Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors worldwide. Traditional and novel
treatments, such as transarterial chemoembolization and stem cell
therapy, are not satisfactory (1–3).
Currently recognized treatment methods, such as immunotherapy, and
anti-programmed cell death protein 1 or anti-cytotoxic T-lymphocyte
protein 4 antibody treatment, were shown to be effective in only
10–20% of patients with HCC (4). The
tumor vaccine approach has seen rapid developments. Tumor vaccine
studies have primarily focusing on identifying new, or modifying
existing, antigens to increase immunogenicity and tumor specificity
(5). The major hepatoma vaccines
include the HCC-associated antigen vaccine (6), the DNA/RNA or hepatitis B virus
(HBV)-based vaccine (7,8), the peptide vaccine (9) and the autologous tumor antigen vaccine
(10). Additionally, the
personalized neoantigen vaccine is a current research hotspot
(11,12). The aforementioned vaccines have shown
varying degrees of anti-cancer effects in the liver. However, they
have not been used in mainstream clinical treatment, which may be
because these antigens are not expressed on all HCC tumor cells.
Therefore, tumor clones without the expression of these antigens
can escape anti-tumor immunity and continue to expand (13). Moreover, due to the prolonged
preparation process and high cost, many patients are unable to
access the vaccine. Only a limited number of studies using the
autologous whole-cell vaccine have obtained successful cancer
immunotherapy results (14),
potentially because the immunogenicity of the untreated whole-cell
vaccines was too poor to induce specific anti-tumor immunity.
Therefore, novel strategies for further enhancement of vaccine
immunogenicity are required (15,16). We
hypothesize that irradiation has the potential to modify or
stimulate HCC cells to express more tumor antigens and trigger a
strong, specific anti-tumor immune response for suppressing tumor
growth. The advantages of the irradiated whole-cell vaccine
approach are: i) It includes a large number of membrane-associated
tumor or mutated antigens; ii) it completely matches the major
histocompatibility complexes of the donor patient; and iii) it is
easy to prepare. Preparing this type of vaccine is less
time-consuming and more practical than preparing other types of
tumor vaccines. T helper (Th)9 cells were first described in the
course of parasitic infection (17).
These cells also possess pleiotropic functions and are involved in
cancer, autoimmunity and other pathologies (18–20). To
determine the effectiveness and possible mechanisms through which
the irradiated whole-cell vaccine exerts its anti-tumor effect, the
present study focused on the role of Th9 cells. Purwar et al
(21) were the first to discover the
anti-tumor effect of Th9 cells. It was found that ROR-γt-deficient
mice were able to increase CD4+interleukin
(IL)-9+ cell numbers, and that the anti-tumor effect was
abolished when using an IL-9 neutralizing antibody. Lu et al
(22) have also verified the
anti-tumor effects of Th9 cells, confirming that the in
vitro transfer of Th9 cells into tumor-bearing animals
suppresses tumor growth.
The differentiation mechanism of Th9 cells remains
unclear. However, the transcription factors (TFs) PU.1, interferon
regulatory factor 4 (IRF4) and basic leucine zipper transcriptional
factor ATF-like (BATF) are indispensable in this process (23). PU.1 and IRF4 have proven critical for
Th9 cell differentiation (24,25).
PU.1 is induced by TGF-β (26),
while IRF4 is induced by IL-4 (27)
in conjunction with antigen receptor stimulation. Furthermore, the
ectopic expression of PU.1 or IRF4 increases IL-9 production in the
polarization of Th9 cell cultures (23). Th9 cells exert their anti-tumor
effects in a variety of ways (28):
i) Th9 cells promote T cell survival and secrete IL-9 and granzyme
B, which directly target tumor cells (29,30); ii)
IL-9 promotes the activation and proliferation of macrophages and
plays a non-specific role in tumor cell destruction (31); and iii) IL-9 promotes the secretion
of chemokine C-C motif chemokine ligand 2, and enhances the
survival and antigen-presentation ability of C-C chemokine receptor
type 6+ dendritic cells (32)
The aim of the present study was to determine
whether a single high-dose-irradiated HCC whole-cell lysate vaccine
could inhibit the growth of HCC, focusing on the role of Th9 cells
in this novel approach to active immunotherapy.
Materials and methods
Cell culture
Murine HCC Hepa1-6 cells (American Type Culture
Collection) were cultured in Dulbecco's modified Eagle's medium,
and murine HCC H22 cells, (Bio-Rad Laboratories, Inc.) in RPMI 1640
at 37°C (5% CO2) in a humidified incubator. The media contained 10%
fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin.
All other reagents were purchased from Thermo Fisher Scientific,
Inc., unless otherwise stated.
