Introduction
Ovarian cancer, the leading cause of mortality among
gynecological malignancies, is the fifth most common type of cancer
in females (1,2). The majority of patients with ovarian
cancer are diagnosed at a late tumor stage, due to the asymptomatic
progression and lack of an efficient screening strategy (3). High-grade serous ovarian cancers exhibit
frequent tumor protein p53 (TP53) mutations, whereas GTPase KRAS
and serine/threonine-protein kinase B-raf mutations are less common
(4). Melanoma cell adhesion molecule,
also termed cluster of differentiation 146 (CD146), and vascular
endothelial growth factor A (VEGFA) are frequently studied
angiogenic factors in other cancer types, and are considered to be
important in the progression of cancer (5).
Tumor growth and metastasis depend on angiogenesis,
which provides oxygen and nutrients to tumor cells (6). CD146 is a non-CA2+-dependent
cell adhesion molecule on the cell membrane surface of
transmembrane glycoproteins, and is involved in neovascularization
(7–11). The mechanism of CDl46-regulated tumor
angiogenesis may involve the activation of matrix
metalloproteinase-2 via VEGFA, leading to extracellular matrix
degradation, angiogenesis and metastasis (12). CD146 on tumor endothelial cells binds
the CD146 ligand on tumor cells to cause cell aggregation, vessel
blocking and adhesion to new vascular endothelia. These processes
all lead to tumor invasion and metastasis (10,13–21).
The biological functions of angiogenesis in ovarian
cancer development remain unknown; there are contradictory studies
regarding the influence of microvessel density in ovarian cancer
prognosis (22–24). Certain studies report that there is no
a clear association between VEGFA and prognosis (25,26),
whereas others have demonstrated a considerable independent
prognostic influence (27). The
abilities of sprouting, migration and tube formation in response to
VEGFA treatment are impaired in the endothelial cells (ECs) of
CD146 EC-knockout (KO) mice. Mechanistic studies further
illustrated that VEGFA-induced VEGF receptor (VEGFR)-2
phosphorylation and RAC-α serine/threonine-protein kinase (Akt)/p38
mitogen-activated protein kinase/nuclear factor-κB pathway
activation were inhibited in these CD146-null endothelial cells,
which may indicate the underlying reason for the observed tumor
angiogenesis inhibition in CD146EC-KO mice (26). Knockdown of CD146 decreased
VEGFR-2/E-cadherin expression and altered focal adhesion kinase
activation in response to VEGF (28).
VEGFA, a primary responder to VEGF signaling, serves
important roles in physiological and pathological angiogenesis.
Tumors which grow quickly generally have increased numbers of
interstitial blood vessels and less fibrous connective tissue
compared with benign tumors that grow slowly (29). The induction of neovascularization is
one of the prerequisites for tumor growth, invasion and
metastasis.
Recently, CD146 has been identified as a VEGFR-2
coreceptor-mediated VEGF/VEGFR2 pathway that is essential in
VEGF-induced cell migration and tube formation, and has been
implicated in the tumor progression of epithelial ovarian cancer
(30,31). The present study aimed to characterize
the expression pattern of CD146 and VEGFA in ovarian cancer and
their role in this cancer type.
Materials and methods
Patients
Epithelial ovarian samples were collected <10 min
following surgery from 22 patients with ovarian cancer, including
14 cases of high-grade serous ovarian carcinoma, eight cases of
low-grade serous ovarian carcinoma and 30 cases diagnosed with
benign disease (control) between January 2014 and January 2016, in
the Cancer Hospital Affiliated to Xinjiang Medical University
(Xinjiang, China). Among them, four cases were <30 years old, 21
were between 30 and 40 years, nine patients were between 40 and 50
years, 14 were between 50 and 60 years and four cases were >60
years. The median age was 50.1 years. A total of 12 patients had
lymph node metastasis. All specimens collected prior to
chemotherapy, radiation therapy or immunotherapy were
histopathologically confirmed to be epithelial ovarian cancer.
Informed consent was obtained from all individual participants
included in the study, and the study was approved by the ethics
committee of the Cancer Hospital Affiliated to Xinjiang Medical
University.
Reagents and instruments
TRIzol® was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA); the
high-efficiency reverse transcription (RT) kit was purchased from
Tiangen Biotech Co., Ltd. (Beijing, China) and SYBR Select Master
mix was purchased from Applied Biosystems (Thermo Fisher
Scientific, Inc.). VEGFA and GAPDH primers (Table I) were synthesized by Quintarabio
Biotechnology Co., Ltd. (Urumqi, China), and the RT-quantitative
polymerase chain reaction (RT-qPCR) instrument was purchased from
Applied Biosystems.
 | Table I.Primers used for reverse
transcriptase-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcriptase-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) | Amplicon size (base
pairs) |
|---|
| CD146 | F:
AGCTCCGCGTCTACAAAGC | 102 |
|
| R:
CTACACAGGTAGCGACCTCC |
|
| VEGFA | F:
AGGGCAGAATCATCACGAAGT | 75 |
|
| R:
AGGGTCTCGATTGGATGGCA |
|
| GAPDH | F:
GGAGCGAGATCCCTCCAAAAT | 197 |
|
| R:
GGCTGTTGTCATACTTCTCATGG |
|
RT-qPCR
RNA extraction was conducted using
TRIzol®, according to the manufacturer's protocol.
