Introduction
Hepatocellular carcinoma is one of the common
malignant tumors in China with a high mortality rate and incidence
rate that is on the increase annually. The common cause of the
disease is infection by hepatitis C viruses (1,2). With the
continuous development of medical technologies and the deepening of
research on the pathogenesis and treatment of hepatocellular
carcinoma, the current treatments for patients with hepatocellular
carcinoma include radiotherapy, chemotherapy, and surgical
resection. Most patients have progressed into the advanced stage at
the time of diagnosis and missed the optimal time to receive
medical treatment. Consequently, medical chemotherapy becomes the
main treatment method, which is of great significance for improving
the quality of life of patients and extending their survival time
(3–6).
At present, either the effect of monotherapy or that
of combined administration for treating the patients with
hepatocellular carcinoma is poor, and the chemotherapy has many
side effects; thus, identification of a therapeutic drug with good
curative effects and few side effects is essential (7). Paeonol, also known as peony phenol, is
the main active ingredient of peony root barks and Paniculate
swallowwort root or the whole herb, which has multiple
pharmacological functions such as immune regulation and
cardiovascular and cerebrovascular protection (8). Previous findings based on in
vitro experiments have shown that paeonol can kill a variety of
malignant tumor cells. Thus, paeonol may be developed into a
potential treatment for malignant tumors (9). Yang et al (10) found that paeonol can effectively
promote the effect of Platinum on the treatment of hepatocellular
carcinoma and reduce the additive dosage of cisplatin. The direct
pharmacological effect of paeonol on hepatocellular carcinoma has
not been studied, and its mechanism of killing cancer cells is
still not clear. It has been found that hepatocellular carcinoma
apoptosis inhibitor-5 (API-5) is closely related to apoptosis.
Nuclear factor-κ-light-chain-enhancer of activated B cells (NF-κB),
as the upstream gene of paeonol, can influence the phosphorylation
of API-5 by activating NF-κB signaling pathways, thus playing
physiological roles (11).
In this study, the effects of paeonol on the
proliferation and apoptosis of human hepatoma (Huh7) cells were
investigated by in vitro experiments, and the effects of
paeonol on the expressions of API-5 and NF-B were observed, in
order to determine the effects of paeonol on hepatocellular
carcinoma cells and reveal its underlying mechanism, thus providing
new ideas and new methods for the clinical treatment of
hepatocellular carcinoma.
Materials and methods
Materials and instruments
Human hepatoma Huh7 cell line (Kunming Cell Bank,
Chinese Academy of Sciences); Dulbecco's modified Eagle medium
(DMEM) (Grand Island Biological Company (GIBCO), Grand Island, NY,
USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); paeonol and
cisplatin (Shanghai Aladdin Biochemical Technology Co., Ltd.);
tumor necrosis factor-α (TNF-α) and dimethyl sulfoxide (DMSO)
(Sigma-Aldrich; Merck KGaA); radioimmunoprecipitation assay (RIPA)
lysate as well as protease inhibitors and phosphatase inhibitors
(Wuhan Google Biotechnology Co., Ltd.); TRIzol kit and reverse
transcription kits (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); rabbit anti-human NF-κB, pretein-API-5
(anti-p-API-5), NF-κB p65/50, glyceraldehyde 3-phosphate
dehydrogenase (anti-GAPDH) polyclonal antibodies and horseradish
peroxidase-labeled goat anti-rabbit secondary polyclonal antibody
(cat nos. 14220-1-AP, 25689-1-AP, 15506-1-AP, 10494-1-AP,
SA00001-2; ProteinTech Group, Inc.; Wuhan Sanying Biotechnology,
Wuhan, China); enhanced chemiluminescence (ECL) solution and
developing powder (Invitrogen; Thermo Fisher Scientific, Inc.);
Hoechst staining kits (Wuhan Google Biotechnology Co., Ltd);
terminal deoxynucleotidyl transferase 2′-deoxyuridine
5′-triphosphate nick end labeling (TUNEL) and flow apoptosis
detection kits (Cell Signaling Technology, Inc., Danvers, MA, USA);
ultraviolet spectrophotometer (Beckman Coulter, Inc., Brea, CA,
USA); electrophoresis apparatus (Corning Incorporated, Corning, NY,
USA); and low-temperature centrifugal machine (Thermo Fisher
Scientific, Inc.) were used in the present study.