Vaccine preparation
Hepa1-6 or H22 cells cultured in 15-cm dishes were
placed on the 1-cm tissue equivalent compensator and exposed to
8-Gy radiation using a linear accelerator (voltage, 6 MV;
direction, 180°; dose rate, 5 Gy min; irradiated volume, 10×10 cm;
distance from source to skin, 100 cm). After 2 days, the cells and
their conditional media were harvested and homogenized using the
Ultrasonic Cell Disruptor (Scientz-IID; NingBo Scientz
Biotechnology Co., Ltd.). The protein concentration of the
homogenized mixtures (irradiated Hepa1-6 or H22 cell cultures) was
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) and adjusted to a final concentration
of 1 mg/ml. The two irradiated cell vaccine preparations were then
used to induce active anti-tumor immunity in the corresponding
tumor-bearing mouse models (i.e., Hepa1-6 vaccine for the
Hepa1-6/C57BL/6 model, and H22 vaccine for the H22/ICR model).
Animal models
C57BL/6 or ICR mice (pathogen-free, female, 6–8
weeks old) were purchased from Slaccas Experimental Animal LLC
(license no. SCXK 2012-0002). A total of 1×106 Hepa1-6
or H22 cells in 0.1 ml each were subcutaneously injected into the
C57BL/6 or ICR mice, respectively. The next day, the mice were
randomly divided into 2 groups (10–16 mice/group, Fig. 1A); the control group was injected
with media alone, and the other group was immunized with the
corresponding vaccine preparation (Hepa1-6 or H22) into two-foot
pads (0.1 ml/site). A vernier caliper was used to measure tumor
length and width. The measurements were taken once or twice a week
depending on the mouse model. Tumor volume was calculated as ½
(length × width2) (33).
At the end of the study period, the mice were anesthetized with
1.5% pentobarbital sodium (40 mg/kg, Merck KGaA) and sacrificed by
cervical dislocation. The experiment was performed twice. All mice
had ad libitum access to a standard diet and water, and
animal wellbeing was closely monitored. All animal experiments were
approved by the Fujian Medical University Institutional Animal
Ethical Committee (approval no. FJMU IACUC 2018-027).
Flow cytometry (FCM)
When tumor growth in the treated mice was
significantly suppressed (~2 weeks after immunization),
immunological parameters were evaluated using FCM. For
myeloid-derived suppressor cell (MDSC) staining, the tail blood was
collected and incubated with anti-mouse CD11b-FITC (cat. no.
101206; BioLegend, Inc.) and anti-mouse Ly-6G/Ly-6C (Gr1)-PE
antibody (cat. no. 108408; BioLegend, Inc.) for 30 min at 4°C. Red
cells were then lysed with ammonium-chloride-potassium (ACK) lysis
buffer (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The remaining cells were assessed by
FCM.
Regulatory T (Treg) cell staining was performed
following the manufacturers' instructions for the one-step protocol
for intracellular (nuclear) proteins. Briefly, the blood cells were
incubated with anti-mouse CD4-APC (cat. no. 17-0041-81;
eBioscience; Thermo Fisher Scientific, Inc.) and anti-mouse
CD25-PE-Cy7 antibodies (cat. no. 25-0251-81; eBioscience; Thermo
Fisher Scientific, Inc.) for 30 min at 4°C, and then lysed with ACK
lysis buffer (Thermo Fisher Scientific, Inc.) to remove red blood
cells. The remaining white blood cells (WBCs) were treated with
fresh fixation/permeabilization working solution (Thermo Fisher
Scientific, Inc.) for 30 min at room temperature, then incubated
with anti-mouse Foxp3-PE antibody (cat. no. 12-5773-82;
eBioscience; Thermo Fisher Scientific, Inc.) in fresh
permeabilization working solution (Thermo Fisher Scientific, Inc.)
for another 30 min at 4°C.
For cytokine staining, blood was diluted two-fold in
RPMI 1640, stimulated with 50 ng/ml phorbol 12-myristate 13-acetate
(PMA) and 1 µg/ml ionomycin (Merck KGaA) for 4 h, and then treated
with 1 µg/ml monensin (Merck KGaA) for another 2 h. Cytokine
staining was subsequently performed following the manufacturers'
instructions for the two-step protocol for intracellular
(cytoplasmic) proteins. Briefly, the stimulated and blocked blood
cells were harvested and incubated with anti-mouse CD3-FITC (cat.
no. 11-0032-80; eBioscience; Thermo Fisher Scientific, Inc.),
anti-mouse CD8a-FITC (cat. no. 11-0081-81; eBioscience; Thermo
Fisher Scientific, Inc.), anti-mouse CD4-APC and anti-mouse
CD25-PE-Cy7 antibodies separately or together for 30 min at 4°C.
Red blood cells were lysed with ACK lysis buffer and the remaining
WBCs were then fixed by 4% paraformaldehyde for another 15 min at
4°C. The cells were washed with permeabilization solution and then
stained with anti-mouse IL-9-PE (cat. no. 514104; BioLegend, Inc.),
anti-mouse IL-4-PE (cat. no. 12-7041-82; eBioscience; Thermo Fisher
Scientific, Inc.), anti-mouse IL-17A-PE (cat. no. 12-7177-81;
eBioscience; Thermo Fisher Scientific, Inc.), anti-mouse IFN-γ-PE
(cat. no. 12-7311-82; eBioscience; Thermo Fisher Scientific, Inc.)
or anti-mouse IL-10-PE antibodies (cat. no. 12-7101-82;
eBioscience; Thermo Fisher Scientific, Inc.) in fresh
permeabilization working solution for another 30 min at 4°C.