Briefly, 100 mg homogenized tissue samples were mixed with 800 µl
TRIzol and 200 µl chloroform. The mixture was vortexed thoroughly
for 2 min, incubated for 20 min at room temperature, and
centrifuged at 8,000 × g for 20 min at 4°C. The upper phase was
carefully transferred into a fresh tube, and precipitated with 600
µl isopropanol at −20°C for 20 h. Following centrifugation, the RNA
pellet was washed with 70% ethanol, air dried and re-suspended in 1
ml diethyl pyrocarbonate-treated water. cDNA was transcribed using
500 ng RNA as the template, according to the RT kit instructions,
and stored at −80°C.
Each RT-qPCR reaction tube contained 12.5 µl SYBR
Green Real-time PCR Master mix, 1 µl of each forward and reverse
primer (Table I), 2 µl cDNA template
and 6.5 µl dH2O. The mixture was centrifuged for 15 sec,
and RT-qPCR was performed using the following protocol: An initial
denaturation step at 95°C for 2 min, 40 cycles of amplification
with denaturation at 95°C for 15 sec, and annealing and extension
at 60°C for 1 min. PCR signals from a total of 40 cycles were
collected at 60°C and fluorescence quantitation was assessed using
RT-qPCR software.
The relative expression levels of target genes were
quantified by normalizing target gene expression to that of the
internal control using the 2−ΔΔCq formula (32).
Measuring the protein expression
levels of CD146 and VEGFA using western blotting
Proteins from ovarian tissues were extracted using
radioimmunoprecipitation assay/phenylmethylsulfonyl fluoride
(100:1) solution, according to the manufacturer's instructions
(Boster Biological Technology, Pleasanton, CA, USA). A total of 50
µg proteins (concentration 2.5 µg/µl) were electro-separated on a
10% SDS-PAGE gel and electro-transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA) for western
blotting. The membrane was rinsed with PBS and the non-specific
binding sites were blocked in a solution of 5% non-fat milk in PBS
with 0.05% Tween-20 (PBST) for 2 h at 37°C, followed by three
washes with PBST. The primary antibodies against CD146 (1:500
dilution; cat. no. 13475S; CST Biological Reagents Co., Ltd.,
Shanghai, China), VEGFA (1:1,000 dilution; cat. no. 2478S; CST
Biological Reagents Co., Ltd.) and β-actin (1:2,500 dilution; cat.
no. ab8227; Abcam, Cambridge, UK) were added and incubated
overnight at 4°C. The secondary Goat Anti-Rabbit-IgG (H+L) antibody
conjugated to horseradish peroxidase (1:10,000 dilution; cat. no.
31460; Pierce; Thermo Fisher Scientific Inc.) was added for a 1-h
incubation at room temperature. Western blotting signals were
developed using an enhanced chemiluminescent kit (cat. no. 32106;
Thermo Fisher Scientific, Inc.). Following color development, the
detection of immunoreactive bands was performed using a ChemiScope
mini chemiluminescence meter (Qinxiang Science Instrument Co.,
Ltd., Shanghai, China).
The protein expression levels were calculated using
the following equation: Protein expression=integral optical density
value of target protein/integral optical density value of β-actin.
Protein levels were quantified using a ChemiScope Mini system
(Qinxiang Science Instrument Co., Ltd.).
Immunohistochemistry (IHC)
Tissue samples were fixed with 4% paraformaldehyde
at room temperature for 12 h and embedded with paraffin. Following
deparaffinization and rehydration, the CD146 and VEGFA antigens
were retrieved by citric acid buffer (pH 6.0); subsequently the
samples were blocked for endogenous peroxidase activity using 3%
hydrogen peroxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 30 min at 37°C and then 2 min at room temperature. Following
washing with potassium-free PBS buffer, the samples were incubated
with monoclonal antibodies against CD146 (catalog no. ab75769;
1:100; Abcam) and VEGFA (catalog no. ab51745; 1:100; Abcam)
overnight at 4°C. Following rewarming, the samples were incubated
with the corresponding secondary antibody (Goat Anti-Rabbit-IgG
(H+L) antibody conjugated to horseradish peroxidase; cat. no.
31460; 1:1,000; Pierce) for 1 h at room temperature. The sections
were subsequently stained with Mayer's hematoxylin for 5 min at
room temperature, followed by dehydration and permeabilization.
Finally, the sections were mounted with neutral resins and
visualized by light microscopy (×100 magnification). Staining
intensity was graded as 0 (negative), 1 (weak), 2 (moderate), and 3
(strong); the percentage of positive cells examined was scored as 0
(negative), 1 (<10%), 2 (11–50%), 3 (51–80%), and 4 (>80%).
