Introduction
Giant cell tumor of bone (GCTB) is characterized by
a large number of osteoclastic giant cells uniformly distributed
amongst mononuclear spindle-like stromal cells and other rounded
monocytes (1,2). It is among the most prevalent tumors of
the bone in East and Southeast Asia, accounting for ~20% of all
cases (3–5). It is classified as an intermediate tumor
(6) with characteristic locally
invasive behavior leading to osteolysis (1,7,8). GCTB is mainly treated by curettage;
however, clinically, the high rate of local recurrence following
curettage (13–65%) poses a major challenge for the treatment of
GCTB (9,10).
Factors contributing to the prognosis of local
recurrence of GCTB require investigation in order to achieve local
control and guidance for surgical approaches. In the present study,
literature concerning molecular prognostic factors for local
recurrence of GCTB was reviewed to identify various potential
prognostic factors. High activities of matrix metalloproteinase
(MMP) and vascular endothelial growth factor (VEGF) have been
linked to the biological aggressiveness of GCTB (11,12). Kumta
et al (13) demonstrated that
elevated levels of VEGF and MMP-9 in GCTB were positively
correlated with local recurrence. In bone tumors, co-overexpression
of receptor activator of nuclear factor-κB (RANK) and RANK ligand
(RANKL) was identified as a potential discriminating factor for a
poor prognosis (14). The expression
of RANKL affected the proliferation of neoplastic GCTB cells in
another study (15). Based on these
studies, it has been hypothesized that GCTB expressing elevated
levels of these proteins may be more prone to recurrence.
As a non-invasive method, magnetic resonance imaging
(MRI) is valuable for the diagnosis and evaluation of bone tumors,
owing to its heightened sensitivity to soft tissue disease and
multiplanar image acquisition (16).
MRI may provide information regarding tumor margins and soft tissue
extension, as well as cystic changes (17–19).
Biological aggressiveness and poor prognoses are associated with
the aforementioned molecular expression patterns of GCTB (11–13), and
may also be evaluated through preoperative MRI. However, to the
best of our knowledge, no previous studies have analyzed the
association between molecular expression patterns, MRI features and
the prognosis of GCTB.
The present study aimed to investigate the
association between prognostic protein expression and preoperative
MRI features, and its value in predicting the local recurrence of
GCTB. The present study was inspired by appeals for the
establishment of a novel multiple-perspective evaluation system
(20). Furthermore, elucidation of
the role of the expression of the aforementioned proteins in the
local recurrence of GCTB will provide insight into the clinical
value of investigating these molecular mechanisms.
Patients and methods
Patients
The present study was approved by the Institutional
Review Board of Ruijin Hospital, Shanghai Jiao Tong University
School of Medicine (Shanghai, China). Written informed consent was
obtained from all patients prior to enrolment in the study. All
enrolled patients had been diagnosed with
histopathologically-confirmed GCTB of the proximal tibia or distal
femur. MRI and computerized tomography scans of all patients were
obtained and analyzed prior to surgery.
Currently, intralesional excision is the preferred
treatment of GCTB, rather than en bloc resection, although the
latter is associated with reduced recurrence with the compromise of
limb function. Both treatments are selective for patients at Ruijin
Hospital.
To examine the factors associated with local
recurrence, patients with ≥2 years of follow-up information
available subsequent to intralesional curettage treatment, which
was performed consistently by orthopedic specialists in Ruijin
Hospital, were selected for retrospective study. A total of 69
patients were prospectively enrolled in the present study between
January 2005 and October 2015. A total of 35 male and 39 female
patients were eligible for inclusion in the present study. The
median age was 29 years (range, 17–64 years). To exclude the impact
of increased complexity of surgical treatment, 5 patients with
pathological fractures and 4 patients with soft tissue masses were
excluded from the present study.
Between March 2013 and July 2016, 36 patients (18
male and 18 female; mean age, 32.56 years; median age, 28.5 years;
age range, 21–61 years) with GCTB around the knee were enrolled for
investigation. Immunohistochemistry (IHC) features, including the
protein expression of VEGF, MMP-9, RANKL and RANK, were studied. A
total of 22 of these patients overlapped with the former group; en
bloc resection was performed in the remaining 14 patients, 2 of
whom were enrolled for investigation on the pathological basis of
specific preoperative MRI features. Adjacent tissues obtained in
seven cases as a negative control.
Immunohistochemistry (IHC)
IHC was performed on formalin-fixed (23–26°C for 24
h), paraffin-embedded tumor tissue samples, cut into 3-µm thick
sections. The sections were deparaffinized with xylene, rehydrated
in a descending ethanol series (100% for 5 min; 100% for 5 min; 95%
for 5 min; 95% for 5 min; 85% for 3 min and 75% for 2 min), and
treated using an automated immunostainer Bench Mark XT (Ventana
Medical Systems, Inc., Tucson, AZ, USA) following antigen retrieval
with citrate buffer (cat. no. ab64214; Abcam, Cambridge, UK) of pH
6.0, microwaved on high power until boiling (93°C) for 5 min. The
slides were kept warm by heating for 10 min at low power (4°C). The
coplin jar was left to sit in the microwave for at least 20 min.
The sections were incubated with the following primary antibodies
for 1 h at 37°C: MMP-9 (dilution, 1:1,000; cat. no. ab38898), VEGF
receptor 1 (dilution, 1:250; cat. no. Y103), RANK (dilution, 1:50;
cat. no. 64C1385) and RANKL (dilution, 1:100; cat. no. 12A668; all
Abcam). The polymeric detection system, ultra View Universal DAB
Detection kit (Ventana Medical Systems, Inc.), was then used
according to the manufacturer's protocol. Finally, the sections
were counter-stained with Gill's modified hematoxylin (6 g/l; cat.
no. GH316; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room
temperature for 17 min, and then cover-slipped with
EUKITT® (ORSAtec GmbH, Bobingen, Germany) mounting
media. Mouse brain tissue (15 days old wild-type mouse embryonic
brain; Department of Laboratory Animal Sciences, Shanghai Jiao tong
University School of Medicine, Shanghai, China) was used as a
positive tissue control for the anti-VEGF antibody, and RAW 264.7
cells [Type Culture Collection of the Chinese Academy of Sciences;
cells were cultured in Dulbecco's modified Eagle's medium (cat. no.