Following FCM (Accuri C6; BD Biosciences), the
percentages of CD4 T cells (CD4+), CD8 T cells
(CD8+), MDSCs (CD11b+Gr1+), Treg
cells (CD4+CD25+Foxp3+), Th1 cells
(CD3+CD4+IFN-γ+), Th2 cells
(CD3+CD4+IL-4+), Th9 cells
(CD3+CD4+IL-9+), Th17 cells
(CD3+CD4+IL-17+) and T regulatory
type 1 cells (Tr1 cells,
CD3+CD4+CD25+IL-10+)
were obtained directly from the forward scatter/side scatter-gated
lymphocyte population, and the data were analyzed using FlowJo7.6
software (FlowJo LLC).
In vitro Th9 cell induction
Spleen and mesentery lymph nodes were harvested from
C57BL/6 or ICR mice, and ACK lysis buffer was used to remove red
cells. The remaining cells were adjusted to a final density of
2.5×106/ml in culture media, either in the presence of
10 ng/ml murine IL-4 (PeproTech, Inc.) and 5 ng/ml human TGF-β
(PeproTech, Inc.), alone or in combination with 5 µg/ml Hepa1-6 or
10 µg/ml H22 cell lysate vaccine. After 6 h, half of the cells were
harvested and stored at −80°C for subsequent analysis, and the
remainder were cultured for a further 3 days. Before harvesting,
the cells were stimulated with 50 ng/ml PMA and 1 µg/ml ionomycin
(Merck KGaA) for 4 h, and then treated with 1 µg/ml monensin (Merck
KGaA) for another 2 h. Following stimulation and blocking, the
cells were harvested and washed with PBS containing 1% BSA, and
incubated with anti-mouse CD4-APC or anti-mouse CD4-FITC antibodies
(cat. no. 11-0041-81; eBioscience; Thermo Fisher Scientific, Inc.)
for 30 min at 4°C. The cells were then stained with anti-mouse
IL-9-PE, following the manufacturers' instructions for the two-step
protocol for intracellular (cytoplasmic) proteins (as
aforementioned). Briefly, the cells were fixed in 4%
paraformaldehyde for another 15 min at 4°C, washed with
permeabilization solution, and then incubated with the anti-mouse
IL-9-PE antibody (also in permeabilization solution) for another 30
min at 4°C. The percentage of Th9 cells
(CD4+IL-9+) was determined by FCM (CytoFLEX;
Beckman Coulter, Inc.), and the data were analyzed using FlowJo7.6
software (FlowJo LLC).
Cytokine assay
Plasma IL-9 was quantified using the mouse IL-9
ELISA Kit [cat. no. EK2092/2-96T; Multisciences (Lianke) Biotech
Co., Ltd.], per the manufacturers' instructions, and measured using
a SpectraMax i3× ELISA reader (Molecular Devices, LLC).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
cDNA synthesis was performed using Supermo III M-MLV Reverse
Transcriptase (Bioteke Corporation) according to the manufacturer's
instructions. The expression of PU.1, IRF4 and BATF mRNA was
quantified in triplicate using RT-qPCR SYBR Green I dye (Promega
Corporation) with the QuantStudio 5 system (Applied Biosystems,
Inc.). The thermocycling conditions were as follows: Initial
activation of Taq polymerase at 95°C for 5 min, followed by 40
cycles of PCR amplification at 95°C for 15 sec and
annealing/elongation at 60°C for 30 sec. Fold changes in gene
expression were calculated using the 2−ΔΔCq method as
previously described (34). β-actin
was used as an internal control for normalizing target gene
expression levels. The primer sequences used were as follows: PU.1
forward, 5′-AGGAGTCTTCTACGACCTGGA−3′ and reverse,
5′-GAAGGCTTCATAGGGAGCGAT−3′; IRF4 forward,
5′-CCGACAGTGGTTGATCGACC-3′ and reverse,
5′-CCTCACGATTGTAGTCCTGCTT-3′; BATF forward,
5′-CACAGAAAGCCGACACCCTT-3′ and reverse,
5′-GCTGTTTGATCTCTTTGCGGA−3′; and β-actin forward,
5′-GGCTGTATTCCCCTCCATCG-3′ and reverse,
5′-CCAGTTGGTAACAATGCCATGT-3′.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation unless stated otherwise. Statistical
significance was determined using the Student's unpaired t-test for
two-group comparisons or one-way analysis of variance followed by
Tukey's HSD test with ranks for multiple-group comparisons. All
statistical analysis analyses were performed using GraphPad Prism 5
(GraphPad Software, Inc.), and P<0.05 was considered to indicate
a statistically significant difference.