The two scores were multiplied and the immunoreactivity score (IRS;
values of 0–12) was determined: Values 0–1 as negative (−); 2–3 as
weak positive (+); values 4–8 as moderate positive (++); and values
9–12 as strongly positive (+++).
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 (SPSS Inc., Chicago, IL, USA). Data are presented as
the mean ± standard deviation. All experiments were repeated three
times. Student's t-test was used to compare the means of two
independent groups in order to determine whether differences
between the means were significant. The Pearson test used for the
independent data revealed the associations among the factors
tested. A Spearman's Rho test was used to determine the correlation
between the IRS of CD146 and VEGFA and the pathological grade of
tumor IRS, and the statistical significance of differences between
tumor tissues and non-tumor tissues. Dot density frequency
analysis, using SigmaPlot 11.1.0 (Systat Software Inc., San Jose,
CA, USA), was performed on the means of CD146 and VEGFA to assess
their sensitivity and specificity (33,34).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Gene and protein expression levels of
CD146 and VEGFA in ovarian tissues
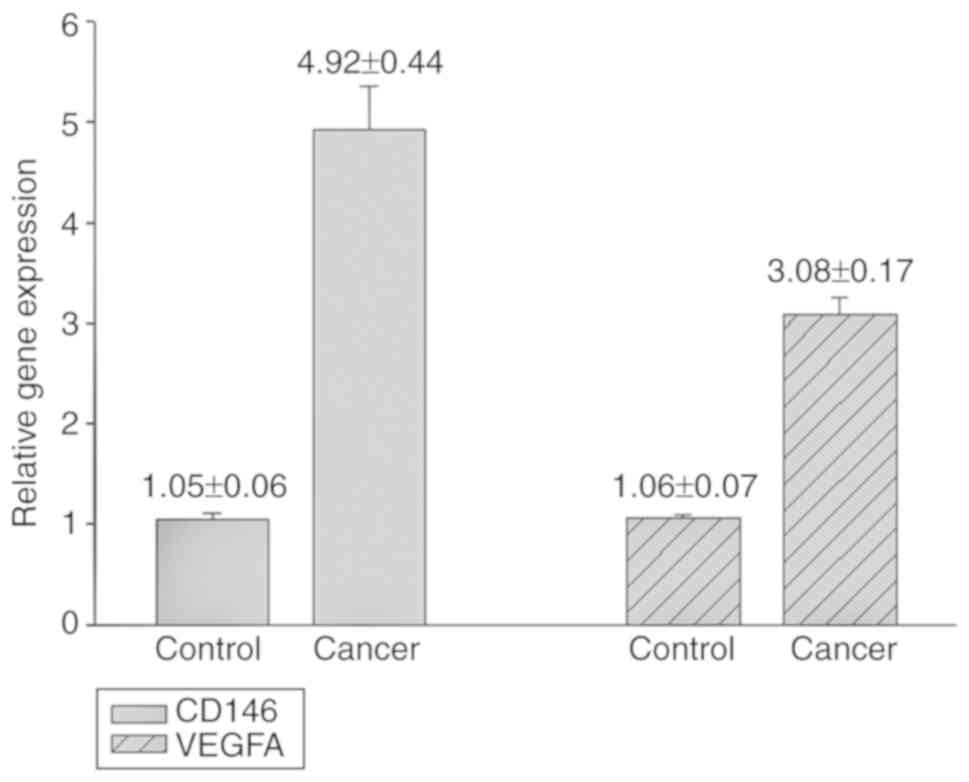
The RT-qPCR results demonstrated that CD146 and
VEGFA gene expression levels in the primary tumor were
significantly increased compared with the control (CD146 4.92±0.44
vs. 1.05±0.06, P<0.01; VEGFA, 3.08±0.17 vs. 1.06±0.07,
P<0.01; Fig. 1). The results of
the western blotting experiments indicated that the protein
expression levels of CD146 and VEGFA in the primary tumors were
significantly higher compared with those of the control (CD146
protein, 0.70±0.02 vs. 0.41±0.07, P<0.01; VEGFA protein,
0.54±0.01 vs. 0.26±0.01, P<0.01; Fig.
2).
Correlation analysis
The Pearson correlation analysis (Table II) demonstrated that there were
significant positive correlations between the gene and protein
expression levels of CD146 (r=0.73, P<0.05), gene and protein
expression levels of VEGFA (r=0.74, P<0.05), gene expression
levels of CD146 and VEGFA (r=0.78, P<0.05), protein expression
levels of CD146 and VEGFA (r=0.69, P<0.05), CD146 gene
expression levels and VEGFA protein expression levels (r=0.77,
P<0.05) and between CD146 protein expression levels and VEGFA
gene expression levels (r=0.81, P<0.05). Although the results of
IHC demonstrated that the protein expression levels of CD146
(IRS=8) and VEGFA (IRS=9) in tumor tissue were significantly higher
compared with those in non-tumor tissue (IRS=2, Fig. 3), the Spearman's Rho value was 0.37,
indicating no strong association between the IRS of CD146 and VEGFA
and the pathological grade of the tumor (P>0.05; data not
shown).
 | Table II.Pearson correlation coefficients (r)
between the gene and protein expression of CD146 and VEGFA. |
Table II.
