Introduction
Laryngeal carcinoma is a common malignant tumor of
the head and neck with high incidence and mortality rates, which
severely threatens the patients' life and health. He et al
(1) have reported that
age-standardized incidence rates of laryngeal carcinoma by Chinese
standard population and by world standard population were 1.18 and
1.19 per 100,000 individuals, respectively, and age-standardized
mortality rates by Chinese standard population and by world
standard population were 0.61 and 0.61 per 100,000 individuals,
respectively, in China in 2015. The conventional treatment options
for laryngeal cancer include surgery (2,3),
radiotherapy (4,5), chemotherapy (6,7) and
biotherapy (8).
Chemotherapy is the main treatment approach for
patients with advanced and postoperative recurrent laryngeal cancer
(9). Cisplatin (CDDP) is often used
as a chemotherapeutic drug for laryngeal cancer, and anticancer
effects have been observed in a number of patients with laryngeal
cancer undergoing chemotherapy with CDDP. However, drug resistance
is one of the main factors limiting the efficacy of chemotherapy in
patients with laryngeal cancer (10,11).
The increase in cell membrane transporter expression
has been demonstrated to be associated with the drug resistance of
tumors, among which the ATP binding-cassette (ABC) proteins are the
major factors (12). ABCB1 and ABCG2
are members of the ABC superfamily and are implicated in drug
resistance (13–16).
Our previous studies have established cell (17) and animal (18) esophageal cancer models with
Adriamycin resistance, in which the association between ABCG2
expression and Adriamycin resistance of esophageal squamous cell
carcinoma has been demonstrated. In vitro and in vivo
experiments have confirmed the involvement of ABCG2 in esophageal
cancer drug resistance (17,18). Based on these results, the present
study aimed to determine relationship between ABCG2 and drug
resistance of laryngeal squamous cell carcinoma. ABCG2 has been
demonstrated to be expressed at high levels in various types of
tumor, such as esophageal, ovarian and breast cancer, as well as
osteosarcoma, and to participate in the development of drug
resistance in tumor cells (18–22);
however, a limited number of reports are currently available on the
effects of ABCG2 in laryngeal squamous cell carcinoma (23). In addition, the mechanism by which
drug-resistant cells affect the drug resistance of neighboring
cells has not been elucidated to date.
Tumor microenvironment serves an important role in
tumor development, drug resistance and cancer therapy (24,25).
Tumor cells create a favorable microenvironment for tumor
development by transferring the information (DNA, RNA and protein)
between cells, which promotes tumor development (26,27).
Therefore, investigating the mechanism of acquired drug resistance
caused by changes in the tumor microenvironment provides a new
direction for studying drug resistance in laryngeal squamous cell
carcinoma.
Extracellular vesicles (EVs) are supermicrocystic
structures that are produced and released by both tumor and normal
cells (28). EVs are bioactive
substances that are secreted by cells and include microvesicles
(MVs) and exosomes (29,30). During the formation of EVs, proteins,
mRNAs and non-coding RNAs are functionally selected from the source
cells; these signaling molecules are released into the target cells
during interaction between EVs and target cells, serving a
functional role by changing the genotype and phenotype of the
target cells (31,32).
A limited number of studies have reported that EVs
released by certain types of tumor cells, such as prostate and lung
cancer cells, can promote cell proliferation, tumor angiogenesis,
metastasis and immune escape by acting on tumor cells, endothelial
cells, tumor-related fibroblasts and immune cells in their
microenvironment, thus promoting the occurrence and development of
tumors (33,34). Takahashi et al (35) have confirmed that the exosomes
secreted by hepatocellular carcinoma cells regulate the biological
activity of target cells through their intrinsic microRNAs and
suggested that long non-coding (lnc)RNAs are also present in EVs.
For example, lncRNA very low-density lipoprotein receptor in EVs
has been demonstrated to regulate acquired drug resistance of
hepatocellular carcinoma cells by acting on ABCG2.
The present study aimed to study the regulatory
effects of EVs released by drug-resistant laryngeal cancer cells on
cell drug resistance, providing a new method for investigating the
drug resistance mechanism in laryngeal cancer.
Materials and methods
Cell lines
Human laryngeal cancer cells AMC-HN-8 were purchased
from Beijing Bnbio Co., Ltd. The AMC-HN-8 cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100
U/l of penicillin and streptomycin (North China Pharmaceutical Co.,
Ltd.) in a humidified atmosphere containing 5% CO2/95%
air at 37°C. A CDDP resistant cell line was established from
AMC-HN-8 cells by continuously exposing the cells to increasing
concentrations of CDDP (0.01–2 µg/ml) for 8 months. At the end of
the exposure, one of the surviving clones was isolated and
designated as AMC-HN-8/CDDP, and was further maintained in medium
containing 2 µg/ml CDDP.
MTT assay and inhibitory rate
calculation
The monolayer of cultured AMC-HN-8, AMC-HN-8/CDDP,
AMC-HN-8-EVs1 and AMC-HN-8-EVs2 cells was digested by 0.25%
trypsin, and DMEM with 10% FBS was added to the mixture to produce
a single-cell suspension. The cell density was adjusted to 1×104
cells/ml, and the cells were inoculated into a 96-well plate and
cultured at 37°C with 5% CO2 in order to achieve
adherence. After 24 h, once the cells firmly adhered to the plate,
various concentrations of CDDP (0, 0.05, 0.1, 0.2, 0.5, 1, 2, 5,
10, 20 and 50 µg/ml) were added. The total reaction volume was 200
µl/well. The cells in the negative control group were incubated
with the medium. In the wells of the blank control group, only the
medium was present. The reactions for each CDDP concentration, the
negative and blank control groups were repeated in three wells.
Following 24-h culture at 37°C with 5% CO2, 20 µl MTT
solution (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each
well and incubated for another 4 h. Subsequently, the solution was
replaced with 180 µl/well DMSO, and the plates were agitated for 10
min. The blank control value was set as 0, and the optical density
of each well was read at 490 nm using a microplate
spectrophotometer. The inhibitory rate (IR) was calculated by the
following formula in order to calculate the 50% inhibitory
concentration (IC50) of the cells: IR (%) = (1 - Test
group A490/Control group A490) × 100%. The resistance index was
determined as the IC50 of the resistant cells/the
IC50 of the parental cells.