Results
Irradiated HCC whole-cell lysate
vaccines suppress HCC tumor growth
Preparation of the irradiated HCC whole-cell lysate
vaccines (briefly, whole-cell vaccines), as well as the vaccine
immunization schedule, is described in Fig. 1A and B. HCC tumor-bearing mouse
models were used to assess the effects of the whole-cell vaccine:
Hepa1-6 cells in C57BL/6 and H22 in ICR mice. HCC tumor growth was
significantly suppressed in mice immunized with the whole-cell
vaccine compared with that in the control mice. In the
Hepa1-6/C57BL/6 model group, tumor volume was 692.4±182.3
mm3 in the control and 138.6±60.17 mm3 in the
immunized group (Fig. 1C and E;
P<0.01). In the H22/ICR tumor model, the tumor volume was
1228±184.6 mm3 in the control and 766.7±96.65
mm3 in the immunized group (Fig. 1D and F; P<0.05). These data
demonstrate that the whole-cell vaccine effectively triggered
active immunity against tumor growth in two mouse models.
Alterations in the immune cell subsets
of tumor-bearing mice immunized with the whole-cell vaccine
Alterations in the immune cell subsets of whole-cell
vaccine-immunized tumor-bearing mice were detected by antibody
staining and FCM. In the H22/ICR mouse model, no significant
differences were detected in the percentages of CD4 cells (Fig. 2B), CD8 cells (Fig. 2C), MDSCs (Fig. 2D), Th1 cells (Fig. 2E) and Th17 cells (Fig. 2G) between the control and immunized
groups. However, the percentages of Th2 (Fig. 2F) and Tr1 (Fig. 2H) cells was increased, while those of
Treg cells (Fig. 2I) were decreased
in the immunized group compared with the control group. Of note,
the percentage of Th9 cells
(CD3+CD4+IL-9+) was significantly
elevated in the immunized groups compared with the control group in
both HCC-bearing mouse models (Fig. 2J
and K).
 | Figure 2.Increased immune cell numbers in
vaccinated HCC tumor-bearing mice. (A) H22 vaccine suppressed H22
tumor growth. Systemic immunological cellular changes of the
H22/ICR tumor-bearing mice immunized with the H22 whole-cell
vaccine were evaluated by FCM. There were no significant
differences in the percentages of (B) CD4 cells, (C) CD8 cells, (D)
MDSCs, (E) Th1 cells and (G) Th17 cells between the control group
and vaccine-immunized groups. However, there were significant
differences in the numbers of (F) Th2, (H) Treg and (I) Tr1 cells
between the two groups. Percentages of blood
CD4+IL-9+ Th9 cells were increased in the
vaccinated groups of the two HCC-bearing mouse models, compared
with (J and K) their control groups. Significant differences
between the two groups were identified by Student's t-test. N≥6.
*P<0.05, **P<0.01 and ***P<0.0001. HCC, hepatocellular
carcinoma; FCM, flow cytometry; MDSCs, myeloid-derived suppressor
cells; Th, T helper; Treg, regulatory T cells; CD, cluster of
differentiation; IL, interleukin; Im, whole-cell vaccine
immunized. |
Plasma IL-9 level is increased in
whole-cell vaccine-immunized tumor-bearing mice
As shown in Fig. 3A and
B, in two HCC tumor-bearing mouse models, the concentration of
plasma IL-9 was increased in the immunized compared with the
control group, indicating that Th9 cells may promote HCC cell
killing by increasing the expression of IL-9.
Vaccine-induced Th9 differentiation
may be associated with the upregulation of TFs PU.1, IRF4 and
BATF
Th0 cells are precursors that selectively
differentiate into different Th cell subsets under divergent