Pearson correlation coefficients (r)
between the gene and protein expression of CD146 and VEGFA.
|
| CD146 gene
expression | CD146 protein
expression | VEGFA gene
expression | VEGFA protein
expression |
|---|
| CD146 gene
expression | 1 | – | – | – |
| CD146 protein
expression | 0.73a | 1 | – | – |
| VEGFA gene
expression | 0.78a | 0.81a | 1 | – |
| VEGFA protein
expression | 0.77a | 0.69a | 0.74a | 1 |
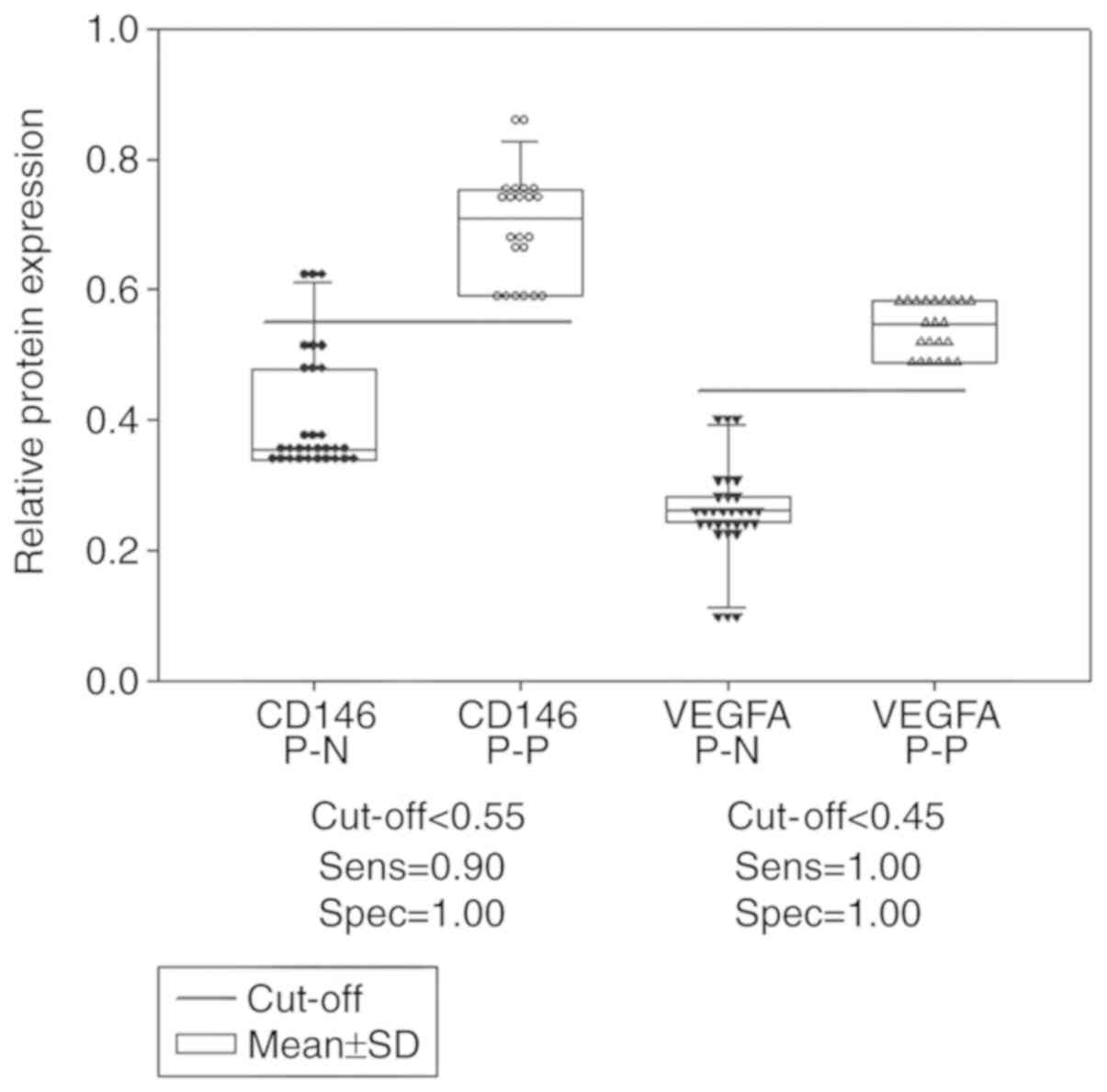
Dot density frequency analysis
The optimal cut off values determined by dot density
frequency analysis were 1.80 and 1.96 for the CD146 and VEGFA gene
expression levels, respectively (Fig.