The study was approved by the Ethics Committee of
the Second Hospital of Shandong University (Jinan, China).
Effects of paeonol on proliferation of
hepatocellular carcinoma cells
After the human hepatoma Huh7 cell line was
resuscitated, it was cultured in an incubator containing 5%
CO2 at 37°C. After sub-culture, the cells were seeded in
96-well plates. The number of cells inoculated per well was
6×103. At 24 h after inoculation, the serum was deprived
for 2 h. Then different concentrations of paeonol (800, 600, 400,
200, 100, 50 and 1 µM) were added, and the blank control and
positive drug groups (cisplatin (2 µg/ml)) were established. After
drug treatment and incubation for 24 h, 1% MTT was added and the
serum was kept in the dark. Cells in the plates were further
incubated in the incubator for 4 h and then removed, and the medium
was discarded. After 150 µl of DMSO was added to each well, the
plates were vibrated for 10 min, and the optical density (OD)
value, at the wavelength of 570 nm, was detected using a microplate
reader (Bio-Rad, Hercules, CA, USA). The survival rate of the cells
was calculated by referring to the OD value of each group/the OD
value of the normal control group, with the latter as the
standard.
Detection of effects of paeonol on the
apoptosis of hepatocellular carcinoma cells by flow cytometry
Huh7 cells under good growth conditions were
selected, and cells were added to 6-well plates after the cell
density was adjusted to 5×106/ml. The cells were divided
into the blank control group (C group), parthenolide group [CE
group (5 µmol/l)], paeonol group [PO group (500 µM)] and paeonol
(600 µM) + TNF-α (104 U/l) group (PN group), in which
parthenolide was an inhibitor of NF-κB and TNF-α was an activator
of NF-κB. After the treatment for 24 h, the cells were washed twice
with pre-cooled phosphate-buffered saline (PBS) and centrifuged at
1,500 × g for 10 min at 4°C after washing. The apoptotic cell
suspension was prepared and operated in accordance with the
instructions of apoptosis detection kits. The cells were
resuspended and centrifuged at 3,000 × g for 8 min at 4°C. Then the
cells were added with the fluorescence solution (Thermo Fisher
Scientific, Inc.) and incubated at room temperature in the dark for
15 min. After that, the detection by flow cytometry was
conducted.
Detection of effects of paeonol on the
apoptosis of hepatocellular carcinoma cells by Hoechst
staining
Huh7 cells under good growth conditions were
selected, and cells were added to 6-well plates after the cell
density was adjusted to 5×106/ml. The cells were divided
into the blank control group (C group), parthenolide group (CE
group), paeonol group (PO group) and paeonol + TNF-α group (PN
group). Concentrations of drugs were set the same as above. After
treatment for 24 h, culture medium was removed, and then 4%
paraformaldehyde was added for the fixation for 5 min. The cells
were washed twice with PBS, 200 µl staining solution A prepared in
a Hoechst-33258 kit was added for the incubation for 15 min in the
dark, and then the washing solution B was added and the cells were
washed twice. Cells were observed under a fluorescence microscope
(Olympus, Tokyo, Japan), which showed that the wavelength was 352
nm.
Detection of the expression levels of
relevant mRNAs by quantitative polymerase chain reaction
(qPCR)
The RNA of treated Huh7 cells in all the above
groups were extracted using TRIzol kits. The RNA integrity was
confirmed by 2% agarose gel electrophoresis, the results of which
showed that the 28S, 18S and 5S bands were clear and the brightness
of the band 28S was ~2-fold brighter than that of 18S, indicating
that the RNA was intact and could be used for subsequent
experiments. The OD value of RNA in each group was detected, and it
was found that the A260/A280 of each group
was between 1.8 and 2.0, which indicated that the extracted RNA had
better quality. cDNA was obtained from the reverse transcription
using reverse transcription kits. The expression levels of NF-κB
and API-5 were detected by semi-quantitative PCR (Sigma-Aldrich;
Merck KGaA) with GAPDH as the internal reference. Reaction
conditions were: Pre-denaturation at 95°C for 5 min, at 95°C for 30
sec, at 64°C for 25 sec and at 72°C for 30 sec, and the process was
repeated for 35 cycles, followed by extension at 72°C for 7 min.