microenvironments. To investigate the effect of the whole-cell
vaccine on Th0- to-Th9 cell differentiation, the whole-cell vaccine
was added to the culture media of lymphocytes from the spleen and
mesenteric lymph nodes of C57BL/6 or ICR mice.
CD4+IL-9+ cells were detected by FCM after 3
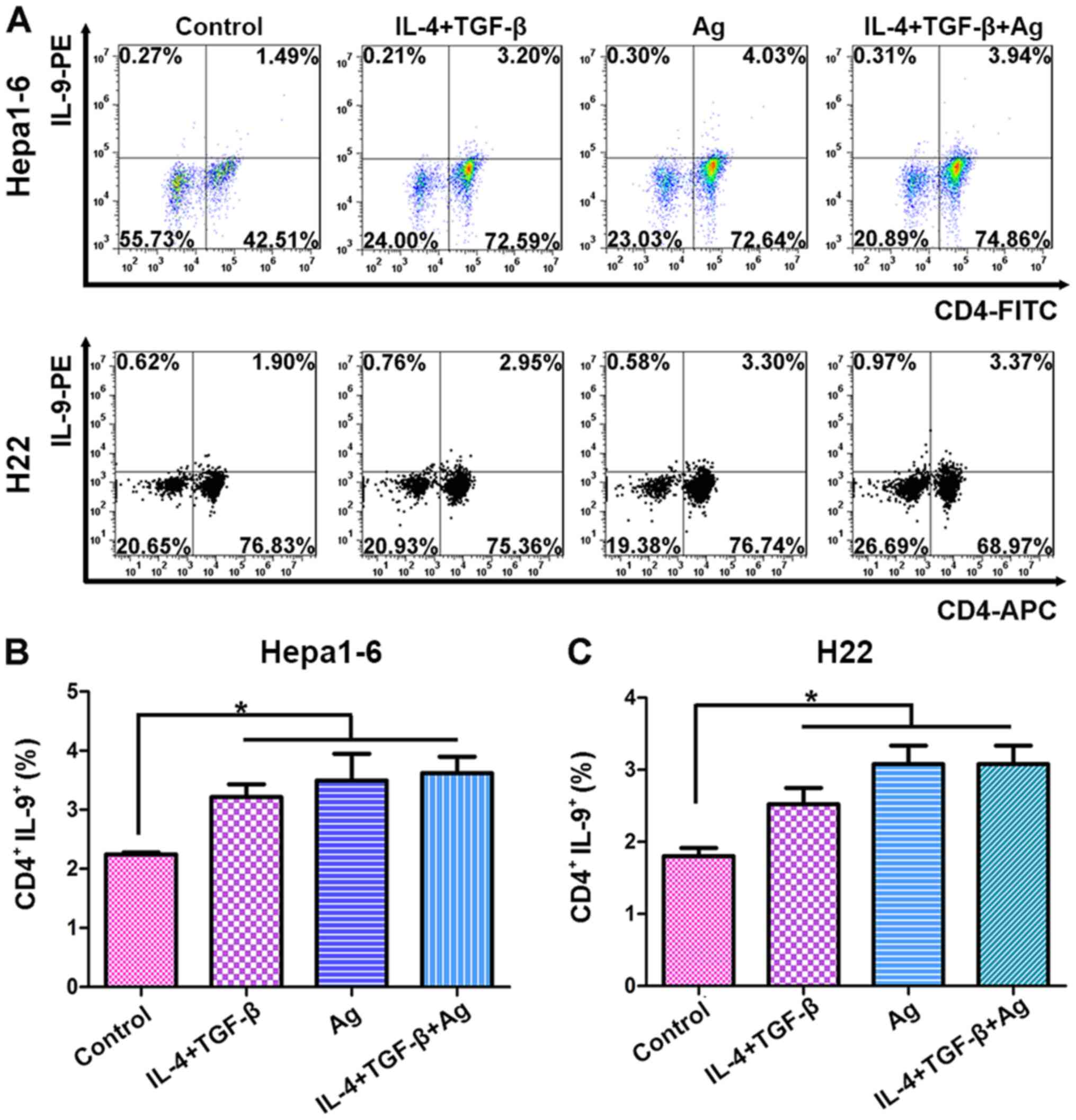
days of culture. Fig. 4A shows that
Th0 cells were transformed into Th9 cells in the presence of the
whole-cell vaccine, TGF-β + IL-4 or a combination of the vaccine +
TGF-β + IL-4. There were no significant differences among the three
treatment groups (Fig. 4B and C),
indicating that the whole-cell vaccine acted in a similar manner to
TGF-β and IL-4 in Th9 cell transformation.
The RT-qPCR results indicated that TFs PU.1
(Fig. 5A), IRF4 (Fig. 5B) and BATF (Fig. 5C) were upregulated in the
Hepa1-6/C57BL/6 model, while only PU.1 and IRF4 were upregulated in
the H22/ICR model.
 | Figure 5.Vaccine-induced Th9 differentiation
upregulates transcription factors PU.1, IRF4 and BATF in
vitro. After 6 h, lymphocytes of the spleen and mesentery lymph
nodes from C57/BL6 or ICR mice were cultured with 10 ng/ml IL-4 and
5 ng/ml TGF-β alone or in combination with a 5 µg/ml Hepa1-6 or 10
µg/ml H22 vaccine. Total RNA was extracted and cDNA was prepared.
Reverse transcription-quantitative PCR was carried out in
triplicate using SYBR Green I dye. Relative quantification was
performed using the 2−ΔΔCq method and normalized to
β-actin expression. Results of (A) PU.1, (B) IRF4 and (C) BATF
expression. Fold up- or downregulation is directly marked above the
histogram of each group. In the control group, the reference sample
value is set as 1. All other groups were compared with the
reference sample. *P<0.05, **P<0.01 and ***P<0.001.
Experiments were repeated >3 times. Th, helper T; IL,
interleukin; TGF, transforming growth factor; IRF4, interferon
regulatory factor 4; BATF, basic leucine zipper transcriptional
factor ATF-like. |
Discussion
Using two HCC tumor-bearing mouse models, the
present study demonstrated that a whole-cell vaccine (prepared with
8 Gy-irradiated HCC cells) exerted promising anti-HCC tumor effects
by promoting Th9 cell expansion. Th9 cell differentiation was also
found to be associated with the TFs PU.1, IRF4 and BATF. Clinical
trials for previous cancer vaccines designed to promote a
HCC-specific immune response (including those derived from
irradiated autologous whole tumor lysates) have been shown to be
well tolerated and relatively safe, but to deliver unsatisfactory
clinical outcomes (14,35,36). We
hypothesize that if tumor cells are killed by irradiation, that the
corresponding antigens will be the same as or similar to those of
live tumor cells in vivo, which are already tolerated by, or
have evaded, host immune surveillance. Herein, a novel approach was
adopted using irradiation as a ‘stressor’ to upregulate neoantigens
that could then trigger anti-tumor immunity. Both the cellular
portion and conditioned media (containing the secreted fraction of
the irradiated cells) were obtained. Previously, exosomes collected
from cells following irradiation stress have been proven to elicit
anti-tumor immunity (37). The
present results suggested that the irradiated vaccine could contain
more neoantigens and inhibit tumor growth more effectively than
traditional tumor vaccines.