Xenograft assay
BALB/c nude mice (age, 5–7 weeks; weight, 19–23 g)
were obtained from Beijing Vital River Laboratory Animal Technology
Co., Ltd., China. A total of 18 mice (9 male and 9 female) were
equally divided into three groups (n=6; 3 male and 3 female per
group) according to their body weight. The mice were housed in a
controlled environment with a temperature of 25±1°C, relative
humidity of 40–60%, a light/dark cycle of 12/12 h and free access
to a standard diet and water. The mice were subcutaneously injected
with 200 µl cancer cell suspension containing 6×106 AMC-HN-8 or
AMC-HN-8-EVs1 cells at the right forelimb. At 5 days, the tumor
formation rate was 100%. The treatment groups were as follows: i)
The blank control group was inoculated with AMC-HN-8 cells and was
intraperitoneally injected with normal saline (NS); ii) the control
group was inoculated with AMC-HN-8 cells and intraperitoneally
injected with 3 mg/kg CDDP; and iii) the test group was inoculated
with AMC-HN-8-EVs1 cells and intraperitoneally injected with 3
mg/kg CDDP. CDDP was injected once every 7 days, and the total
duration of the treatment was 2 weeks. The volume of the tumors was
calculated as follows: V = a × b2/2, where a and b represent the
long and short diameter of the tumor, respectively. Mice were
sacrificed by cervical dislocation at the end of the treatment
period; death was confirmed by the sound of cervical spine fracture
and the absence of breathing. All animal experiments were performed
in compliance with the regulations and guidelines of The Fourth
Hospital of Hebei Medical University institutional animal care and
according to the AAALAC and IACUC guidelines. The Ethics Committee
of the Fourth Hospital of Hebei Medical University (Shijiazhuang,
China) approved this study (approval no. 2018MEC106).
Subcutaneous tumor single cell
suspension
After dissection of the subcutaneous tumors, single
cell suspension was immediately prepared using the mesh rubbing
method, and the cell density was adjusted to 1×107 cells/ml. The
main steps of mesh rubbing method were as follows: Tumor tissue was
placed on a 150-mesh sieve and washed with PBS for three times. A
dish was placed under the sieve, and ophthalmic scissors were used
to cut the tissues. Ophthalmic tweezers were used to lightly rub
the tissue. Samples were then rinsed with PBS and passed through a
300-mesh nylon sieve, and the cell suspension was collected.
Reverse transcription-quantitative
(RT-q)PCR
The RNA Isolater (Vazyme Biotech Co., Ltd.) was used
to extract the total RNA from AMC-HN-8, AMC-HN-8/CDDP,
AMC-HN-8-EVs1, AMC-HN-8-EVs2 cells and EVs. For mouse subcutaneous
tumor analysis, 50 mg of tumor tissue was sampled, washed with
precooled PBS twice and mixed with 1 ml RNA Isolater solution; the
total RNA was extracted according to routine one-step assay
(36). The cDNA was synthesized
using the HiScript II First Strand cDNA Synthesis kit (Vazyme
Biotech Co., Ltd) according to the manufacturer's protocol. Human
GAPDH was used as an internal reference. SYBR® Green I
(Vazyme Biotech Co., Ltd.) was used as fluorescent dye. qPCR was
using the SYBR Green Master Mix kit (Vazyme Biotech Co., Ltd.)
according to the manufacturer's protocol. The thermocycling
conditions were as follows: 95°C for 5 min (pre-denaturation),
followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec, and
dissociation at 95°C for 15 sec, 60°C for 1 min and 95°C for 15
sec. The experiments for each sample were repeated thrice. The
primers used were as follows: ABCG2 forward,
5′-GGTCAGAGTGTGGTTTCTGTAGCA-3′ and reverse,
5′-GTGAGAGATCGATGCCCTGCTTTA-3′; and GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
The relative expression levels of ABCG2 mRNA were calculated using
the 2-∆∆Cq method (37).
Protein expression analysis by flow
cytometry (FCM)
The AMC-HN-8, AMC-HN-8/CDDP, AMC-HN-8-EVs1 and
AMC-HN-8-EVs2 cells were collected and washed with cold
phosphate-buffered saline (PBS) twice. A volume of 0.1 ml single
cell suspension (containing 1×106 cells) was incubated with 10 µl
FITC-labeled ABCG2 antibody (undiluted; cat. no. 332014; Biolegend,
Inc.) in the dark for 30 min at room temperature. In addition, 0.1
ml subcutaneous tumor single cell suspension was prepared as
aforementioned and incubated with 10 µl FITC-labeled ABCG2 antibody
(undiluted) in the dark for 30 min at room temperature. The cells
were washed with PBS, and the protein expression levels were
determined using an FC-500 type flow cytometer (Beckman Coulter,
Inc.). Immunofluorescence data was measured using the EXPO 32 ADC
v1.2 software (Beckman Coulter, Inc.). The protein expression level
was expressed as the mean fluorescence intensity.
EV extraction from AMC-HN-8 and
AMC-HN-8/CDDP cell culture medium
The AMC-HN-8 and AMC-HN-8/CDDP cells were cultured
for 48 h until they reached the logarithmic phase. The EVs were
collected from the medium of the AMC-HN-8/CDDP and AMC-HN-8 cells
via gradient centrifugation and were termed EVs1 and EVs2,
respectively. The EVs were collected by the following steps: i)
Supernatant was obtained by gradient centrifugation (600 × g, 10
min; 1,500 × g, 30 min; 10,000 × g, 1 h) at 4°C; ii) this
supernatant was centrifuged at 110,000 × g for 16 h at 4°C; iii)
the supernatant was discarded, and the deposit of EVs was obtained
by dissolving it in PBS at 4°C; and iv) the EVs were quantified by
Nanodrop (Thermo Fisher Scientific, Inc.).
AMC-HN-8 cell incubation with EVs
The AMC-HN-8 cells were collected by trypsin
digestion. The density of the cell suspension was adjusted to 1×105
cells/ml, and 3 ml cell suspension was seeded into 6-well culture
plates in triplicate. The cell groups were treated with 50 µl EVs1,
EVs2 or normal saline; following 48-h treatment of AMC-HN-8 cells
with EVs1 and EVs2 at 37°C with 5% CO2, the cells were
termed AMC-HN-8-EVs1 and AMC-HN-8-EVs2 cells, respectively, and the
saline-treated group was used as the control. The concentration of
all EV solutions was 50 µg/ml. Following treatment, the density of
the single-cell suspension was adjusted to 1×106 cells/ml.