Primers were produced by TianGen BioTech (Beijing) Co., Ltd.
(Table I). Subsequently, 2% agarose
gel electrophoresis was applied, and the sequences were observed
under an ultraviolet imaging system. The expression levels of NF-κB
and API-5 mRNAs were detected by semi-quantification of the band
brightness/GAPDH brightness in each group.
 | Table I.PCR primer sequences. |
Table I.
PCR primer sequences.
| Gene name | Primer sequences |
|---|
| NF-κB | F:
5′-GGTAGTCCTGATCATGAACTCCC-3′ |
|
| R:
5′-CCTGGTGCAAATCGTACACAGGC-3′ |
| API-5 | F:
5′-CCTGGTGCATGAACTAACTG-3′ |
|
| R:
5′-GGTCTGTGCAACTGTAACC-3′ |
| GAPDH | F:
5′-GATGATTGGCATGGCTTT −3′ |
|
| R:
5′-CACCTTCCGTTCCAGTTT-3′ |
Detection of the expression levels of
relevant proteins by western blot analysis
Treated Huh7 cells placed in 6-well plates were
selected, the culture medium was discarded and 70 µl RIPA lysate
containing 1% protease inhibitor and 1% phosphatase inhibitor was
added to each well for lysis. After the cells were centrifuged at
3,000 × g at 12°C for 10 min, the supernatant was discarded and 12
µl protein was quantified by bicinchoninic acid (BCA) protein assay
kit (Invitrogen; Thermo Fisher Scientific, Inc.). After that,
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) was conducted using 12% gel. The film was transferred at
100 V for 90 min, and the target protein was transferred to the
polyvinylidene difluoride (PVDF) membrane and blocked for 2 h. Then
the target bands were cut and incubated overnight at 4°C with
NF-κB, p-API-5 andNF-κB p65/50 primary antibodies (1:1,000). Then
the bands were washed with Tris-buffered saline with Tween-20
(TBST) three times, and 5 min after each washing, secondary
antibodies (1:5,000) were incubated at room temperature for 2 h.
After the washing with TBST for an additional three times, an
appropriate amount of ECL solutions were added in the dark (uniform
mixture of A and B solution at a ratio of 1:1), and tablet pressing
was performed. According to the fluorescence intensity of protein
bands, the time of tablet pressing was determined. The fixation was
performed after development, and after the bands were scanned, the
gray value was analyzed using ImageJ version 1.36b software (NIH,
Bethesda, MD, USA).
Statistical analysis
Data in this study were expressed as mean ± standard
deviation and analyzed by Statistical Product and Service Solutions
(SPSS) 19.0 software (SPSS Inc., Chicago, IL, USA). The t-test was
used for comparisons between two groups, while the analysis of
variance was used for comparisons among multiple groups. If the
variance was homogeneous, the Bonferroni correction was used for
pairwise comparisons, but if the variance was heterogeneous, the
Welch's method was used for pairwise comparisons and Dunnett's T3
method for comparisons among multiple groups. P<0.05 indicated
there were no statistically significant differences.
Results
Detection of paeonol on the viability
of hepatocellular carcinoma cells by MTT assay
The effects of paeonol at different concentration
gradients on the viability of Huh7 cells were detected by MTT
assay. As shown in Fig. 1, following
treatment of Huh7 cells with 200–800 µM paeonol, the viability of
Huh7 cells was significantly inhibited compared with that in the
blank control group (P<0.01), indicating that based on
concentrations paeonol can effectively inhibit the proliferation of
Huh7 cells. Compared with the control group, the viability of Huh7
cells treated with 200 and 400 µM paeonol was significantly
decreased (P<0.05). After treatment with 600 and 800 µM, the
cell viability decreased more significantly, and the effect
intensity was dose-dependent (P<0.01). When the concentration of
paeonol was 563 µM, the viability of Huh7 cells was only 50% of
that of the control group (data not shown).