4), and 0.55 and 0.45 for the CD146 and VEGFA protein
expression levels, respectively (Fig.
5).
Discussion
Expression of CD146 and VEGFA is necessary for tumor
occurrence and development. CD146 promotes cancer progression,
enhanced migration and invasion in melanoma, gallbladder
adenocarcinoma, breast cancer and prostate cancer (6,35). It has
been recognized as a biomarker to predict poor prognosis in gastric
cancer, lung adenocarcinoma, malignant pleural mesothelioma and
non-small-cell lung cancer (36–39). CD146
is associated with an advanced tumor stage in melanoma, prostate
cancer and ovarian cancer (6).
Inhibition of CD146 gene expression via RNA interference reduces
in vitro perineural invasion in a high-metastatic adenoid
cystic carcinoma cell line (ACC-M) (40). In triple-negative breast cancer
samples, high expression levels of CD146 are strongly associated
with E-cadherin downregulation, suggesting that CD146 promotes
breast cancer progression due to the induction of
epithelial-mesenchymal transition via the activation of ras homolog
family member A and the upregulation of snail family
transcriptional repressor 2 (41). A
CD146 immunohistochemical study revealed that its overexpression
was positively and significantly correlated with the pathological
subtype of cervical cancer, with the histological grade and depth
of myometrial invasion in endometrial cancer, yet not with patient
age or the pathological type of the tumor (42).
VEGFA expression in patients with ovarian cancer at
stages III and IV is significantly higher compared with that at
stages I and II (43). VEGFA
represents a potent cytokine in ovarian cancer progression. High
VEGFA production from primary tumors was hypothesized to correlate
with increased metastasis and a worse prognosis compared with low
VEGFA-producing tumors (44). In
addition, VEGFA secretion has recently been proposed as one of the
major factors responsible for defective immune function in patients
with cancer (44). Patients with
early-stage cancer (stages I and II) display a poorer prognosis
when VEGFA expression is increased in the tumor (45), and elevated expression levels of the
VEGFA gene predict a poor prognosis; notably, this does not appear
to be associated with microvessel density, which contradicts
previous studies (25,45). A tissue microarray study indicated
that high VEGFA expression levels in epithelial ovarian cancer may
be associated with serous morphology, high grade and advanced
stage. Among 78 cases of primary malignant epithelial ovarian
neoplasms that exhibited high VEGFA expression, 23 were serous
carcinomas (46).
The present study confirmed that the gene and
protein expression levels of CD146 and VEGFA in cancer tissues were
increased significantly, and were positively correlated with each
other. Dot density frequency analysis revealed that gene expression
levels of CD146 and VEGFA are superior compared with protein
expression levels as potential biological indicators. Furthermore,
protein quantification is costly and time-consuming. The cut off
value of the gene expression levels, based on the mean, were higher
compared with the control, indicating that a gene expression
approach may be used in the first instance.
The Pearson test was used to compare the gene/gene,
gene/protein and protein/protein expression levels, and it was
confirmed that CD146 and VEGFA are co-expressed, yet their
expression levels in the tumor tissue are not associated with
pathological grade of ovarian serous carcinomas. This result is
consistent with the results of Premalata et al (47), as the results of that study suggested
that the high expression levels of VEGFA in epithelial ovarian
cancer may be associated with serous morphology, high grade and
advanced stage. Though a certain level of VEGFA expression was
observed in the majority of ovarian carcinomas, high expression
levels were only observed in one-third of patients. High VEGFA
expression levels occurred in a small proportion of patients with
ovarian cancer, and this may be used as an independent predictor of
poor prognosis; patients with tumors that express high levels of
VEGFA had worse survival rates compared with those with medium, low
or no VEGFA expression (48).
Spearman's Rho analysis failed to suggest that the
overexpression of CD146 and VEGFA was associated with high
histopathological grade. In fact, it was only associated with
static changes in the tumor, including the tumor size, growth rate,
infiltration and metastatic progression in contrast to dramatic
changes. CD146 interacts with VEGF-C directly to control lymphatic
sprouting in lymphangiogenesis. CD146 is expressed in lymphatic
endothelial cells (LEC) and regulates LEC activation induced by
VEGF-C. It has been demonstrated in vitro and in vivo
that CD146 is a receptor of VEGF-C, regulating lymphangiogenesis at
the sprouting step independently of vasculogenesis (49).
CD146 and VEGFA expression levels are directly
associated with dynamic tumor behavior. Thus, to a certain extent,
these factors influence the biological status (proliferation or
inhibition) of the tumor cells in the body; therefore, they are
more likely to be the dynamic indicators of tumor alterations,
including the potential growth rate, prognosis and the effects of
anticancer agents (6). All of these
will provide an important reference value in the clinic.