Cell cycle assay
A total of 1 ml single-cell suspension was collected
from each group following treatment with EVs. The cells were washed
with ice-cold PBS and fixed with 70% alcohol at 4°C for 24 h.
Following washing twice with ice-cold PBS, the cells were suspended
in 100 µl PBS, and 1 ml propidium iodide (BD Biosciences) was added
to the suspension for staining at 4°C for 30 min prior to cell
cycle detection with an FC-500 type flow cytometer (Beckman
Coulter, Inc.). The data were analyzed using the Multicycle AV
software version 275 (Phoenix Flow Systems, Inc.). The
proliferation index (PI) was calculated using the following
formula: PI = (S + G2/M)/(G0/1 + S +
G2/M) × 100%.
Apoptosis assay
A total of 1 ml single-cell suspension from each
AMC-HN-8 cell group following treatment with EVs or 0.1 ml
subcutaneous tumor single-cell suspension was collected and
resuspended in 100 µl PBS. The cells were washed with ice-cold PBS
and suspended in 100 µl 1X binding buffer (BD Biosciences).
Subsequently, 10 µl of Annexin V-FITC (BD Biosciences) was added,
and the mixture was placed on ice for 15 min in the dark prior to
the addition of 380 µl 1X binding buffer and 10 µl propidium
iodide. The cells were incubated on ice for 15 min in the dark,
washed once with cold PBS, resuspended in 1 ml PBS and analyzed
using a FC-500 type flow cytometer (Beckman Coulter, Inc.). Early
and late apoptotic cells were assessed. The apoptotic rate was
measured using the EXPO 32 ADC v1.2 software (Beckman Coulter,
Inc.).
Statistical analysis
Statistical analysis was performed using SPSS 21
software (IBM Corp.). Data are presented as the mean ± standard
deviation. Multi-group comparisons were performed by one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Establishment of drug-resistant
laryngeal cancer cells and the drug-resistant phenotype of
AMC-HN-8/CDDP cells
Following 24-h treatment with CDDP, the
IC50 of CDDP in AMC-HN-8 cells was detected by MTT
method. The IC50 of CDDP in AMC-HN-8 cells was 6.47±0.16
µg/ml (data not shown). Based on the IC50 value, the
CDDP concentrations ranging between 0.01 and 2 µg/ml were used to
treat the AMC-HN-8 cells in order to establish the drug-resistant
cell line AMC-HN-8/CDDP.
The AMC-HN-8/CDDP cell line was successfully
established from the parental CDDP-sensitive cell line AMC-HN-8 by
continuous exposure to increasing concentrations of CDDP for 8
months. One of the surviving clones was isolated and termed
AMC-HN-8/CDDP. The IC50 value of CDDP in the
AMC-HN-8/CDDP cells was 36.22±1.62 µg/ml. The resistance index of
AMC-HN-8/CDDP cells to CDDP was 5.60 (data not shown).
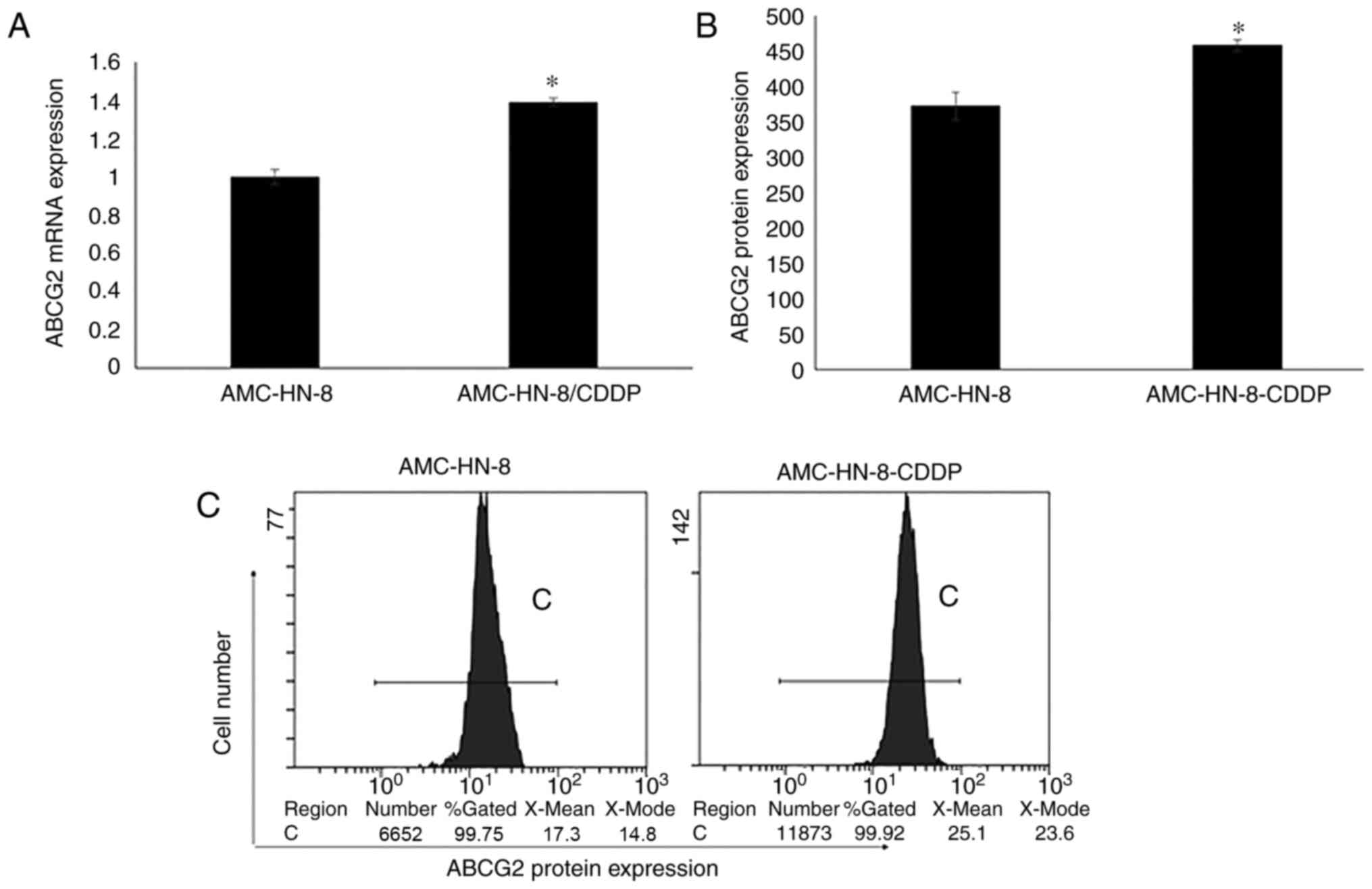
The ABCG2 gene and the protein expression levels in
AMC-HN-8 and AMC-HN-8/CDDP cells were detected by RT-qPCR and FCM.