Detection of paeonol on the apoptosis
of Huh7 cells by flow cytometry
The effect of paeonol on the apoptosis of Huh7 cells
was detected by flow cytometry. Following treatment with 563 µM
paeonol, the apoptotic levels of cells in the PO and CE groups were
significantly increased compared with that in the C group, and
those in the PO group and CE group were higher than that in the PN
group (Fig. 2). The differences were
statistically significant (P<0.01).
Detection of paeonol on the apoptosis
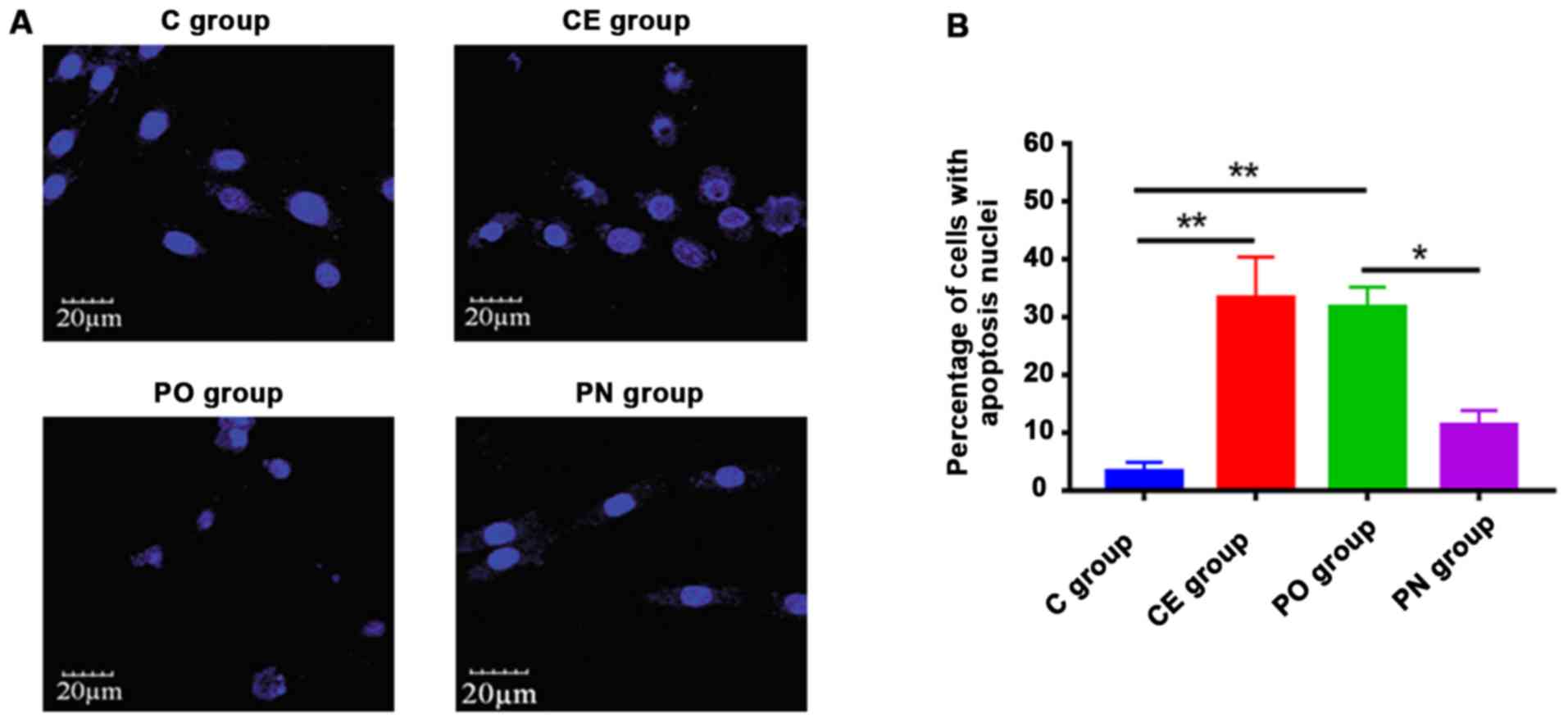
of hepatocellular carcinoma cells by Hoechst staining
The effect of paeonol on the apoptosis of human Huh7
cells was detected by Hoechst-33258 staining. As shown in Fig. 3, the apoptotic levels of cells in the
CE and PO groups were significantly higher than that in the C group
following treatment with 563 µM paeonol (P<0.01), and those in
the PO group were significantly increased compared with that in the
PN group (P<0.05).