In a xenograft model using immunodeficient
NOD/Shi-scid IL-2Rγ-null mice, CD146+ cells purified
from malignant rhabdoid tumor (MRT) cell lines or a primary tumor
displayed the unique ability to form tumors in vivo.
Blocking CD146-associated mechanisms, including short hairpin RNA
knockdown or treatment with a polyclonal antibody against CD146,
effectively suppressed the tumor growth of MRT cells in
vitro and in vivo, via the induction of apoptosis by
inactivating Akt (50).
CD146 and VEGFA cut-off values determined from dot
density frequency may predict the biological status of tumor cells
based on sensitivity and specificity. Analysis of the dot density
graphs indicated that for the identification of ovarian cancer and
the control group, the cut-off for the gene expression was 1.80
(CD146) and 1.96 (VEGFA) respectively; the cut-off for the protein
expression was 0.55 (CD146) and 0.45 (VEGFA) respectively. It was
postulated that elevated cellular production of CD146 and VEGFA may
serve as a potential indicator of tumor alterations, and that these
factors may correlate with a poorer prognosis for patients with
ovarian carcinoma. These indicators may facilitate the
investigation of whether tumor cells are under either proliferative
or inhibitory conditions. VEGFA is a key regulator of angiogenesis,
which drives endothelial cell survival, proliferation and migration
while increasing vascular permeability. VEGFA not only serves an
important function in the physiology of normal ovaries, but has
also been implicated in the pathogenesis of ovarian cancer. It is
understood that CD146 serves a potent role in angiogenesis, and
VEGFA may also serve a central and specific function in
angiogenesis (51).
In conclusion, CD146 and VEGFA expression levels are
positively correlated with dynamic tumor biology, and they are
co-overexpressed in ovarian cancer as indicators of tumor activity.
Their expression levels may be important indicators in the
diagnosis, treatment and prognosis of epithelial ovarian
cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by grant no. Y14130032,
from the Science and Technology Board of Wulumuqi City. Project
name: Study on the correlation between CD146, VEGF-A and the
invasion and metastasis of epithelial ovarian cancer; local
level.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and JY conceived the study, designed the
experiments and wrote the manuscript. PZ, TX, JC and FL performed
laboratory tests and acquired the data. TQ analyzed the data and
performed the statistical analysis. JY conducted a literature
search and gave suggestions on revising the manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from all individual
participants included in the study, and the study was approved by
the Ethics Committee of the Cancer Hospital Affiliated to Xinjiang
Medical University.
Patient consent for publication
Consent to the publication of material was obtained
from all participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bowtell DD: The genesis and evolution of
high-grade serous ovarian cancer. Nat Rev Cancer. 10:803–808. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rechsteiner M, Zimmermann AK, Wild PJ,
Caduff R, von Teichman A, Fink D, Moch H and Noske A: TP53
mutations are common in all subtypes of epithelial ovarian cancer
and occur concomitantly with KRAS mutations in the mucinous type.
Exp Mol Pathol. 95:235–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedlander LT, Hussani H, Cullinan MP,
Seymour GJ, De Silva RK, De Silva H, Cameron C and Rich AM: VEGF
and VEGFR2 in dentigerous cysts associated with impacted third
molars. Pathology. 47:446–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zabouo G, Imbert AM, Jacquemier J, Finetti
P, Moreau T, Esterni B, Birnbaum D, Bertucci F and Chabannon C:
CD146 expression is associated with a poor prognosis in human
breast tumors and with enhanced motility in breast cancer cell
lines. Breast Cancer Res. 11:R12009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shih IM: The role of CDl46 (Mel-CAM) in
biology and pathology. J Pathol. 189:4–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan X, Lin Y, Yang D, Shen Y, Yuan M,
Zhang Z, Li P, Xia H, Li L, Luo D, et al: A novel anti-CDl46
monoclonal antibody, AA98, inhibits angiogenesis and tumor growth.
Blood. 102:184–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu GJ, Varma VA, Wu MW, Wang SW, Qu P,
Yang H, Petros JA, Lim SD and Amin MB: Expression of a human cell
adhesion molecule, MUCl8, in prostate cancer cell lines and
tissues. Prostate. 48:305–315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bardin N, Anfosso F, Massé JM, Cramer E,
Sabatier F, Le Bivic A, Sampol J and Dignat-George F:
Identification of CDl46 a component of the endothelial junction
involved in the control of cell-cell cohesion. Blood. 98:3677–3684.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang Y, Wang F, Feng J, Yang D, Yang X and
Yan X: Knockdown of CDl46 reduces the migration and proliferation
of human endothelial cells. Cell Res. 16:313–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Campbell NE, Kellenberger L, Greenaway J,
Moorehead RA, Linnerth-Petrik NM and Petrik J: Extracellular matrix
proteins and tumor angiogenesis. J Oncol. 2010:5869052010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vainio O, Dunon D, Aïssi F, Dangy JP,
McNagny KM and Imhof BA: HEMCAM, an adhesion molecule expressed by
c-kit+ hemopoietic progenitors. J Cell Biol. 135:1655–1668. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bowen MA, Patel DD, Li X, Modrell B,
Malacko AR, Wang WC, Marquardt H, Neubauer M, Pesando JM, Francke
U, et al: Cloning, mapping, and characterization of activated
leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med.