The results revealed that the ABCG2 mRNA and protein expression
levels in AMC-HN-8/CDDP cells were significantly higher compared
with those in AMC-HN-8 cells (P<0.01; Fig. 1). The expression trends of the
protein and mRNA levels in these cells were consistent.
Drug-resistant phenotype of AMC-HN-8
cells is modulated by EVs in vitro
The ABCG2 mRNA expression in EVs was detected by
RT-qPCR, and the results revealed that the ABCG2 mRNA expression
levels in EVs1 derived from drug-resistant AMC-HN-8/CDDP cells were
significantly higher compared with those in EVs2 derived from the
parental CDDP-sensitive AMC-HN-8 cells (P<0.01; Fig. 2A).
Following 48-h treatment with EVs and 24-h exposure
to CDDP, the IC50 value of AMC-HN-8 cells was detected
by the MTT method. As presented in Fig.
2B, the IC50 value in the AMC-HN-8-EVs1 group was
significantly higher compared with those in the AMC-HN-8-EVs2 and
AMC-HN-8 groups (P<0.01; Fig.
2B). No significant differences were observed between the
IC50 values in the AMC-HN-8-EVs2 and the AMC-HN-8
control groups (P>0.05; Fig. 2B).
The resistance index of AMC-HN-8-EVs1 cells to CDDP was 4.32 (data
not shown).
Following 48-h treatment with EVs, the ABCG2 gene
and protein expression levels in AMC-HN-8 cells were detected by
RT-qPCR and FCM, respectively. The ABCG2 mRNA and protein
expression levels in the AMC-HN-8-EVs1 group were significantly
higher compared with those in the AMC-HN-8-EVs2 and AMC-HN-8
control groups (P<0.01; Fig.
2C-E). The ABCG2 mRNA and protein expression levels in EVs2
group exhibited no significant differences compared with those in
the control group (P>0.05; Fig.
2C-E). Therefore, the ABCG2 expression levels were increased
following treatment with EVs1 derived from drug-resistant
AMC-HN-8/CDDP cells.
EVs modulate the cell cycle and
apoptotic rate of AMC-HN-8 cells
The changes in the cell cycle distribution in
AMC-HN-8 cells treated with EVs were determined by flow cytometry.
In the G0/1 and G2/M phases of the cell
cycle, the percentages of cells in the EVs1 group were lower
compared with those in the EVs2 and control groups (P<0.01); the
EVs2 group exhibited no differences compared with the control group
(P>0.05). In the S phase, the percentage of cells in the EVs1
group was higher compared with that in the EVs2 and control groups
(P<0.01), and the EVs2 group exhibited no significant
differences compared with the control group (P>0.05). The cell
PI in the EVs1 group was higher compared with those in the EVs2 and
control groups (P<0.01), whereas the EVs2 group exhibited no
differences compared with the control group (P>0.05) (Fig. 3A and B).
The EVs1 group exhibited a lower apoptotic rate
compared with that in the control and EVs2 groups (P<0.01). No
significant differences were observed in the apoptotic rates
between the EVs2 and control groups (P>0.05) (Fig. 3C and D).
Drug-resistant phenotype of AMC-HN-8
cells is modulated by EVs in vivo
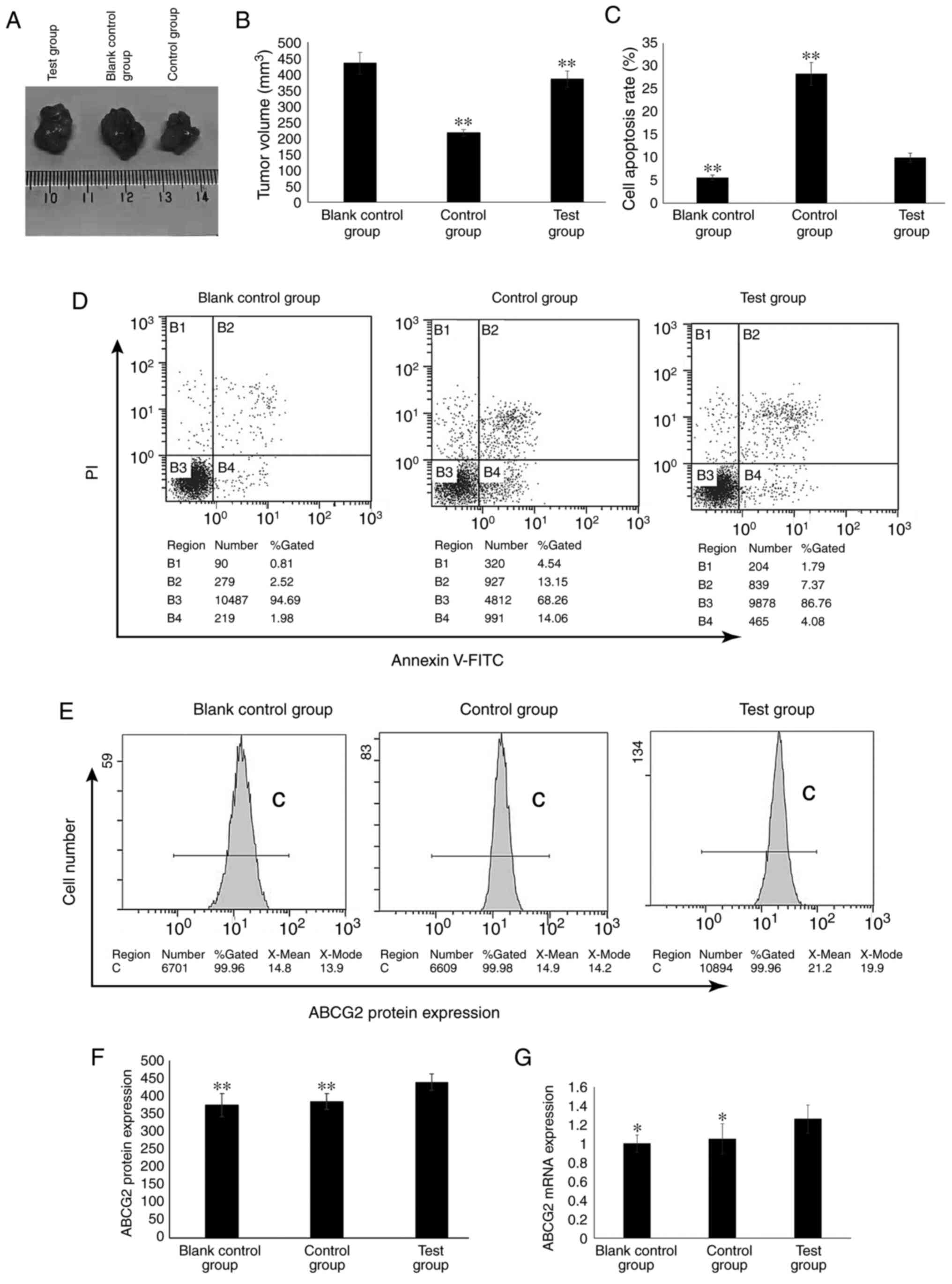
The nude mouse xenografts models of human laryngeal
carcinoma were successfully established. The tumors started to grow
from day 5 post-inoculation of AMC-HN-8 and AMC-HN-8-EVs1 cells,
and the tumor volume gradually increased with a tumor formation
rate of 100% (Fig. 4A). The drug
administration began after the tumors grew >0.5 cm.
Compared with that in the control group, the mean
volume of the subcutaneously implanted tumors in the test group was
significantly higher (P<0.01; Fig.
4B). In the control group, the volume inhibitory rate of CDDP
on the growth of the subcutaneously implanted tumor was 50.06%. In
the test group, the volume inhibitory rate of CDDP on the growth of
the subcutaneously implanted tumor was 11.41% (data not shown).
Compared with those in the control group, the subcutaneously
implanted tumor cells in the test group were resistant to CDDP.
The apoptotic rate of the cells isolated from the