Detection of the expression level of
mRNA by semi-quantitative PCR
The expression levels of NF-κB and API-5 mRNAs were
detected by semi-quantitative PCR. The expression levels of NF-κB
and API-5 in the CE and PO groups were significantly decreased
compared with those in the C group (P<0.01), and those in the PO
group were lower than those in the PN group (P<0.05) (Fig. 4).
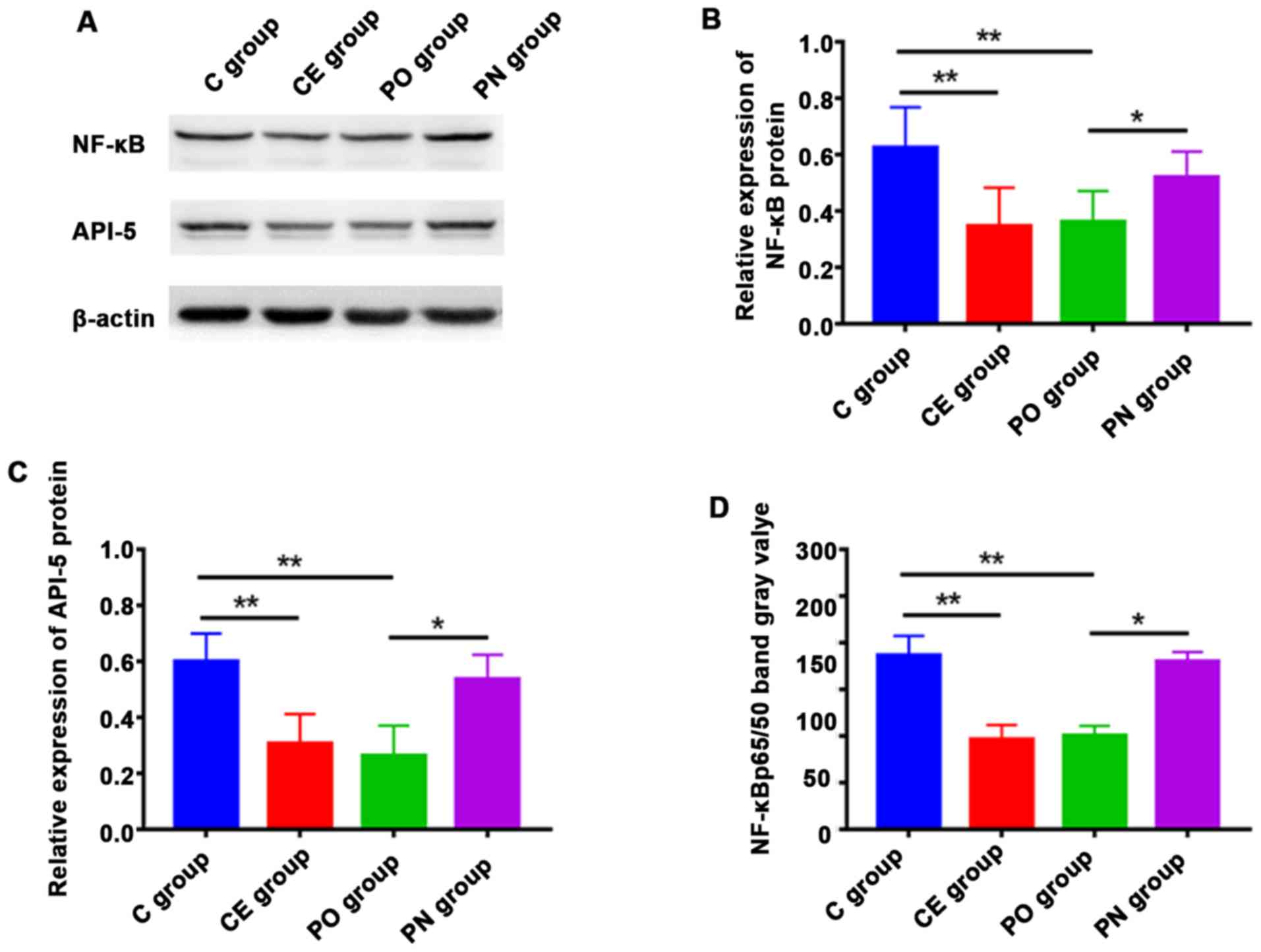
Detection of the expression levels of
proteins via western blot analysis
Western blot analysis was used to detect the
expression levels of NF-κB and p-API-5 proteins. The expression
levels of NF-κB and p-API-5 in the CE and PO groups were
significantly lower those in the C group, and those in the PO group
were lower than those in the PN group. The differences were
statistically significant (P<0.01) (Fig. 5). The expression level of NF-κB p65/50
in each group was detected. The results indicated that the
expression levels of NF-κB p65/50 in the CE and PO groups were
significantly lower than that in the C group (P<0.05), and those
in the CE and PO groups were significantly lower than that in the
PN group. The differences were statistically significant
(P<0.01).
Discussion
Early-onset hepatocellular carcinoma often lacks
typical clinical symptoms. Except for early investigation, many
patients have progressed into the middle or advanced stage at the
time of diagnosis. The treatment for advanced hepatocellular
carcinoma often lacks surgical conditions, and chemotherapy and
radiotherapy are generally applied. At present, chemotherapeutics
for hepatocellular carcinoma have many side effects with poor
curative effects and other shortcomings (12). Previous findings have shown that
paeonol has many significant pharmacological effects such as
preventing and treating cancer, promoting cancer cell apoptosis and
inhibiting cancer cell proliferation and migration (13). Longo et al (14) found that the expression level of