12800017) and 10% fetal bovine serum (cat. no. 10100147) (both from
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an
atmosphere containing 5% CO2 at 37°C] were used as the
positive control for the other antibodies. IHC on adjacent tissues
in the absence of the primary antibody were used as a negative
control. The sections were analyzed with an Olympus light
microscope (Olympus Corporation, Tokyo, Japan) using ×10 and ×40
objectives. Independent experienced pathologists FY and QZ, blinded
to the clinical details of the individual patients, evaluated the
immunoreactivity of VEGF, RANKL, RANK and MMP-9, using a scoring
system. The expression levels of VEGF, RANKL, RANK and MMP-9 were
semi-quantified using a visual grading system based on the extent
of staining: grade 0, virtually no immunoreactivity; grade 1,
patchy to diffuse weak immunoreactivity; grade 2, patchy to diffuse
moderate immunoreactivity; and grade 3, patchy to diffuse strong
immunoreactivity, as previously described (21,22).
However, according to the results of the IHC analysis, there were
very few cases of grade 0 and grade 3; therefore, 0–1 cases were
classified into a low-grade group, and grade 2–3 cases into a
high-grade group.
Imaging procedures
MRI was performed using a 1.5-T superconducting
whole-body imager (SIGNA; GE Healthcare, Chicago, IL, USA) with
dedicated extremity coils. A combination of axial, sagittal and
coronal images was obtained using the following sequences:
Spin-echo T1-weighted (TR range/TE range, 450–600 ms/15–20 ms),
fast spin-echo T2-weighted (TR range/TE range, 3,500-4,000/80–120
ms) and fat-suppressed fast spin-echo T2-weighted (TR range/TE
range, 3,500-4,000/80–120 ms). The field of view, slice thickness
and inter slice gap varied depending on the region of interest and
tumor size. The slice thickness was <5 mm and inter slice gap
was 0.5 to 2.0 mm. The imaging matrix ranged from 192×256 to
256×256 pixels. For follow-up, the patients underwent routine
anterior-posterior and lateral plain imaging.
Objective features and groups
A total of 4 imaging features, namely, cystic
changes, the ‘paint brush borders’ sign, peritzmoral edema and
adjacent soft tissue invasion, were evaluated and interpreted by 3
senior musculoskeletal radiologists (LD, JZ and XD, with 17, 22 and
25 years of experience, respectively) until a diagnostic consensus
was reached. However, if a mutual consensus could not be reached,
the majority opinion was used. GCTB cases were classified into
positive and negative groups according to the aforementioned
preoperative imaging features. GCTB margins are observed as
well-defined, irregularly penetrating margins. The ‘paint brush
borders’ sign is defined as penetrating irregular margins with a
‘paint brush’ appearance protruding towards the bone. This is
visible on MRI scans as they offer a stark contrast between the
high signal intensity of the bone marrow and the low signal
intensity of tumor tissue. T1-weighted images provide a good
demonstration of manifesting irregular protrusions compared with
other sequences. Patients were classified as positive for the
‘paint brush borders’ if such margins were noted, irrespective of
tumor number and shape. Cystic changes indicate liquefactive
necrosis. This feature may be associated with a high cellular or
protein content, and manifests as a homogeneous high signal
intensity on T2-weighted images. Peritumoral edema is identifiable
by a very high signal intensity on fat-suppressed MRI scans, which
are used for the evaluation of joint effusion and edema. GCTBs were
classified into 2 grades according to the presence of edema: Grade
A, high signal intensity on fat-suppressed T2-weighted images and
grade B, minimally increased signal intensity on fat-suppressed
T2-weighted images. In GCTB, adjacent soft tissue invasion is
indicative of tumor aggressiveness. Specifically, this feature is
an indication of peripheral soft tissue involvement, coupled with
loss of bone cortex, defined by low signal intensity on MRI scans
(23).
Intralesional curettage procedure
All 60 patients were treated with intralesional
curettage, which was performed by senior orthopedic surgeons. The
tumor tissue was removed with a curette following creation of a
wide cortical window. The remainder of the tumor cavity was
eliminated with a high-speed burr. Phenol (5%) was applied to the
borders of the cavity with cotton-tipped applicators at room
temperature for 6 min, and then neutralized with 75% ethanol twice
in 33 cases at room temperature. The remaining 27 cases were
treated without phenol. Finally, the tumorcavity was carefully
packed with polymethyl methacrylate filling. Surgical specimens
were obtained from 2 selected patients treated with en bloc
resection.
Follow-up and recurrence
All patients were re-examined by radiography or MRI
annually for ≥2 years, regardless of their symptomatic status. For
patients who were unable to undergo re-examination in Ruijin
hospital, telephone, written or home visit follow-up was performed.
Enlargement of the radiolucent zone was considered a reliable
indicator of possible local recurrence. Recurrent tumor sex hibited
high signal intensity around the polymethyl methacrylate filling on
T2-weighted images. Patients were also re-examined immediately if
any abnormal pain or swelling occurred following surgery.
Statistical analysis
Statistical analyses were performed using SPSS
(version 22.0; IBM Corp., Armonk, NY, USA). Categorical variables
are presented as the percentage and absolute number of patients.
The associations between dichotomous attributes and local
recurrence were analyzed using the χ2 test. Fisher's
exact test was used if the number of cases was <40. Independent
factors were determined by multivariate logistic regression
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
All patient groups were classified according to the
preoperative MRI features. The positive rates of the ‘paint brush
borders’ sign, peritumoral edema, cystic changes and adjacent soft
tissue invasion were 41.89, 51.35, 68.92 and 71.62%, respectively.
Tissue specimens from 36 patients were investigated by IHC. The
clinical characteristics of all enrolled patients are detailed in
Tables I and II.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Number (%) |
|---|
| Age |
|
| ≤28
years | 37 (50.00) |
| >28
years | 37 (50.00) |
| Sex |
|
|
Male | 35 (47.30) |
|
Female | 39 (52.70) |
| Location |
|
|
Proximal tibia | 34 (45.96) |
| Distal
femur | 40 (54.04) |
| ‘Paint brush
borders’ sign |
|
|
Present | 31 (41.89) |
|
Absent | 43 (58.11) |
| Peritumoral
edema |
|
| Grade
A | 38 (51.35) |
| Grade
B | 36 (48.65) |
| Adjacent soft
tissue invasion |
|
|
Yes | 53 (71.62) |
| No | 21 (28.38) |
| Cystic changes |
|
|
Yes | 51 (68.92) |
| No | 23 (31.08) |
 | Table II.Comparison of patients in terms of
preoperative MRI characteristics and immunohistochemistry
scores. |
Table II.