tumors in the test group was significantly lower compared with that
in the cells from the control group, but significantly higher
compared with that in the cells from the blank control group
(P<0.01; Fig. 4C and D).
The ABCG2 mRNA and protein expression levels in the
test group were significantly higher compared with those in the
blank control and control groups (P<0.01; Fig. 4E-G).
Discussion
Chemotherapy is one of the main methods for the
treatment of malignant tumors, especially for patients with late
and postoperative recurrence (9).
The main disadvantages of chemotherapy include the side effects of
chemotherapy drugs and drug resistance (38,39);
drug resistance severely affects the efficacy of chemotherapy.
To date, the following major mechanisms of tumor
drug resistance have been described: i) Drug efflux pump mediated
by membrane transporters, where the level of antitumor drugs in
multidrug-resistant cells is reduced through the drug pump function
of transmembrane transporter (40);
ii) enzyme-mediated resistance, where the activation of cell
oxidation and the glutathione-related detoxification enzyme system
result in direct inactivation of drugs or acceleration of drug
excretion, e.g. by protein kinase C and glutathione transferase
(41); and iii) apoptotic
gene-mediated resistance, where p53 gene mutation reduces the
expression levels of apoptotic genes and proteins, and increases
the expression levels of antiapoptotic factors, such as Bcl-2,
allowing multidrug-resistant cells to resist and escape the
apoptosis induced by antitumor drugs (42). In particular, the mechanism of
membrane transporter-mediated drug efflux pump has been thoroughly
studied.
The ABC transporter family is associated with tumor
drug resistance (43–45) and serves a role of drug efflux pump
mediated by membrane transporters; ABC transporters reduce the
effective drug concentration in cells and induce drug resistance
(46,47). For example, Hofman et al
(46) have demonstrated that ABCG2
served as a drug (mitoxantrone) efflux transporter to induce drug
resistance in A431 cells in vitro. Zhang et al
(48) have demonstrated that ABCG2
mediates drug (mitoxantrone) resistance of colon cancer S1-M1-80
cells. Bar-Zeev et al (49)
have reported that the drug (mitoxantrone) resistance of gastric
cancer EPG85-257RNOV cells is mediated by ABCG2 and reversed by
inhibiting the ABCG2-dependent efflux pumps. Ge et al
(50) have demonstrated that high
expression levels of ABCG2 mediate the drug (doxorubicin)
resistance of lung cancer H460/MX20 cells and colorectal cancer
S1-MI-80 cells. Baxter et al (51) have reported that ABCG2 is commonly
and significantly upregulated in breast cancer following treatment
with neoadjuvant endocrine therapy in three separate cohorts
comprising a total of 200 patients, and ABCG2 upregulation is
significantly associated with tumor chemoresistance.
In our previous study, ABCG2 was demonstrated to be
abnormally highly expressed in esophageal cancer tissues and to be
involved in the development of drug resistance in esophageal cancer
(18). Laryngeal cancer is a common
cancer of the head and neck, and determining the mechanism of drug
resistance is of great significance to improve the therapeutic
effects and the survival rate of patient with laryngeal cancer. To
achieve this, in the present study, a CDDP-resistant laryngeal
cancer cell line AMC-HN-8/CDDP was established by culture with
increasing CDDP concentrations. The ABCG2 gene and protein
expression levels in the AMC-HN-8/CDDP cells were significantly
increased compared with those in the parental cells, suggesting
that ABCG2 may be involved in the development of drug resistance in
laryngeal cancer. However, the absence of clinical data (e.g.,
expression levels in tissues and association with patient
prognosis) is a limitation of the present study: In our future
studies, clinical data will be used to verify the results.