Due to its high recurrence rate and serious
complications, none of the current therapeutic regimens for liver
cancer produce satisfactory results. The whole-cell vaccine
generated in the present study is easy to prepare and exerts a
strong anti-tumor effect. Combined with surgery, radiotherapy or
chemotherapy, this novel approach may indicate a novel therapeutic
strategy to combat HCC recurrence.
Systemic immunity commonly destroys liver cancer
cells via CD8 T or NK cells (9,38). There
is now a consensus that Th9 cells comprise a subset of anti-tumor
immune cells (18). Abdul-Wahid
et al (31) found that the
induction of antigen-specific Th9 cell immunity accompanied by mast
cell activation blocked in vivo tumor cell engraftment in an
IL-9-dependent manner. When IL-9 was depleted, the vaccine was
found to have no effect on tumor suppression (31). The present data demonstrated that the
whole-cell vaccine increased Th9 cell numbers, though the
percentage of CD8 cells did not change in vivo. At the same
time, plasma IL-9 was also increased. These results indicated that
Th9 cells destroy HCC cells by secreting IL-9. In patients with
HCC, Th9 cells are reportedly tumor-promoting through the C-C motif
chemokine ligand 2 and signal transducer and activator of
transcription 3 pathways (39). In
China, the majority of patients with HCC are also believed to be
chronically infected with HBV, a conclusion that was drawn based on
virus-free mouse models, which admittedly differ from HCC patients
with HBV. The effects of whole-cell vaccine and HBV
cross-interaction on Th9 cells should be further studied to
identify possible dual effects of Th9 cells in HCC (40–43).
The mechanisms underlying Th9-associated IL-9
production were further explored during treatment with the vaccine.
The whole-cell vaccine comprised a number of different antigens,
and as such, its composition was complex. It was therefore
difficult to determine the exact antigen responsible for Th9 cell
induction. The current co-culture study demonstrated that the
whole-cell vaccine had the same capacity to transform Th0 cells
into Th9 cells as IL-4 and TGF-β, revealing a new mechanism through
which the whole-cell vaccine exerts its anti-tumor effects.
TFs are the key molecules that determine the
differentiation of Th0 cells. For example, TGF-β activates Foxp3,
resulting in Th0-to-Treg cell differentiation (44). TGF-β also promotes Th0 -to-Th9
differentiation by inducing PU.1, which is encoded by Sfpi1
(26), as well as IRF4, activated by
IL-4 (27). BATF is another TF
necessary for Th9 cell differentiation (45). When naive CD4 cells were in a
BATF-deficient environment, Th9 cell differentiation was
significantly inhibited (45). The
TFs required for the differentiation of Th0 cells may also be
strain/cell-dependent. In Hepa1-6 cells of the C57BL/6 model,
antigens primarily regulated BATF, while H22 antigens mainly
regulated PU.1 and IRF4, promoting Th9 cell differentiation in the
ICR model of the present study. Thus, Th9 cell differentiation is a
complex process involving the interaction of multiple regulatory
networks.
In conclusion, in the present study, a whole-cell
vaccine was found to be a new approach for promoting active
immunotherapy, which works by triggering the production of Th9
cells and their related TF pathways. These Th9-associated TF
pathways will be studied in greater detail, as clinical use of the
whole-cell vaccine requires further investigation.
Acknowledgements
The authors would like to thank Ms. Daisy E. Johnson
from the Joint Pathology Center (Bethesda, USA) for her critical
scientific and English editing of this manuscript.
Funding
The present study was supported in part by grants
from the Fujian Medical University Qihang Grants (grant no.
2017XQ1093), the Scientific Research Project of Education
Department of Fujian Province (grant no. JAT170232), Fujian Medical
University funds (grant no. 0000-081919) and Science and Technology
Program of Fujian Province (grant no. 2018Y2003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JC and LZ conceived and designed the study, and are
responsible for confirming the authenticity of the raw data. JC,
YD, FH, RL, RC, ZW, WH, JL and BW performed the experiments. JC,
YY, JH and LF analyzed the data. JC, LZ, WZ and JH interpreted the
experimental results. JC and ZW prepared the figures. JC drafted
the manuscript. JC, LZ, ZW, FH, JH and WZ edited and revised
manuscript. All authors approved the content of the final version
of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Fujian
Medical University Institutional Animal Ethical Committee (approval
no. FJMU IACUC 2018-027).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
Th cells
|
helper T cells
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
IL
|
interleukin
|
|
IR
|
irradiation
|
|
FCM
|
flow cytometry
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
Treg cells
|
regulatory T cells
|
|
Tr1 cells
|
T regulatory type 1 cells
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
HBV
|
hepatitis B virus
|
|
TF
|
transcription factor
|
References
|
1
|
Han K and Kim JH: Transarterial
chemoembolization in hepatocellular carcinoma treatment: Barcelona
clinic liver cancer staging system. World J Gastroenterol.