Comparison of patients in terms of
preoperative MRI characteristics and immunohistochemistry
scores.
|
|
|
| Preoperative MRI
feature |
Immunohistochemistry staining score |
|---|
|
|
|
|
|
|
|---|
| Patient number | Surgical
approach | Local
recurrence | PE | ASTI | PS | CC | RANKL | MMP-9 | VEGF | RANK |
|---|
| 1 | Curettage | No | B | No | No | Yes | 1 | 2 | 2 | 1 |
| 2 | Curettage | No | A | Yes | No | No | 2 | 1 | 1 | 0 |
| 3 | Curettage | No | A | Yes | No | Yes | 2 | 1 | 0 | 1 |
| 4 | Curettage | No | B | Yes | No | Yes | 2 | 2 | 1 | 1 |
| 5 | Curettage | No | B | Yes | No | No | 1 | 1 | 1 | 1 |
| 6 | Curettage | No | B | No | No | No | 1 | 1 | 1 | 1 |
| 7 | Curettage | No | A | Yes | No | No | 1 | 1 | 2 | 2 |
| 8 | Curettage | No | B | No | No | No | 1 | 1 | 0 | 0 |
| 9 | Curettage | No | A | No | No | No | 2 | 1 | 1 | 1 |
| 10 | Curettage | No | B | Yes | No | No | 1 | 1 | 2 | 1 |
| 11 | Curettage | No | B | Yes | Yes | No | 1 | 0 | 3 | 0 |
| 12 | Curettage | Yes | A | No | Yes | Yes | 2 | 2 | 3 | 2 |
| 13 | Curettage | Yes | A | Yes | No | Yes | 1 | 1 | 0 | 2 |
| 14 | Curettage | Yes | A | Yes | No | Yes | 2 | 2 | 2 | 2 |
| 15 | Curettage | Yes | B | Yes | No | Yes | 2 | 0 | 1 | 1 |
| 16 | Curettage | Yes | A | Yes | No | Yes | 1 | 1 | 2 | 1 |
| 17 | Curettage | Yes | B | Yes | Yes | Yes | 2 | 2 | 2 | 0 |
| 18 | Curettage | Yes | A | Yes | Yes | Yes | 1 | 0 | 0 | 1 |
| 19 | Curettage | Yes | A | Yes | Yes | Yes | 3 | 2 | 1 | 0 |
| 20 | Curettage | Yes | B | Yes | Yes | Yes | 1 | 0 | 1 | 1 |
| 21 | Curettage | Yes | B | Yes | Yes | No | 3 | 2 | 2 | 1 |
| 22 | Curettage | Yes | A | Yes | Yes | Yes | 2 | 2 | 2 | 2 |
| 23 | Resection | / | B | Yes | No | Yes | 1 | 0 | 1 | 0 |
| 24 | Resection | / | B | Yes | No | Yes | 1 | 2 | 1 | 2 |
| 25 | Resection | / | B | Yes | Yes | Yes | 3 | 2 | 2 | 1 |
| 26 | Resection | / | B | No | No | No | 2 | 0 | 1 | 1 |
| 27 | Resection | / | B | Yes | No | Yes | 2 | 1 | 1 | 2 |
| 28 | Resection | / | B | No | No | Yes | 1 | 1 | 2 | 1 |
| 29 | Resection | / | B | No | No | Yes | 2 | 1 | 2 | 1 |
| 30 | Resection | / | A | Yes | Yes | No | 1 | 1 | 1 | 0 |
| 31 | Resection | / | B | Yes | No | No | 2 | 1 | 1 | 2 |
| 32 | Resection | / | A | Yes | No | No | 2 | 1 | 1 | 3 |
| 33 | Resection | / | B | Yes | Yes | No | 2 | 2 | 2 | 1 |
| 34 | Resection | / | A | Yes | Yes | Yes | 1 | 2 | 1 | 1 |
| 35 | Resection | / | B | Yes | Yes | Yes | 1 | 0 | 1 | 2 |
| 36 | Resection | / | B | Yes | No | Yes | 2 | 2 | 2 | 2 |
Associations between preoperative MRI
features and protein expression
High-grade MMP-9 expression was positively
associated with cystic changes (P<0.05) and the ‘paint brush
borders’ sign (P<0.05). MMP-9 expression also associated with
the expression of RANKL (P<0.05) and VEGF (P<0.05; Table III). However, VEGF, RANK and RANKL
expression were not associated with any preoperative MRI features
of GCTB (P>0.05; Figs. 1 and
2).
 | Table III.Association between MRI features and
protein expression. |
Table III.
Association between MRI features and
protein expression.
|
| High-grade
immunohistochemistry |
|---|
|
|
|
|---|
|
| VEGF | MMP-9 | RANK | RANKL |
|---|
|
|
|
|
|
|
|---|
| MRI feature | Number (%) | P-value | Number (%) | P-value | Number (%) | P-value | Number (%) | P-value |
|---|
| ‘Paint brush
borders’ positive group | 7 (53.8) | 0.31 | 8 (61.54) | 0.03a | 3 (23.1) | 0.71 | 7 (53.8) | 1.00 |
| ‘Paint brush
borders’ negative group | 8 (34.8) |
| 5 (21.74) |
| 8 (34.8) |
| 12 (52.2) |
|
| Cystic change
positive group | 10 (45.5) | 0.73 | 11 (50.0) | 0.40a | 8 (36.4) | 0.47 | 12 (54.5) | 1.00 |
| Cystic change
negative group | 5 (35) |
| 2 (14.3) |
| 3 (21.4) |
| 7 (50) |
|
| Peritumoral edema
high level group | 5 (35.7) | 0.73 | 5 (35.7) | 1.00 | 6 (42.9) | 0.27 | 8 (57.1) | 0.74 |
| Peritumoral edema
low level group | 10 (45.5) |
| 8 (36.4) |
| 5 (22.7) |
| 11 (50.0) |
|
| Soft tissue
invasion positive group | 11 (39.3) | 0.69 | 11 (39.3) | 0.68 | 10 (35.7) | 0.39 | 15 (53.6) | 1.00 |
| Soft tissue
invasion negative group | 4 (50.0) |
| 2 (25.0) |
| 1 (12.5) |
| 4 (50) |
|
| High-grade
MMP-9 | 9 (69.2) | 0.02a |
|
|
|
|
|
|
| Low-grade
MMP-9 | 6 (26.1) |
|
|
|
|
|
|
|
| High-grade
RANK | 5 (45.5) | 1.00 | 5 (45.5) | 0.48 |
|
|
|
|
| Low-grade RANK | 10 (40.0) |
| 8 (32.0) |
|
|
|
|
|
| High-grade
RANKL | 9 (47.4) | 0.52 | 10 (52.6) | 0.04a | 7 (36.8) | 0.48 |
|
|
| Low-grade
RANKL | 6 (35.3) |
| 3 (17.6) |
| 4 (23.5) |
|
|
|
Follow-up and local recurrence
A total of 55 patients (91.67%) treated within
tralesional curettage were successfully followed up, while the
remaining 5 patients were lost to follow-up. A total of 32 patients
did not experience recurrence. A total of 23 patients (41.18%) were
diagnosed with recurrent GCTB; 20 of these patients (86.96%)
experienced recurrence within 2 years (mean, 14.7 months). Of the
remaining 3 recurrent cases, 2 experienced recurrence after 5 years
and one after 8 years. The follow-up period ranged between 2 and 10
years (mean, 5.61 years).