181:2213–2220. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anfosso F, Bardin N, Francès V, Vivier E,
Camoin-Jau L, Sampol J and Dignat-George F: Activation of human
endothelial cells via S-endo 1 antigen (CDl46) stimulates the
tyrosine phosphorylation of focal adhesion kinase p125(FAK). J Biol
Chem. 273:26852–26856. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bardin N, Francès V, Combes V, Sampol J
and Dignat-George F: CDl46: Biosynthesis and production of a
soluble form in human cultured endothelial cells. FEBS Lett.
421:12–14. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang Y, Wang F, Feng J, Yang D, Yang X and
Yan X: Knockdown of CDl46 reduces the migration and proliferation
of human endothelial cells. Cell Res. 16:313–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schrage A, Loddenkemper C, Erben U, Lauer
U, Hausdorf G, Jungblut PR, Johnson J, Knolle PA, Zeitz M, Hamann A
and Klugewitz K: Murine CDl46 is widely expressed on endothelial
cells and is recognized by the monoclonal antibody ME-9F1.
Histochem Cell Biol. 129:441–451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bardin N, Blot-Chabaud M, Despoix N, Kebir
A, Harhouri K, Arsanto JP, Espinosa L, Perrin P, Robert S, Vely F,
et al: CDl46 and its soluble form regulate monocyte
transendothelial migration. Arterioscler Thromb Vasc Biol.
29:746–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng C, Qiu Y, Zeng Q, Zhang Y, Lu D,
Yang D, Feng J and Yan X: Endothelial CDl46 is required for in
vitro tumor-induced angiogenesis: The role of a disulfide bond in
signaling and dimerization. Int J Biochem Cell Bio. 41:2163–2172.
2009. View Article : Google Scholar
|
|
21
|
Satyamoorthy K, Muyrers J, Meier F, Patel
D and Herlyn M: Mel-CAM-specific genetic suppressor elements
inhibit melanoma growth and invasion through loss of gap junctional
communication. Oneogene. 20:4676–4684. 2001. View Article : Google Scholar
|
|
22
|
Hollingsworth HC, Kohn EC, Steinberg SM,
Rothenberg ML and Merino MJ: Tumor angiogenesis in advanced stage
ovarian carcinoma. Am J Pathol. 147:33–41. 1995.PubMed/NCBI
|
|
23
|
Gasparini G, Bonoldi E, Viale G, Verderio
P, Boracchi P, Panizzoni GA, Radaelli U, Di Bacco A, Guglielmi RB
and Bevilacqua P: Prognostic and predictive value of tumor
angiogenesis in ovarian carcinomas. Int J Cancer. 69:205–211. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orre M, Lotfi-Miri M, Mamers P and Rogers
PA: Increased microvessel density in mucinous compared with
malignant serous and benign tumours of the ovary. Br J Cancer.
77:2204–2209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakanishi Y, Kodama J, Yoshinouchi M,
Tokumo K, Kamimura S, Okuda H and Kudo T: The expression of
vascular endothelial growth factor and transforming growth
factor-beta associates with angiogenesis in epithelial ovarian
cancer. Int J Gynecol Pathol. 16:256–262. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto S, Konishi I, Mandai M, Kuroda H,
Komatsu T, Nanbu K, Sakahara H and Mori T: Expression of vascular
endothelial growth factor (VEGF) in epithelial ovarian neoplasms:
Correlation with clinicopathology and patient survival, and
analysis of serum VEGF levels. Br J Cancer. 76:1221–1227. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Komatsu H, Oishi T, Itamochi H, Shimada M,
Sato S, Chikumi J, Sato S, Nonaka M, Sawada M, Wakahara M, et al:
Serum vascular endothelial growth factor-A as a prognostic
biomarker for epithelial ovarian cancer. Int J Gynecol Cancer.
27:1325–1332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jouve N, Bachelier R, Despoix N, Blin MG,
Matinzadeh MK, Poitevin S, Aurrand-Lions M, Fallague K, Bardin N,
Blot-Chabaud M, et al: CD146 mediates VEGF-induced melanoma cell
extravasation through FAK activation. Int J Cancer. 137:50–60.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Yang ZL, Liu JQ, Jiang S and Miao
XY: Identification of CD146 expression, angiogenesis, and
lymphangiogenesis as progression, metastasis, and poor-prognosis
related markers for gallbladder adenocarcinoma. Tumour Biol.