21:10327–10335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakisaka M, Haruta M, Komohara Y, Umemoto
S, Matsumura K, Ikeda T, Takeya M, Inomata Y, Nishimura Y and Senju
S: Therapy of primary and metastatic liver cancer by human iPS
cell-derived myeloid cells producing interferon-β. J Hepatobiliary
Pancreat Sci. 24:109–119. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-Pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, et al:
Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1
checkpoint blockade. Cell. 170:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waidmann O: Recent developments with
immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther.
18:905–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Butterfield LH, Ribas A, Potter DM and
Economou JS: Spontaneous and vaccine induced AFP-specific T cell
phenotypes in subjects with AFP-positive hepatocellular cancer.
Cancer Immunol Immunother. 56:1931–1943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin Y and Liao P: Hepatitis B virus
vaccine breakthrough infection: Surveillance of S gene mutants of
HBV. Acta Virol. 62:115–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Ding J, Zhao X and Qi G: Combination
therapy of hepatocellular carcinoma by DNA shuffling-based VEGF
vaccine and doxorubicin. Immunotherapy. 10:951–969. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang F, Chen J, Lan R, Wang Z, Chen R,
Lin J, Zhang L and Fu L: δ-Catenin peptide vaccines repress
hepatocellular carcinoma growth via CD8+ T cell
activation. Oncoimmunology. 7:e14507132018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han Q, Wang Y, Pang M and Zhang J:
STAT3-blocked whole-cell hepatoma vaccine induces cellular and
humoral immune response against HCC. J Exp Cancer Res. 36:1562017.
View Article : Google Scholar
|
|
11
|
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J,
Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al: An
immunogenic personal neoantigen vaccine for patients with melanoma.
Nature. 547:217–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sahin U, Derhovanessian E, Miller M, Kloke
BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B,
et al: Personalized RNA mutanome vaccines mobilize poly-specific
therapeutic immunity against cancer. Nature. 547:222–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buonaguro L, Petrizzo A, Tagliamonte M,
Tornesello ML and Buonaguro FM: Challenges in cancer vaccine
development for hepatocellular carcinoma. J Hepatol. 59:897–903.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Z, Zhu Y, Xia J, Sawakami T, Kokudo N
and Zhang N: Status of and prospects for cancer vaccines against
hepatocellular carcinoma in clinical trials. BioScience Trends.
10:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petrizzo A, Tagliamonte M, Mauriello A,
Costa V, Aprile M, Esposito R, Caporale A, Luciano A, Arra C, Arra
C, et al: Unique true predicted neoantigens (TPNAs) correlates with
anti-tumor immune control in HCC patients. J Transl Med.
16:2862018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Chen J, Zhou S, Yang N, Duan S,
Zhang Z, Su J, He J, Zhang Z, Lu X and Zhao Y: Mouse IP-10 gene
delivered by folate-modified chitosan nanoparticles and
dendritic/tumor cells fusion vaccine effectively inhibit the growth
of hepatocellular carcinoma in mice. Theranostics. 7:1942–1952.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Végran F, Apetoh L and Ghiringhelli F: Th9
cells: A novel CD4 T-cell subset in the immune war against cancer.
Cancer Res. 75:475–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vargas TR, Humblin E, Végran F,
Ghiringhelli F and Apetoh L: Th9 cells in anti-tumor immunity.
Semin Immunopathol. 39:39–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chauhan SR, Singhal PG, Sharma U, Bandil
K, Chakraborty K and Bharadwaj M: Th9 cytokines curb cervical
cancer progression and immune evasion. Hum Immunol. 80:1020–1025.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salazar Y, Zheng X, Brunn D, Raifer H,
Picard F, Zhang Y, Winter H, Guenther S, Weigert A, Weigmann B, et
al: Microenvironmental Th9 and Th17 lymphocytes induce metastatic
spreading in lung cancer. J Clin Invest. 130:3560–3575. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Purwar R, Schlapbach C, Xiao S, Kang HS,
Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo
VK, et al: Robust tumor immunity to melanoma mediated by
interleukin-9-producing T cells. Nature Medicine. 18:1248–1253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu Y, Hong S, Li H, Park J, Hong B, Wang
L, Zheng Y, Liu Z, Xu J, He J, et al: Th9 cells promote antitumor
immune responses in vivo. J Clin Invest. 11:4160–4171. 2012.
View Article : Google Scholar
|
|
23
|
Vegran F, Martin F, Apetoh L and
Ghiringhelli F: Th9 cells: A new population of helper T cells. Med
Sci (Paris). 32:387–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rivera Vargas T, Cai Z, Shen Y, Dosset M,
Benoit-Lizon I, Martin T, Roussey A, Flavell RA, Ghiringhelli F and
Apetoh L: Selective degradation of PU.1 during autophagy represses
the differentiation and antitumour activity of Th9 cells. Nat
Commun. 8:5592017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamiya T, Ichiyama K, Kotani H, Fukaya T,
Sekiya T, Shichita T, Honma K, Yui K, Matsuyama T, Nakao T, et al:
Smad2/3 and IRF4 play a cooperative role in IL-9-producing T cell
induction. J Immunol. 191:2360–2371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Veldhoen M, Uyttenhove C, van Snick J,
Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C and Stockinger
B: Transforming growth factor-beta ‘reprograms’ the differentiation
of T helper 2 cells and promotes an interleukin 9-producing subset.