EVs are bioactive substances secreted by cells;
during the process of EV formation, the proteins, mRNAs and
non-coding RNAs from the source cells are functionally selected and
released into the target cells following interaction between EVs
and target cells, and they serve a functional role by altering the
phenotype and genotype of the target cells (31). The EVs in the tumor microenvironment
regulate the biological characteristics of target cells, thus
affecting their biological behavior (52). Previous studies have reported that
the EVs in the tumor microenvironment regulate drug resistance of
various types of tumor cells, such as breast cancer, glioma and
lung cancer cells (53,54). Wang et al (55) have demonstrated that the
chemotherapeutic drug vincristine stimulates drug-resistant KBv200
cells to release EVs containing ABCB1, which in turn lead to a
significant increase of ABCB1 intercellular transfer to develop a
drug-resistant phenotype. Bouvy et al (56) have reported that EVs from HL60/AR
interact with HL60 cells and, at least partially, transfer their
chemoresistance. Soekmadji et al (57) have reviewed that the role of EVs in
mediating drug (mitoxantrone) resistance in prostate cancer cell
PC3, particularly the role of EVs mediating drug resistance in
advanced prostate cancer. Ma et al (58) have demonstrated that short transient
receptor potential channel 5-containing circulating EVs may
transfer the Adriamycin chemoresistance property to chemosensitive
MCF-7/WT recipient cells. In our previous study, EVs with high
expression levels of ABCG2 were demonstrated to regulate the drug
resistance of esophageal cancer (59). In the present study, whether EVs
released by drug-resistant cells may affect the drug resistance of
their surrounding cells was studied in order to provide novel ideas
for the study of drug resistance in laryngeal cancer. In
vitro experiment results demonstrated that the ABCG2 expression
levels in the EVs released by AMC-HN-8/CDDP cells were
significantly increased compared with those in the EVs from
AMC-HN-8 cells, suggesting that EVs may modulate the drug
resistance of cells via ABCG2 present in EVs. Following treatment
of the parental cells with EVs released by AMC-HN-8/CDDP cells, the
parental cells exhibited drug resistance, decreased apoptotic rate
and reduced cell proliferation compared with those cells in the
control group or cells treated with EVs released by AMC-HN-8 cells,
suggesting that exposure to EVs may affect cell drug resistance. To
further verify the role of EVs with high levels of ABCG2 in the
regulation of drug resistance, a subcutaneous tumor model of
laryngeal cancer drug-resistant cells was established in the
present study. The subcutaneously transplanted tumor was
established by injecting laryngeal cancer cells treated with EVs
with high levels of ABCG2. The results of the xenograft experiment
demonstrated that the subcutaneously transplanted tumors of the
test group (inoculated with AMC-HN-8-EVs1 cells) were resistant to
CDDP, and ABCG2 was highly expressed in the cells isolated from the
tumors. These results suggested that EVs may upregulate the
expression levels of ABCG2 in the target cells, leading to drug
resistance of the target cells.
The exact mechanism of action of ABCG2 in drug
resistant cells regulating the drug resistance of the surrounding
cells has not been elucidated to date. In the present study, the
regulatory effects of EVs on the drug resistance of laryngeal
cancer cells were explored. The absence of other laryngeal cancer
cell lines and ABCG2-knockdown experiments were potential
limitations of the present study; in future studies, additional
laryngeal cancer cell lines and knockdown ABCG2 expression
experiment should be used to verify the results.
In conclusion, the results of the present study
demonstrated ABCG2 was involved in the drug resistance of laryngeal
squamous cell carcinoma, and high expression levels of ABCG2 in EVs
released by drug-resistant laryngeal cancer cells modulated the
drug resistance of target cells. EVs with high levels of ABCG2
upregulated the expression of ABCG2 in the target cells, inducing
drug resistance. The results of the present study suggested that
drug-resistant laryngeal cancer cells may induce drug resistance by
EVs released into the tumor microenvironment, providing an
experimental basis to further study the drug resistance mechanism
of laryngeal cancer. The molecular mechanisms underlying drug
resistance modulated by EVs are complex, necessitating further
study in the future. The function of ABCG2 in reducing the
intracellular effective drug concentration will be analyzed in
future experiments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ performed the experiments and wrote the
manuscript. YC and JW performed the experiments and statistical
analysis. LL designed the study, performed the experiments and
revised the manuscript. YZ and LL confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Fourth Hospital of Hebei
Medical University (Shijiazhuang, China) approved this study
(approval no. 2018MEC106).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He Y, Liang D, Li D, Shan B, Zheng R,
Zhang S, Wei W and He J: Incidence and mortality of laryngeal
cancer in China, 2015. Chin J Cancer Res. 32:10–17. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang D, Tao L, Zhou L, Zhang M, Wu H, Li
X, Chen X, Li C, Xie M and Cheng L: Retrospective analysis of 659
laryngeal squamous cell carcinoma patients treated with open
laryngeal function-preserving operations. Acta Otolaryngol.
138:1043–1050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou J, Zhou L, Tao L, Zhang M, Wu H, Chen
X, Li X, Li C and Gong H: Oncological outcomes of surgical
treatment for T3 supraglottic laryngeal squamous cell carcinoma
patients. Acta Otolaryngol. 138:1028–1034. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adeel M, Faisal M, Rashid A, Rasheed S,
Hussain R, Malik KI, Hameed MY and Jamshed A: Outcomes of
definitive radiotherapy for early laryngeal cancer in terms of
survival and patterns of failure. J Laryngol Otol. 133:1087–1091.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozdemir Y and Topkan E: Second primary
malignancies in laryngeal carcinoma patients treated with
definitive radiotherapy. Indian J Cancer. 56:29–34. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsieh CH, Lin CY, Hsu CL, Fan KH, Huang
SF, Liao CT, Lee LY, Ng SK, Yen TC, Chang JT, et al: Incorporation
of Astragalus polysaccharides injection during concurrent
chemoradiotherapy in advanced pharyngeal or laryngeal squamous cell
carcinoma: Preliminary experience of a phase II double-blind,
randomized trial. J Cancer Res Clin Oncol. 146:33–41. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spector ME, Rosko AJ, Swiecicki PL, Chad
Brenner J and Birkeland AC: From VA Larynx to the future of
chemoselection: Defining the role of induction chemotherapy in
larynx cancer. Oral Oncol. 86:200–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng H, Huang Y, Chen L, Li H and Ma X:
Exploration and validation of the effects of robust co-expressed
immune-related genes on immune infiltration patterns and prognosis
in laryngeal cancer. Int Immunopharmacol. 85:1066222020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li R, Chen S, Zhan J, Li X, Liu W, Sheng
X, Lu Z, Zhong R, Chen L, Luo X, et al: Long noncoding RNA
FOXD2-AS1 enhances chemotherapeutic resistance of laryngeal
squamous cell carcinoma via STAT3 activation. Cell Death Dis.