Preoperative MRI features and protein
expression for predicting local recurrence
Among the 55 patients successfully followed up, 37
exhibited cystic changes, and of these, 22 patients (59.46%)
developed recurrence. Of the 18 patients who did not exhibit cystic
changes, only 1 (5.56%) experienced recurrence
(χ2=14.461; P<0.05). Furthermore, the ‘paint brush
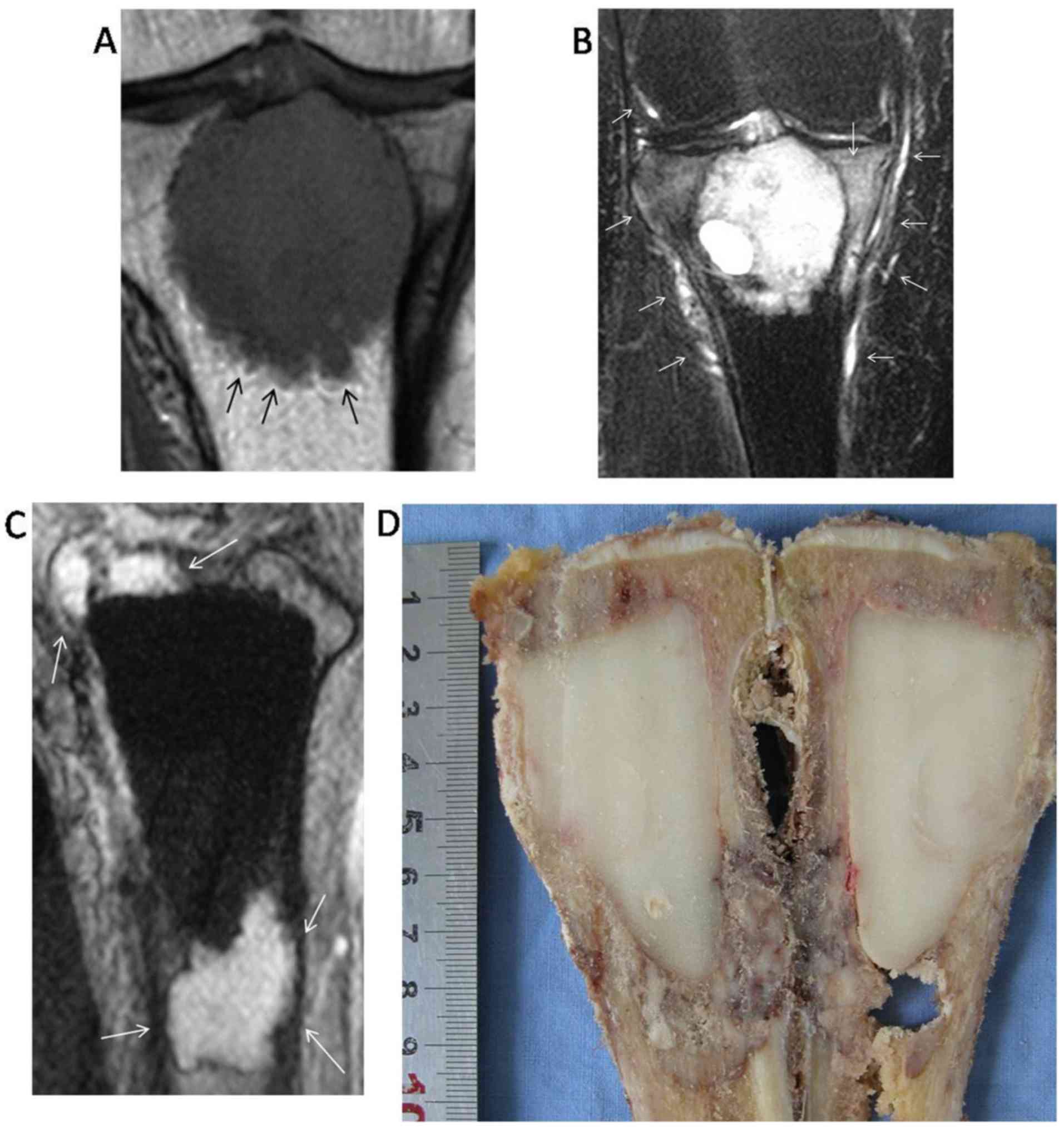
borders’ sign was observed in 21 patients (Figs. 3 and 4);
16 (76.19%) of these patients experienced relapse during follow-up
(Fig. 4). Of the 34 patients who did
not exhibit the ‘paint brush borders’ sign, only 7 (20.59%)
developed local recurrence (χ2=16.496; P<0.05).
Furthermore, adjacent soft tissue invasion was also revealed to be
associated with local recurrence (χ2=4.935; P<0.05).
Neither peritumoral edema, nor MMP-9, RANKL or VEGF expression were
associated with local recurrence (P>0.05; Table IV).
 | Table IV.Predicting local recurrence of GCTB
and confounding variable analysis. |
Table IV.