Nat Immunol. 9:1341–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdelaziz MH, Wang H, Cheng J and Xu H:
Th2 cells as an intermediate for the differentiation of naïve T
cells into Th9 cells, associated with the Smad3/Smad4 and IRF4
pathway. Exp Ther Med. 19:1947–1954. 2020.PubMed/NCBI
|
|
28
|
Chandwaskar R and Awasthi A: Emerging
roles of Th9 cells as an anti-tumor helper T cells. Int Rev
Immunol. 38:204–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang Y, Chen J, Bi E, Zhao Y, Qin T, Wang
Y, Wang A, Gao S, Yi Q and Wang S: TNF-α enhances Th9 cell
differentiation and antitumor immunity via TNFR2-dependent
pathways. J Immunother Cancer. 7:282019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang Y, Chen X, Bai Q, Qin C, Mohamud AO,
Zhu Z, Ball TW, Ruth CM, Newcomer DR, Herrick EJ and Nicholl MB:
IL-9 inhibits HTB-72 melanoma cell growth through upregulation of
p21 and TRAIL. J Surg Oncol. 111:969–974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abdul-Wahid A, Cydzik M, Prodeus A, Alwash
M, Stanojcic M, Thompson M, Huang EH, Shively JE, Gray-Owen SD and
Gariépy J: Induction of antigen-specific TH9 immunity accompanied
by mast cell activation blocks tumor cell engraftment. Int J
Cancer. 139:841–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Y, Chu X, Chen J, Wang Y, Gao S,
Jiang Y, Zhu X, Tan G, Zhao W, Yi H, et al: Dectin-1-activated
dendritic cells trigger potent antitumour immunity through the
induction of Th9 cells. Nat Commun. 7:123682016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim S, Zhang Y, Tang S, Qin C, Karelia D,
Sharma A, Jiang C and Lu J: Optimizing live-animal bioluminescence
imaging prediction of tumor burden in human prostate cancer
xenograft models in SCID-NSG mice. Prostate. 79:949–960. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta CT) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Papaioannou NE, Beniata OV, Vitsos P,
Tsitsilonis O and Samara P: Harnessing the immune system to improve
cancer therapy. Ann Transl Med. 4:2612016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Copier J and Dalgleish A: Whole-cell
vaccines: A failure or a success waiting to happen? Curr Opin Mol
Ther. 12:14–20. 2010.PubMed/NCBI
|
|
37
|
Damo M, Wilson DS, Simeoni E and Hubbell
JA: TLR-3 stimulation improves anti-tumor immunity elicited by
dendritic cell exosome-based vaccines in a murine model of
melanoma. Sci Rep. 5:176222015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu P, Chen L and Zhang H: Natural killer
cells in liver disease and hepatocellular carcinoma and the NK
cell-based immunotherapy. J Immunol Res. 4:12067372018.
|
|
39
|
Tan H, Wang S and Zhao L: A
tumour-promoting role of Th9 cells in hepatocellular carcinoma
through CCL20 and STAT3 pathways. Clin Exp Pharmacol Physiol.
44:213–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qin SY, Lu DH, Guo XY, Luo W, Hu BL, Huang
XL, Chen M, Wang JX, Ma SJ, Yang XW, et al: A deleterious role for
Th9/IL-9 in hepatic fibrogenesis. Sci Rep. 6:186942016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cui M, Lv Y, Lu J, Zhang W, Duan Y, Huang
Y, Yang L, Li M, Liu W, Liu D and Yan H: Decreased frequency of
circulating Th9 cells in patients with chronic hepatitis B
infection. J Clin Lab Anal. 32:e222462018. View Article : Google Scholar
|
|
42
|
Yu X, Zheng Y, Deng Y, Li J, Guo R, Su M,
Ming D, Lin Z, Zhang J and Su Z: Serum Interleukin (IL)-9 and
IL-10, but not T-Helper 9 (Th9) cells, are associated with survival
of patients with acute-on-chronic hepatitis b liver failure.
Medicine (Baltimore). 95:e34052016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen T, Guo J, Cai Z, Li B, Sun L, Shen Y,
Wang S, Wang Z, Wang Z, Wang Z, et al: Th9 cell differentiation and
its dual effects in tumor development. Front Immunol. 11:10262020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen W, Jin W, Hardegen N, Lei KJ, Li L,
Marinos N, McGrady G and Wahl SM: Conversion of peripheral
CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta
induction of transcription factor Foxp3. J Exp Med. 198:1875–1886.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sundrud MS and Hogan SP: What's old is new
again: BATF transcription factors and Th9 cells. Mucosal Immunol.
12:583–585. 2019. View Article : Google Scholar : PubMed/NCBI
|