–74746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itkonen HM, Brown M, Urbanucci A, Tredwell
G, Lau Ho C, Barfeld S, Hart C, Guldvik IJ, Takhar M, Heemers HV,
et al: Lipid degradation promotes prostate cancer cell survival.
Oncotarget. 8:38264–38275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Opoku-Acheampong AB, Unis D, Henningson
JN, Beck AP and Lindshield BL: Preventive and therapeutic efficacy
of finasteride and dutasteride in TRAMP mice. PLoS One.
8:e777382013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senkel S, Lucas B, Klein-Hitpass L and
Ryffel GU: Identification of target genes of the transcription
factor HNF1beta and HNF1alpha in a human embryonic kidney cell
line. Biochim Biophys Acta. 1731:179–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stevens VL, Ahn J, Sun J, Jacobs EJ, Moore
SC, Patel AV, Berndt SI, Albanes D and Hayes RB: HNF1B and JAZF1
genes, diabetes, and prostate cancer risk. Prostate. 70:601–607.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itkonen HM, Engedal N, Babaie E, Luhr M,
Guldvik IJ, Minner S, Hohloch J, Tsourlakis MC, Schlomm T and Mills
IG: UAP1 is overexpressed in prostate cancer and is protective
against inhibitors of N-linked glycosylation. Oncogene.
34:3744–3750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itkonen HM, Minner S, Guldvik IJ, Sandmann
MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T and Mills IG:
O-GlcNAc transferase integrates metabolic pathways to regulate the
stability of c-MYC in human prostate cancer cells. Cancer Res.
73:5277–5287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gelman IH: How the TRAMP model
revolutionized the study of prostate cancer progression. Cancer
Res. 76:6137–6139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gingrich JR, Barrios RJ, Morton RA, Boyce
BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM and Greenberg
NM: Metastatic prostate cancer in a transgenic mouse. Cancer Res.
56:4096–4102. 1996.PubMed/NCBI
|
|
22
|
Wei L, Wang J, Lampert E, Schlanger S,
DePriest AD, Hu Q, Gomez EC, Murakam M, Glenn ST, Conroy J, et al:
Intratumoral and intertumoral genomic heterogeneity of multifocal
localized prostate cancer impacts molecular classifications and
genomic prognosticators. Eur Urol. 71:183–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu DD, Guo SW, Jing YY, Dong YL and Wei
LX: A review on hepatocyte nuclear factor-1beta and tumor. Cell
Biosci. 5:582015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu YL, Zhong D, Pang F, Ning QY, Zhang YY,
Li G, Wu JZ and Mo ZN: HNF1b is involved in prostate cancer risk
via modulating androgenic hormone effects and coordination with
other genes. Genet Mol Res. 12:1327–1335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mallik I, Davila M, Tapia T, Schanen B and
Chakrabarti R: Androgen regulates Cdc6 transcription through
interactions between androgen receptor and E2F transcription factor
in prostate cancer cells. Biochim Biophys Acta. 1783:1737–1744.
2008. View Article : Google Scholar : PubMed/NCBI
|