Predicting local recurrence of GCTB
and confounding variable analysis.
| A, Preoperative MRI
features (n=55). |
|---|
|
|---|
|
| Local
recurrence |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Yes (n=23) | No (n=32) | χ2 | P-value |
|---|
| Peritumoral
edema |
|
|
|
|
| A | 15 | 17 | 0.804 | 0.37 |
| B | 8 | 15 |
|
|
| Cystic
changea |
|
|
|
|
|
Yes | 22 | 15 | 14.461 | 0.02 |
| No | 1 | 17 |
|
|
| ‘Paint brush
borders’ sign |
|
|
|
|
|
Yes | 16 | 5 | 16.496 | <0.01 |
| No | 7 | 27 |
|
|
| Adjacent soft
tissue invasiona |
|
|
|
|
|
Yes | 20 | 19 | 4.935 | 0.04 |
| No | 3 | 13 |
|
|
| Confounding
variable analysis |
|
|
|
|
|
Application of phenol |
|
|
|
|
|
Applied | 12 | 18 | 0.090 | 0.77 |
|
Not applied | 11 | 14 |
|
|
| Study
span (January 2005 to October 2015) |
|
|
|
|
|
First 5 years | 11 | 13 | 0.282 | 0.56 |
|
Last 5 years | 12 | 19 |
|
|
|
| B,
Immunohistochemical analysis (n=22). |
|
|
| Local
recurrence |
|
|
|
|
|
|
|
|
Variable | Yes
(n=11) | No
(n=11) |
χ2 | P-value |
|
| RANKL |
|
|
|
|
|
High-grade | 7 | 4 |
| 0.40 |
|
Low-grade | 4 | 7 |
|
|
| RANK |
|
|
|
|
|
High-grade | 4 | 1 |
| 0.32 |
|
Low-grade | 7 | 10 |
|
|
| VEGF |
|
|
|
|
|
High-grade | 6 | 4 |
| 0.67 |
|
Low-grade | 5 | 7 |
|
|
| MMP-9 |
|
|
|
|
|
High-grade | 6 | 2 |
| 0.18 |
|
Low-grade | 5 | 9 |
|
|
Analysis of confounding factors
A total of 30/33 patients who had undergone
additional adjuvant treatment were successfully followed up. There
was no significant difference in the recurrence rates of patients
treated with or without additional adjuvant therapy (P>0.05).
Furthermore, no significant differences were observed in the
recurrence rates of patients enrolled between January 2005 and
January 2010, and those enrolled between January 2010 and October
2015 (P>0.05; Table IV).
Confirmation of preoperative MRI
features with pathological analysis
Gross pathological sections from patients treated
with en bloc resection were visually matched to the acquired MRI
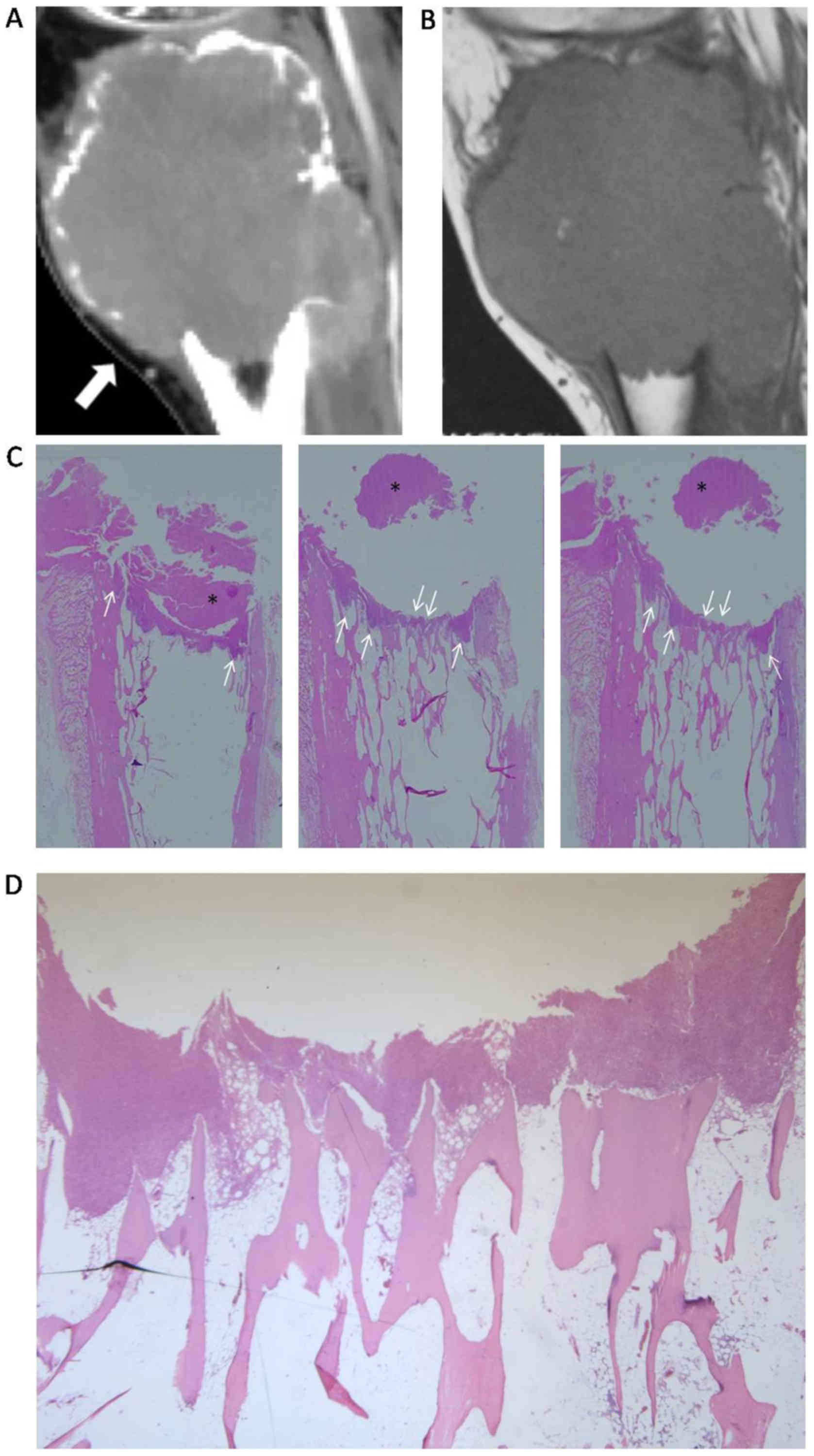
scans. The lengths of the protrusions of GCTB varied between 1.5
and 3.6 mm on the MRI scans. In the patients treated with en bloc
resection, the lengths of the protrusions varied between 1.2 and
3.3 mm in gross pathological sections, while the MRI scans
exhibited lengths between 1.6 and 3.2 mm. A number of
multinucleated giant cells uniformly distributed amongst monocytes
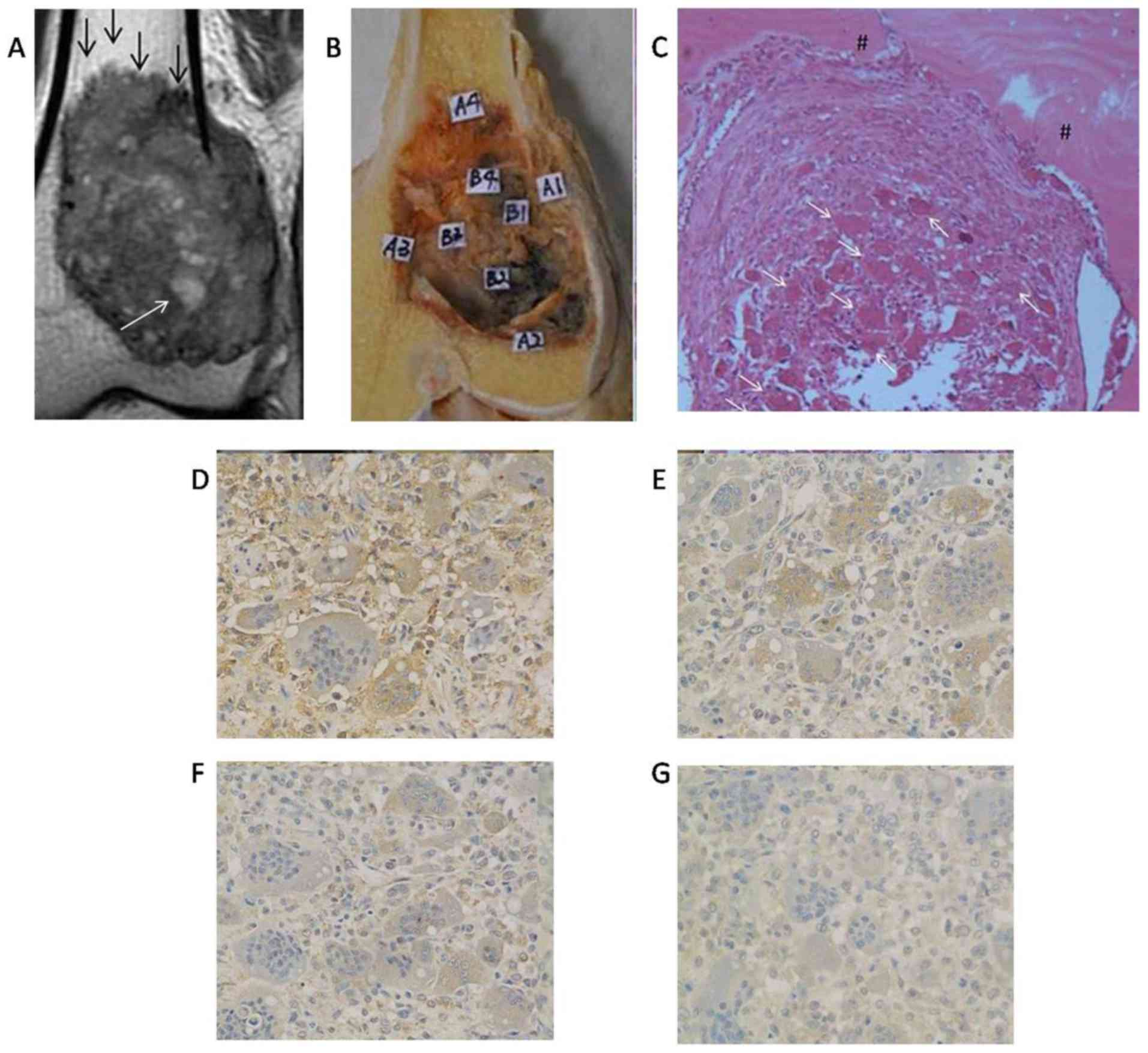
were observed in the protrusions (Figs.
2 and 5), and the ‘paint brush
borders’ sign identified by preoperative MRI was also observable in
the gross pathological sections (Fig.
5). Furthermore, the cystic changes and adjacent soft tissue
invasion observed by MRI were visually matched to tissue samples
corresponding to the GCTB coronal sections. Unfortunately, edema
could not be evaluated pathologically as the sections were
dehydrated.
Discussion
The primary purpose of the present study was to
investigate the association between RANK, RANKL, VEGF and MMP-9