11:412020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu M, Yin F, Yang L, Chen S, Chen R, Zhou
X, Jing W, Fan X, Jia R, Wang H, et al: Contribution of TIP30 to
chemoresistance in laryngeal carcinoma. Cell Death Dis.
5:e14682014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng SL, Luo HB, Cai L, Zhang J, Wang D,
Chen YJ, Zhan HX, Jiang ZH and Xie Y: Ginsenoside Rg5 overcomes
chemotherapeutic multidrug resistance mediated by ABCB1
transporter: In vitro and in vivo study. J Ginseng Res. 44:247–257.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ke B, Wei T, Huang Y, Gong Y, Wu G, Liu J,
Chen X and Shi L: Interleukin-7 Resensitizes Non-Small-Cell Lung
Cancer to Cisplatin via Inhibition of ABCG2. Mediators Inflamm.
2019:72414182019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sinha BK, Perera L and Cannon RE: Reversal
of drug resistance by JS-K and nitric oxide in ABCB1- and
ABCG2-expressing multi-drug resistant human tumor cells. Biomed
Pharmacother. 120:1094682019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie ZY, Wang FF, Xiao ZH, Liu SF, Tang SL
and Lai YL: Overexpressing microRNA-34a overcomes ABCG2-mediated
drug resistance to 5-FU in side population cells from colon cancer
via suppressing DLL1. J Biochem. 167:557–564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Zuo LF and Guo JW: ABCG2 gene
amplification and expression in esophageal cancer cells with
acquired adriamycin resistance. Mol Med Rep. 9:1299–1304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Liu L, Chen Y, Du Y, Wang J and
Liu J: Correlation between adenosine triphosphate (ATP)-binding
cassette transporter G2 (ABCG2) and drug resistance of esophageal
cancer and reversal of drug resistance by artesunate. Pathol Res
Pract. 214:1467–1473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He M, Wu H, Jiang Q, Liu Y, Han L, Yan Y,
Wei B, Liu F, Deng X, Chen H, et al: Hypoxia-inducible factor-2α
directly promotes BCRP expression and mediates the resistance of
ovarian cancer stem cells to adriamycin. Mol Oncol. 13:403–421.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim CK, Oh S, Kim SJ, Leem SH, Heo J and
Chung SH: Correlation of IGF1R expression with ABCG2 and CD44
expressions in human osteosarcoma. Genes Genomics. 40:381–388.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang YL, Wu CH, Kang CY, Lin CN, Shih NY,
Lin SH, Chen YC and Hsu KF: Downregulated Salt-inducible Kinase 3
Expression Promotes Chemoresistance in Serous Ovarian Cancer via
the ATP-binding Cassette Protein ABCG2. J Cancer. 10:6025–6036.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Das S, Mukherjee P, Chatterjee R, Jamal Z
and Chatterji U: Enhancing Chemosensitivity of Breast Cancer Stem
Cells by Downregulating SOX2 and ABCG2 Using
Wedelolactone-encapsulated Nanoparticles. Mol Cancer Ther.
18:680–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen B, Dong P, Li D and Gao S: Expression
and function of ABCG2 in head and neck squamous cell carcinoma and
cell lines. Exp Ther Med. 2:1151–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denton AE, Roberts EW and Fearon DT:
Stromal Cells in the Tumor Microenvironment. Adv Exp Med Biol.
1060:99–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu T and Dai Y: Tumor microenvironment and
therapeutic response. Cancer Lett. 387:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akhtar M, Haider A, Rashid S and Al-Nabet
ADMH: Paget's ‘Seed and Soil’ Theory of Cancer Metastasis: An Idea
Whose Time has Come. Adv Anat Pathol. 26:69–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashida T, Jinno H and Kitagawa Y:
Epithelial-mesenchymal transition and tumor microenvironment
propose the seed and soil theory of the present age. Nihon Rinsho.
70 (Suppl 7):203–207. 2012.(In Japanese). PubMed/NCBI
|
|
28
|
Mulcahy LA, Pink RC and Carter DR: Routes
and mechanisms of extracellular vesicle uptake. J Extracell
Vesicles. Aug 4–2014.doi: 10.3402/jev.v3.24641. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haraszti RA, Didiot MC, Sapp E, Leszyk J,
Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA,
et al: High-resolution proteomic and lipidomic analysis of exosomes
and microvesicles from different cell sources. J Extracell
Vesicles. 5:325702016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lawson C, Vicencio JM, Yellon DM and
Davidson SM: Microvesicles and exosomes: New players in metabolic
and cardiovascular disease. J Endocrinol. 228:R57–R71. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Becker A, Thakur BK, Weiss JM, Kim HS,
Peinado H and Lyden D: Extracellular vesicles in cancer:
Cell-to-cell mediators of metastasis. Cancer Cell. 30:836–848.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lindenbergh MFS and Stoorvogel W: Antigen
presentation by extracellular vesicles from professional
antigen-presenting cells. Annu Rev Immunol. 36:435–459. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joncas FH, Lucien F, Rouleau M, Morin F,
Leong HS, Pouliot F, Fradet Y, Gilbert C and Toren P: Plasma
extracellular vesicles as phenotypic biomarkers in prostate cancer
patients. Prostate. 79:1767–1776. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Dong L, Zhong H, Yang L, Li Q, Su
C, Gu W and Qian Y: Extracellular vesicles (EVs) from lung
adenocarcinoma cells promote human umbilical vein endothelial cell
(HUVEC) angiogenesis through yes kinase-associated protein (YAP)
transport. Int J Biol Sci. 15:2110–2118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takahashi K, Yan IK, Wood J, Haga H and
Patel T: Involvement of extracellular vesicle long noncoding RNA
(linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer
Res. 12:1377–1387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou T, Li Y, Yang L, Liu L, Ju Y and Li
C: Silencing of ANXA3 expression by RNA interference inhibits the
proliferation and invasion of breast cancer cells. Oncol Rep.