breast cancer tissue NF-κB is significantly increased, and
inhibiting NF-κB signaling pathway can effectively increase cell
apoptosis. In addition, API-5 participates in inhibiting the
apoptosis of hepatocellular carcinoma cells, promotes the
proliferation and migration of hepatocarcinoma cells, and
cooperates with other oncogenes to regulate the occurrence and
development of hepatocellular carcinoma (15,16). The
pharmacological effects of paeonol on hepatocarcinoma cells and its
mechanism remain to be determined, and whether API-5 and NF-B
signaling pathways are involved in the above process is still
unclear.
In this study, the effect of paeonol on the
proliferation of Huh7 cells was studied by in vitro
experiments, and its possible mechanism was discussed. The results
showed that paeonol inhibited the proliferation of Huh7 cells, and
its effect intensity was dose-dependent. With the increase of
paeonol concentration, the inhibitory effect of paeonol on the
proliferation of Huh7 cells was stronger, and 200–800 µM paeonol
inhibited Huh7 cell proliferation. A large number of studies have
shown that paeonol plays a role in promoting apoptosis in a variety
of tumor cells (17,18). In this study, parthenolide and TNF-α
were used as controls, and TNF-α was an activator of NF-κB while
parthenolide was an inhibitor of NF-κB. The effect of NF-κB
signaling pathway in paeonol promoting the apoptosis of
hepatocellular carcinoma cells was studied using flow cytometry and
Hoechst-33258 staining. The results showed that the apoptotic
levels of cells in the CE and PO groups were significantly
increased compared with that in the C group, and those in the CE
and PO groups were significantly higher than that in the PN group.
The differences were statistically significant (P<0.01). The
above results indicated that NF-κB was involved in the promotion of
paeonol on the apoptosis of hepatocellular carcinoma cells.