protein expression and preoperative MRI features identified in
patients with GCTB. An association between MMP-9 and the ‘paint
brush borders’ sign or cystic changes observable on MRI was
demonstrated. Furthermore, specific preoperative MRI features were
identified as prognostic factors in predicting local recurrence of
GCTB.
The MMPs are a family of enzymatic proteins that are
often overexpressed in the tumor microenvironment (24). MMP-9 is regarded as a gelatinase B,
type IV collagenase and is highly expressed during the early stages
of osteoclast development, as well as in mature osteoclasts that
resorb bone (25). The primary
function of MMP-9 in GCTB is to stimulate bone re sorption by giant
cells (1). In the present clinical
study of preoperative MRI features, the ‘paint brush borders’ sign
was visually matched with the corresponding gross pathological
sections (Fig. 5). The pathological
basis of the ‘paint brush borders’ sign on preoperative MRI has
been identified as an invasion of the bone around the lesions.
Therefore, it has been hypothesized that the ‘paint brush borders’
sign may be indicative of osteolytic destruction. The present study
revealed an association between the expression of MMP-9 and the
‘paint brush borders’ sign, which may contribute to the
characterization of the molecular basis of this feature of the GCTB
border.
To the best of our knowledge, the present study was
the first to identify the ‘paint brush borders’ sign as a
prognostic factor for local recurrence of GCTB following curettage.
The success of intralesional surgical treatments is limited by the
associated high rate of local recurrence. The present study
revealed that GCTB tumors exhibiting the ‘paint brush borders’ sign
on preoperative MRI scans were more likely to recur following
intralesional curettage.
The pathological basis of the ‘paint brush borders’
sign was investigated using tissue sections. Invasion of the bone
around GCTB may be interpreted as an identifying feature of
residual tumor (9,10). Penetrating tumor margins may reduce
the effect of intralesional surgical procedures. The present study
provided evidence that supports the view that residual tumors may
be responsible for local recurrence, and revealed the ‘paint brush
borders’ sign as a risk factor for local recurrence of GCTB. This
suggested that surgeons should increase their awareness of the
‘paint brush borders’ sign, and should pay more attention to ensure
thorough elimination of residual tumors in regions of irregular
protrusions by using more aggressive interventions. In the present
study, the lengths of the protrusions observable by MRI were
consistent with those measured in gross pathological sections. It
should be emphasized that a more detailed assessment of the
location of the penetrating irregular margins may aid in guiding
aggressive surgical treatment. The present study suggested that
thorough evaluation of preoperative MRI features may be useful in
guiding surgical treatment and reduce local relapse.