37:388–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leung HW, Lau EYT, Leung CON, Lei MML, Mok
EHK, Ma VWS, Cho WCS, Ng IOL, Yun JP, Cai SH, et al: NRF2/SHH
signaling cascade promotes tumor-initiating cell lineage and drug
resistance in hepatocellular carcinoma. Cancer Lett. 476:48–56.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong P, Chen X, Guo R, Chen X, Chen Z,
Wei C, Li Y, Wang W, Zhou Y and Qin L: Folic acid-modified
nanoerythrocyte for codelivery of paclitaxel and tariquidar to
overcome breast cancer multidrug resistance. Mol Pharm.
17:1114–1126. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paškevičiūtė M and Petrikaitė V:
Overcoming transporter-mediated multidrug resistance in cancer:
Failures and achievements of the last decades. Drug Deliv Transl
Res. 9:379–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ganapathi RN and Ganapathi MK: Mechanisms
regulating resistance to inhibitors of topoisomerase II. Front
Pharmacol. 4:892013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nagraj J, Chatterjee S, Pal T, Sakpal AS,
Gota V, Ramaa CS and Ray P: A novel series of di-fluorinated
propanedione derivatives synergistically augment paclitaxel
mediated caspase 3 activation in ovarian cancer cells. J Cancer Res
Ther. 10:701–709. 2014.PubMed/NCBI
|
|
43
|
Ambjorner SEB, Wiese M, Kohler SC, Svindt
J, Lund XL, Gajhede M, Saaby L, Brodin B, Rump S, Weigt H, et al:
The pyrazolo[3,4-d]pyrimidine derivative, SCO-201, reverses
multidrug resistance mediated by ABCG2/BCRP. Cells. 9:6132020.
View Article : Google Scholar
|
|
44
|
Gao Q, Li XX, Xu YM, Zhang JZ, Rong SD,
Qin YQ and Fang J: IRE1α-targeting downregulates ABC transporters
and overcomes drug resistance of colon cancer cells. Cancer Lett.
476:67–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu CP, Lusvarghi S, Tseng PJ, Hsiao SH,
Huang YH, Hung TH and Ambudkar SV: MY-5445, a phosphodiesterase
type 5 inhibitor, resensitizes ABCG2-overexpressing
multidrug-resistant cancer cells to cytotoxic anticancer drugs. Am
J Cancer Res. 10:164–178. 2020.PubMed/NCBI
|
|
46
|
Hofman J, Sorf A, Vagiannis D, Sucha S,
Kammerer S, Küpper JH, Chen S, Guo L, Ceckova M and Staud F:
Brivanib exhibits potential for pharmacokinetic drug-drug
interactions and the modulation of multidrug resistance through the
inhibition of human ABCG2 drug efflux transporter and CYP450
biotransformation enzymes. Mol Pharm. 16:4436–4450. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bhardwaj B, Baidya ATK, Amin SA, Adhikari
N, Jha T and Gayen S: Insight into structural features of
phenyltetrazole derivatives as ABCG2 inhibitors for the treatment
of multidrug resistance in cancer. SAR QSAR Environ Res.
30:457–475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang YK, Wang YJ, Lei ZN, Zhang GN, Zhang
XY, Wang DS, Al-Rihani SB, Shukla S, Ambudkar SV, Kaddoumi A, et
al: Regorafenib antagonizes BCRP-mediated multidrug resistance in
colon cancer. Cancer Lett. 442:104–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bar-Zeev M, Kelmansky D, Assaraf YG and
Livney YD: β-Casein micelles for oral delivery of SN-38 and
elacridar to overcome BCRP-mediated multidrug resistance in gastric
cancer. Eur J Pharm Biopharm. 133:240–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ge C, Wang F, Cui C, Su X, To KKW, Wang X,
Zhang H, Song X and Fu L: PCI29732, a Bruton's tyrosine kinase
inhibitor, enhanced the efficacy of conventional chemotherapeutic
agents in ABCG2-overexpressing cancer cells. Cell Physiol Biochem.
48:2302–2317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baxter DE, Kim B, Hanby AM, Verghese ET,
Sims AH and Hughes TA: Neoadjuvant endocrine therapy in breast
cancer upregulates the cytotoxic drug pump ABCG2/BCRP, and may lead
to resistance to subsequent chemotherapy. Clin Breast Cancer.
18:481–488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han L, Lam EW and Sun Y: Extracellular
vesicles in the tumor microenvironment: Old stories, but new tales.
Mol Cancer. 18:592019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yousafzai NA, Wang H, Wang Z, Zhu Y, Zhu
L, Jin H and Wang X: Exosome mediated multidrug resistance in
cancer. Am J Cancer Res. 8:2210–2226. 2018.PubMed/NCBI
|
|
55
|
Wang X, Qiao D, Chen L, Xu M, Chen S,
Huang L, Wang F, Chen Z, Cai J and Fu L: Chemotherapeutic drugs
stimulate the release and recycling of extracellular vesicles to
assist cancer cells in developing an urgent chemoresistance. Mol
Cancer. 18:1822019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bouvy C, Wannez A, Laloy J, Chatelain C
and Dogné JM: Transfer of multidrug resistance among acute myeloid
leukemia cells via extracellular vesicles and their microRNA cargo.
Leuk Res. 62:70–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Soekmadji C and Nelson CC: The emerging
role of extracellular vesicle-mediated drug resistance in cancers:
Implications in advanced prostate cancer. BioMed Res Int.
2015:4548372015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ma X, Chen Z, Hua D, He D, Wang L, Zhang
P, Wang J, Cai Y, Gao C, Zhang X, et al: Essential role for
TrpC5-containing extracellular vesicles in breast cancer with
chemotherapeutic resistance. Proc Natl Acad Sci USA. 111:6389–6394.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen Y, Liu L, Li J, Du Y, Wang J and Liu
J: Effects of long noncoding RNA (linc-VLDLR) existing in
extracellular vesicles on the occurrence and multidrug resistance
of esophageal cancer cells. Pathol Res Pract. 215:470–477. 2019.
View Article : Google Scholar : PubMed/NCBI
|