Inhibiting NF-κB could effectively increase the apoptosis of
hepatocellular carcinoma cells and inhibit the proliferation of
hepatocellular carcinoma cells. NF-κB signaling pathway and the
expression of its downstream proteins were studied using
semi-quantitative PCR and western blot analysis. The results showed
that the expression levels of NF-κB, p-API-5 and NF-κB p65/50 in
the PO group were significantly lower than those in the C group,
indicating that paeonol can inhibit the expression and activation
of NF-κB. The expression level of p-API-5 can be affected by
regulating NF-κB through its inhibitors and activators, suggesting
that API-5 may be the downstream gene of NF-κB signaling pathway.
Paeonol inhibits NF-κB expression to decrease the level of p-API-5,
thus regulating the apoptosis of hepatocellular carcinoma cells.
NF-κB is transformed from p50 homodimer to p60/p50 heterodimer with
transcriptional activity. The activated NF-κB is transferred from
the cytoplasm to the nucleus for transcription. It can transcribe a
series of oncogenes related to cell growth and promote the
production of tumors, so the activation condition of the NF-κB
signaling pathway can be evaluated by NF-κB p65/50 (19,20).
However, there are some shortcomings in this experiment. Besides
the NF-κB signaling pathway, whether there are other ways of
affecting the apoptosis of hepatocellular carcinoma cells needs to
be determined. In addition, the effect of paeonol at the effective
concentration on healthy liver cells is not clear. These issues are
to be the focus of further studies. In conclusion, it has been
verified from many perspectives in this study that paeonol can
inhibit the NF-κB signaling pathway, reducing the expression level
of API-5 and further promoting the apoptosis of hepatocellular
carcinoma cells by inhibiting the expression of NF-κB. Therefore,
there is great potential for paeonol being developed into a drug
for treating hepatocellular carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL drafted this manuscript. QL and YZ carried out
the MTT assay. QL and JS were instrumental in performing flow
cytometry. QL and QB were responsible for the PCR and western blot
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Hospital of Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Korean Liver Cancer Study Group (KLCSG);
National Cancer Center, Korea (NCC), . 2014 Korean Liver Cancer
Study Group-National Cancer Center Korea practice guideline for the
management of hepatocellular carcinoma. Korean J Radiol.
16:465–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Z, Chen T, Thorgeirsson SS, Zhan Q,
Chen J, Park JH, Lu P, Hsia CC, Wang N, Xu L, et al: Dramatic
reduction of liver cancer incidence in young adults: 28 year
follow-up of etiological interventions in an endemic area of China.
Carcinogenesis. 34:1800–1805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berretta M, Stanzione B, Di Francia R and
Tirelli U: The expression of PD-L1 APE1 and P53 in hepatocellular
carcinoma and its relationship to clinical pathology. Eur Rev Med
Pharmacol Sci. 19:4207–4209. 2015.PubMed/NCBI
|
|
4
|
Chang ET, Yang J, Alfaro-Velcamp T, So SK,
Glaser SL and Gomez SL: Disparities in liver cancer incidence by
nativity, acculturation, and socioeconomic status in California
Hispanics and Asians. Cancer Epidemiol Biomarkers Prev.