MMPs are capable of degrading the entire
extracellular matrix (26). In the
present study, MMP-9 protein expression was demonstrated to be
associated with the presence of cystic changes identified by
preoperative MRI. Furthermore, cystic changes were identified as an
independent risk factor for local recurrence. Based on these
results, it is speculated that GCTB tumors exhibiting elevated
MMP-9 protein expression levels maybe more prone to cystic changes.
The recurrence rate in patients with GCTB with cystic changes was
59.46%, whereas recurrence developed in only 1 case (5.56%) in
which cystic changes were not observed. This suggests differing
properties in tumors with and without cystic changes. MRI is an
important tool for identifying tumor properties, including cystic
changes, which are associated with a poor prognosis.
A study on sacral chordoma demonstrated that
positive expression of MMP-9 may be indicative of local recurrence
(27). However, in the present study,
high expression of MMP-9 was not positively associated with local
recurrence, nor was expression of VEGF, RANK or RANKL. These
negative results may be due to the limited sample size. A number of
studies have demonstrated that co-expression of VEGF and MMP-9 is
correlated with angiogenesis and invasiveness of human bone tumors
(13,27–29). In
the present study, MMP-9 protein expression was associated with
that of VEGF and RANKL (Table III).
The activation of nuclear factor-κB signaling may activate MMP
expression in the tumor and surrounding stromal cells (29). Therefore, the upregulation of MMP-9
may be triggered by RANKL, which is expressed by spindle-like
stromal cells in GCTB. Therefore, despite the lack of association
with preoperative MRI features, the expression of RANKL in GCTB
requires further investigation. Denosumab, a human monoclonal
antibody that inhibits RANKL, has been demonstrated to be safe and
effective in the treatment of GCTB (30).
Our previous study (17) demonstrated the role of soft tissue
masses in the prognosis of GCTB. In the present study, a similar
conclusion was made regarding adjacent soft tissue invasion. If the
surgeons are able to identify the window of poor bone cortex
integrity to perform the surgery in these patients with adjacent
soft tissue invasions, it would theoretically decrease the
contribution of adjacent soft tissue invasion to the risk of local
recurrence.
Peritumoral edema was not identified as a predictor
of local recurrence in the present study. Peritumoral edema is a
reaction to tumor activity and is associated with its
aggressiveness and poor prognosis (31). It is speculated that the unexpected
results of the present study may be due to other confounding
factors. For example, the use of phenol to prevent local relapse is
controversial (9), and it may be
considered a confounding factor. Furthermore, surgeons have always
aimed to improve the surgical procedure in the long run, thereby
lowering the recurrence rate. However, the rates of local
recurrence in the first and last five years were 45.83 and 38.71%,
respectively, which are not significantly different.
The strengths of the present study include that it
was standardized to patients with GCTB located around the knee. To
exclude the impacts of pathological fracture (32) and soft tissue masses (17) on predicting local recurrence, the
present study did not include patients with GCTB with evidence of
these conditions.
As peritumoral edema was demonstrated in the
majority of study patients, it was predicted that it was associated
with recurrence. However, the classification of peritumoral edema
may have been inaccurate or subjective. Furthermore, as the
pathological sections were dehydrated, it was not possible to
pathologically confirm edema. Additionally, the number of patients
was relatively small to allow for IHC analysis. To the best of our
knowledge, GCTB usually recurs within 2 years (33–35).
However, as 3 patients in the present study developed recurrence
after ≥2 years of follow-up, the possibility of recurrence in other
patients cannot be excluded without a longer period of
follow-up.
In conclusion, IHC staining of MMP-9 was
demonstrated to be associated with preoperative MRI features of the
‘paint brush borders’ sign and cystic changes, which, coupled with
adjacent soft tissue invasion, were identified as risk factors for
local recurrence of GCTB. These results may contribute to the
prediction of local recurrence of GCTB, and may provide further
insight into these preoperative features and expression patterns.
The present study indicated that increased awareness and meticulous
attention of surgeons, along with thorough preoperative MRI
analysis, are important to reduce local recurrence of GCTB.
Acknowledgements
Not applicable.
Funding
The present study received funding from the Science
and Technology Commission of Shanghai Municipality (grant no.
134119a2402).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author, on reasonable
request.
Authors' contributions
The integrity of entire study was guaranteed by XD.
The study designed by XD and YH. The literature was researched by
YH and YZ. The clinical studies were performed by YH and YZ. The
experimental studies were performed by FY, JW, QZ and JL. Data was
acquired by JW, YH and YZ. Imaging interpretation was performed by
XD, LD, YH and YZ. Statistical analyses were performed by YH and
YZ. The manuscript was prepared by YH. The manuscript was edited by
YH and YZ. The manuscript was reviewed by XD and JZ. JX made
substantial contributions to conception and design.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of Ruijin Hospital, Shanghai Jiao Tong University
School of Medicine (Shanghai, China). Written informed consent was
obtained from all patients prior to enrolment in the study.
Consent for publication
The participants of the present study provided
written informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cowan RW and Singh G: Giant cell tumor of
bone: A basic science perspective. Bone. 52:238–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishimura M, Yuasa K, Mori K, Miyamoto N,
Ito M, Tsurudome M, Nishio M, Kawano M, Komada H, Uchida A and Ito
Y: Cytological properties of stromal cells derived from giant cell
tumor of bone (GCTSC) which can induce osteoclast formation of
human blood monocytes without cell to cell contact. J Orthop Res.
23:979–987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siddiqui MA, Seng C and Tan MH: Risk
factors for recurrence of giant cell tumours of bone. J OrthopSurg
(Hong Kong). 22:108–110. 2014. View Article : Google Scholar
|
|
4
|
van der Heijden L, Dijkstra PD, van de
Sande MA, Kroep JR, Nout RA, van Rijswijk CS, Bovée JV, Hogendoorn
PC and Gelderblom H: The clinical approach toward giant cell tumor
of bone. Oncologist. 19:550–561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu P, Zhao L, Zhang H, Yu X, Wang Z, Ye Z,
Wu S, Guo S, Zhang G, Wang J, et al: Recurrence rates and risk
factors for primary giant cell tumors around the knee: A
multicentre retrospective study in china. Sci Rep. 6:363322016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zambo I and Vesely K: WHO classification
of tumours of soft tissue and bone 2013: The main changes compared
to the 3rd edition. Cesk Patol. 50:64–70. 2014.(In Czech).