33:173–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang T, Zhuang J, Duan H, Luo Y, Zeng Q,
Fan K, Yan H, Lu D, Ye Z, Hao J, et al: CD146 is a coreceptor for
VEGFR-2 in tumor angiogenesis. Blood. 120:2330–2339. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aldovini D, Demichelis F, Doglioni C, Di
Vizio D, Galligioni E, Brugnara S, Zeni B, Griso C, Pegoraro C,
Zannoni M, et al: M-CAM expression as marker of poor prognosis in
epithelial ovarian cancer. Int J Cancer. 119:1920–1926. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weddell JC and Imoukhuede PI: Quantitative
characterization of cellular membrane-receptor heterogeneity
through statistical and computational modeling. PLoS One.
9:e972712014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kornbrot D: Statistical software for
microcomputers: SigmaPlot 2000 and SigmaStat2. Br J Math Stat
Psychol. 53:335–337. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu GJ, Wu MW, Wang SW, Liu Z, Qu P, Peng
Q, Yang H, Varma VA, Sun QC, Petros JA, et al: Isolation and
characterization of the major form of human MUC18 cDNA gene and
correlation of MUC18 overexpression in prostate cancer cell lines
and tissues with malignant progression. Gene. 279:17–31. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu WF, Ji SR, Sun JJ, Zhang Y, Liu ZY,
Liang AB and Zeng HZ: CD146 expression correlates with
epithelial-mesenchymal transition markers and a poor prognosis in
gastric cancer. Int J Mol Sci. 13:6399–6406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oka S, Uramoto H, Chikaishi Y and Tanaka
F: The expression of CD146 predicts a poor overall survival in
patients with adenocarcinoma of the lung. Anticancer Res.
32:861–864. 2012.PubMed/NCBI
|
|
38
|
Sato A, Torii I, Okamura Y, Yamamoto T,
Nishigami T, Kataoka TR, Song M, Hasegawa S, Nakano T, Kamei T and
Tsujimura T: Immunocytochemistry of CD146 is useful to discriminate
between malignant pleural mesothelioma and reactive mesothelium.
Mod Pathol. 23:1458–1466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kristiansen G, Yu Y, Schlüns K, Sers C,
Dietel M and Petersen I: Expression of the cell adhesion molecule
CD146/MCAM in non-small cell lung cancer. Anal Cell Pathol.
25:77–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen W, Zhang HL, Jiang YG, Li JH, Liu BL
and Sun MY: Inhibition of CD146 gene expression via RNA
interference reduces in vitro perineural invasion on ACC-M Cell. J
Oral Pathol Med. 38:198–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeng Q, Li W, Lu D, Wu Z, Duan H, Luo Y,
Feng J, Yang D, Fu L and Yan X: CD146, an epithelial-mesenchymal
transition inducer, is associated with triple-negative breast
cancer. Proc Natl Acad Sci USA. 109:1127–1132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
American Cancer Society. Cancer Facts
& Figures 2012 Atlanta: American Cancer Society; 2012
|
|
43
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
44
|
Lu Y, Xu Q, Zuo Y, Liu L, Liu S, Chen L,
Wang K, Lei Y, Zhao X and Li Y: Isoprenaline/β2-AR activates
Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells
to promote tumor angiogenesis. BMC Cancer. 17:8752017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paley PJ, Staskus KA, Gebhard K, Mohanraj
D, Twiggs LB, Carson LF and Ramakrishnan S: Vascular endothelial
growth factor expression in early stage ovarian carcinoma. Cancer.
80:98–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen GH, Ghazizadeh M, Kawanami O, Shimizu
H, Jin E, Araki T and Sugisaki Y: Prognostic significance of
vascular endothelial growth factor expression in human ovarian
carcinoma. Br J Cancer. 83:196–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Premalata CS, Umadevi K, Shobha K,
Anurekha M and Krishnamoorthy L: Expression of VEGF-A in epithelial
ovarian cancer: Correlation with morphologic types, grade and
clinical stage. Gulf J Oncolog. 1:49–54. 2016.PubMed/NCBI
|
|
48
|
Duncan TJ, Al-Attar A, Rolland P, Scott
IV, Deen S, Liu DT, Spendlove I and Durrant LG: Vascular
endothelial growth factor expression in ovarian cancer: A model for
targeted use of novel therapies? Clin Cancer Res. 14:3030–3035.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan H, Zhang C, Wang Z, Tu T, Duan H, Luo
Y, Feng J, Liu F and Yan X: CD146 is required for VEGF-C-induced
lymphatic sprouting during lymphangiogenesis. Sci Rep. 7:74422017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nodomi S, Umeda K, Saida S, Kinehara T,
Hamabata T, Daifu T, Kato I, Hiramatsu H, Watanabe KI, Kuwahara Y,
et al: CD146 is a novel marker for highly tumorigenic cells and a
potential therapeutic target in malignant rhabdoid tumor. Oncogene.
35:5317–5327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsiolakidou G, Koutroubakis IE, Tzardi M
and Kouroumalis EA: Increased expression of VEGF and CD146 in
patients with inflammatory bowel disease. Dig Liver Dis.
40:673–679. 2008. View Article : Google Scholar : PubMed/NCBI
|