19:3106–3118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moris D, Vernadakis S, Papalampros A,
Petrou A, Dimitroulis D, Spartalis E, Felekouras E and Fung JJ: The
effect of Guidelines in surgical decision making: The paradigm of
hepatocellular carcinoma. J BUON. 21:1332–1336. 2016.PubMed/NCBI
|
|
6
|
Barbier-Torres L, Delgado TC,
García-Rodríguez JL, Zubiete-Franco I, Fernández-Ramos D, Buqué X,
Cano A, Gutiérrez-de Juan V, Fernández-Domínguez I, et al:
Stabilization of LKB1 and Akt by neddylation regulates energy
metabolism in liver cancer. Oncotarget. 6:2509–2523. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Wu QJ, Xie L, Chow WH, Rothman N,
Li HL, Gao YT, Zheng W, Shu XO and Xiang YB: Prospective cohort
studies of association between family history of liver cancer and
risk of liver cancer. Int J Cancer. 135:1605–1614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li SS, Li GF, Liu L, Jiang X, Zhang B, Liu
ZG, Li XL, Weng LD, Zuo T and Liu Q: Evaluation of paeonol
skin-target delivery from its microsponge formulation: In vitro
skin permeation and in vivo microdialysis. PLoS One. 8:e798812013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye JM, Deng T and Zhang JB: Influence of
paeonol on expression of COX-2 and p27 in HT-29 cells. World J
Gastroenterol. 15:4410–4414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Q, Wang S, Xie Y, Wang J, Li H, Zhou
X and Liu W: Effect of salvianolic acid B and paeonol on blood
lipid metabolism and hemorrheology in myocardial ischemia rabbits
induced by pituitruin. Int J Mol Sci. 11:3696–3704. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koci L, Chlebova K, Hyzdalova M, Hofmanova
J, Jira M, Kysela P, Kozubik A, Kala Z and Krejci P: Apoptosis
inhibitor 5 (API-5; AAC-11; FIF) is upregulated in human carcinomas
in vivo. Oncol Lett. 3:913–916. 2012.PubMed/NCBI
|
|
12
|
Chen X and Calvisi DF: Hydrodynamic
transfection for generation of novel mouse models for liver cancer
research. Am J Pathol. 184:912–923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horng CT, Shieh PC, Tan TW, Yang WH and
Tang CH: Paeonol suppresses chondrosarcoma metastasis through
up-regulation of miR-141 by modulating PKCδ and c-Src signaling
pathway. Int J Mol Sci. 15:11760–11772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Longo DM, Selimkhanov J, Kearns JD, Hasty
J, Hoffmann A and Tsimring LS: Dual delayed feedback provides
sensitivity and robustness to the NF-κB signaling module. PLoS
Comput Biol. 9:e10031122013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pekow J, Meckel K, Dougherty U, Butun F,
Mustafi R, Lim J, Crofton C, Chen X, Joseph L and Bissonnette M:
Tumor suppressors miR-143 and miR-145 and predicted target proteins
API5, ERK5, K-RAS, and IRS-1 are differentially expressed in
proximal and distal colon. Am J Physiol Gastrointest Liver Physiol.
308:G179–G187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baxter PA, Lin Q, Mao H, Kogiso M, Zhao X,
Liu Z, Huang Y, Voicu H, Gurusiddappa S, Su JM, et al: Silencing
BMI1 eliminates tumor formation of pediatric glioma
CD133+ cells not by affecting known targets but by
down-regulating a novel set of core genes. Acta Neuropathol Commun.
2:1602014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Xie YH, Yang Q, Wang SW, Zhang BL,
Wang JB, Cao W, Bi LL, Sun JY, Miao S, et al: Cardioprotective
effect of paeonol and danshensu combination on
isoproterenol-induced myocardial injury in rats. PLoS One.
7:e488722012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Musselman CA and Kutateladze TG: Methyl
fingerprinting of the nucleosome reveals the molecular mechanism of
high-mobility group nucleosomal-2 (HMGN2) association. Proc Natl
Acad Sci USA. 108:12189–12190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hämäläinen M, Nieminen R, Vuorela P,
Heinonen M and Moilanen E: Anti-inflammatory effects of flavonoids:
Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and
NF-kappaB activations, whereas flavone, isorhamnetin, naringenin,
and pelargonidin inhibit only NF-kappaB activation along with their
inhibitory effect on iNOS expression and NO production in activated
macrophages. Mediators Inflamm. 2007:456732007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szukiewicz D, Wojciechowska M, Bilska A,
Stangret A, Szewczyk G, Mittal TK, Watroba M and Kochanowski J:
Aspirin action in endothelial cells: Different patterns of response
between chemokine CX3CL1/CX3CR1 and TNF-alpha/TNFR1 signaling
pathways. Cardiovasc Drugs Ther. 29:219–229. 2015. View Article : Google Scholar : PubMed/NCBI
|