PubMed/NCBI
|
|
7
|
Enneking WF: A system of staging
musculoskeletal neoplasms. Clin Orthop Relat Res. 9–24.
1986.PubMed/NCBI
|
|
8
|
Wülling M, Engels C, Jesse N, Werner M,
Delling G and Kaiser E: The nature of giant cell tumor of bone. J
Cancer Res Clin Oncol. 127:467–474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klenke FM, Wenger DE, Inwards CY, Rose PS
and Sim FH: Giant cell tumor of bone: Risk factors for recurrence.
Clin Orthop Relat Res. 469:591–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arbeitsgemeinschaft Knochentumoren, ;
Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, Hovy
L, Matejovsky Z, Szendroi M, et al: Local recurrence of giant cell
tumor of bone after intralesional treatment with and without
adjuvant therapy. J Bone Joint Surg Am. 90:1060–1067. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masui F, Ushigome S and Fujii K: Giant
cell tumor of bone: An immunohistochemical comparative study.
Pathol Int. 48:355–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Z, Yin H, Liu T, Yan W, Li Z, Chen J,
Chen H, Wang T, Jiang Z, Zhou W and Xiao J: MiR-126-5p regulates
osteoclast differentiation and bone resorption in giant cell tumor
through inhibition of MMP-13. Biochem Biophys Res Commun.
443:944–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumta SM, Huang L, Cheng YY, Chow LT, Lee
KM and Zheng MH: Expression of VEGF and MMP-9 in giant cell tumor
of bone and other osteolytic lesions. Life Sci. 73:1427–1436. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quattrini I, Pollino S, Pazzaglia L, Conti
A, Novello C, Ferrari C, Pignotti E, Picci P and Benassi MS:
Prognostic role of nuclear factor/IB and bone remodeling proteins
in metastatic giant cell tumor of bone: A retrospective study. J
Orthop Res. 33:1205–1211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang T, Zhang G, Lau CP, Zheng LZ, Xie XH,
Wang XL, Wang XH, He K, Patrick Y, Qin L and Kumta SM: Effect of
water-soluble P-chitosan and S-chitosan on human primary
osteoblasts and giant cell tumor of bone stromal cells. Biomed
Mater. 6:0150042011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CS, Lou JH, Liao JS, Liao JS, Ding
XY, Du LJ, Lu Y, Yan L and Chen KM: Recurrence in giant cell tumour
of bone: Imaging features and risk factors. Radiol Med.
118:456–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Ding XY, Wang CS, Si MJ, Du LJ,
Zhang WB and Lu Y: In-depth analysis of local recurrence of giant
cell tumour of bone with soft tissue extension after intralesional
curettage. Radiol Med. 119:861–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klenke FM, Wenger DE, Inwards CY, Rose PS
and Sim FH: Recurrent giant cell tumor of long bones: Analysis of
surgical management. Clin Orthop Relat Res. 469:1181–1187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prosser GH, Baloch KG, Tillman RM, Carter
SR and Grimer RJ: Does curettage without adjuvant therapy provide
low recurrence rates in giant-cell tumors of bone? Clin Orthop
Relat Res. 211–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Wan N and Hu Y: Giant cell tumour
of bone: A new evaluating system is necessary. Int Orthop.
36:2521–2527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Moraes M, de Matos FR, de Souza LB, de
Almeida Freitas R and de Lisboa Lopes Costa A: Immunoexpression of
RANK RANKL, OPG, VEGF and vWF in radicular and dentigerous cysts. J
Oral Pathol Med. 42:468–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanematsu M, Semelka RC, Leonardou P,
Mastropasqua M, Armao D, Vaidean G, Firat Z and Woosley JT:
Angiogenesis in hepatocellular nodules: Correlation of MR imaging
and vascular endothelial growth factor. J Magn Reson Imaging.
20:426–434. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He Y, Zhang J and Ding X: Prognosis of
local recurrence in giant cell tumour of bone: What can we do?
Radiol Med. 122:505–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reponen P, Sahlberg C, Munaut C, Thesleff
I and Tryggvason K: High expression of 92-kD type IV collagenase
(gelatinase B) in the osteoclast lineage during mouse development.
J Cell Biol. 124:1091–1102. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lynch CC and Matrisian LM: Matrix
metalloproteinases in tumor-host cell communication.
Differentiation. 70:561–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen KW, Yang HL, Lu J, Wang GL, Ji YM, Wu
GZ, Zhu LF, Liu JY, Chen XQ and Gu YP: Expression of vascular
endothelial growth factor and matrix metalloproteinase-9 in sacral
chordoma. J Neurooncol. 101:357–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaya M, Wada T, Kawaguchi S, Nagoya S,
Yamashita T, Abe Y, Hiraga H, Isu K, Shindoh M, Higashino F, et al:
Increased pre-therapeutic serum vascular endothelial growth factor
in patients with early clinical relapse of osteosarcoma. Br J
Cancer. 86:864–869. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumour of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu CX, Lin GS, Lin ZX, Zhang JD, Chen L,
Liu SY, Tang WL, Qiu XX and Zhou CF: Peritumoral edema on magnetic
resonance imaging predicts a poor clinical outcome in malignant
glioma. Oncol Lett. 10:2769–2776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Donnell RJ, Springfield DS, Motwani HK,
Ready JE, Gebhardt MC and Mankin HJ: Recurrence of giant-cell
tumors of the long bones after curettage and packing with cement. J
Bone Joint Surg Am. 76:1827–1833. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scully SP, Mott MP, Temple HT, O'Keefe RJ,
O'Donnell RJ and Mankin HJ: Late recurrence of giant-cell tumor of
bone. A report of four cases. J Bone Joint Surg Am. 76:1231–1233.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lausten GS, Jensen PK, Schiodt T and Lund
B: Local recurrences in giant cell tumour of bone. Long-term follow
up of 31 cases. Int Orthop. 20:172–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kivioja AH, Blomqvist C, Hietaniemi K,
Trovik C, Walloe A, Bauer HC, Jorgensen PH, Bergh P and Follerås G:
Cement is recommended in intralesional surgery of giant cell
tumors: A Scandinavian Sarcoma Group study of 294 patients followed
for a median time of 5 years. Acta Orthop. 79:86–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|