Introduction
Breast cancer is the most common female malignant
tumor worldwide (1). Breast cancer
can be divided into different subtypes according to cancer cell
morphology and cell surface receptors, including Luminal type,
human epidermal growth factor receptor 2 (HER2) type and triple
negative breast cancer (2). Bone
tissue is the most common site of breast cancer metastasis
(3). Depending on the stages of
breast cancer, the incidence of bone metastasis can reach 75%
(4–6). Even early breast cancer has a 22%
incidence of bone metastasis, and bone metastases occurs after ~8.4
years (7). The average overall
survival rate for patients with breast cancer and bone metastasis
is only 40 months (6).
Triple negative breast cancer (TNBC) is a specific
subtype of breast cancer, which is characterized by a negative
expression of estrogen receptor (ER), progesterone receptor (PR)
and HER2 receptor (8,9). TNBC is more common in premenopausal
women, and the age of onset is earlier than other types of breast
cancer (10). TNBC is an independent
clinicopathological type with strong invasiveness and poor
prognosis. TNBC accounts for 10–20% of all breast cancers (11,12).
Although this subtype of breast cancer is sensitive to chemotherapy
(13), the clinical outcome and
prognosis remain poor following standard treatment. Because
patients with TNBC have negative expression for ER, PR and HER2,
they cannot benefit from endocrine therapy and targeted therapy
against HER2. Immunotherapy, which is a new method to treat tumors,
has been gradually applied to the treatment of TNBC. For example,
suppression against immune checkpoint PD-L1 can be used as a
therapeutic approach (14). At
present, TNBC has become a new hotspot of breast cancer research in
the world.
Numerous active principles from Chinese herbal
medicines have been found to have anti-cancer effects, and some
have been approved by the Food and Drug Administration (15). For example, betulinic acid (BA) has
been widely studied because of its safety and effect on numerous
biological functions. Previous studies have demonstrated that BA
has some therapeutic effects, including anti-inflammation (16,17) and
treatment of diabetes by selective activation of Tgr5 receptors
(18,19). BA and its derivative compounds show
antiplasmodial activity against chloroquine-resistant Plasmodium
falciparum parasites (20). BA also
has a certain therapeutic effect on tumors, and it was reported
that it can promote the apoptosis and regulate the cell cycle of
glioma cancer cells (21,22).
Because of the poor solubility and the low
bioavailability of BA, 35 BA derivatives were obtained and the
compound 20 (SH-479) inhibitory effects of RANKL-induced
osteoclastogenesis of these derivatives was evaluated in TNBCs.
Compound 20 has been reported to exhibit a dramatic increase in
inhibitory potency compared with BA (23). In order to maintain BA activity, the
water solubility of the derivatives was enhanced and the
bioavailability of the derivatives was subsequently improved.
The activity and migratory ability of tumor cells
can directly affect the occurrence of distant metastasis. Tumor
cells are compared to the “seeds” of plants. Only when they reach
the proper “soil”, which is the organs with metastatic lesions, can
the seeds grow, and metastasis occurs (24). Bone marrow microenvironment plays an
important role in the development of bone metastasis. Osteoclasts
can decompose bone, release increased growth factors and promote
the proliferation of tumor cells, ultimately forming a vicious
circle (25). Simultaneously,
immunosuppressive cells are recruited into the bone marrow
microenvironment, such as Treg and bone marrow-derived suppressor
cells (MDSCs) (26,27). Immune cells in the bone marrow
microenvironment are inhibited when bone metastasis occurs,
promoting tumor cell immune escape. Breast cancer, lung cancer and
multiple myeloma inhibit CD8+T lymphocytes by recruiting MDSC and
thus promote tumor development (28). The present study aimed to investigate
the effect of the BA derivative SH-479 on breast cancer and bone
microenvironment.
Materials and methods
Cell culture
The cell lines MDA-MB-231, MCF-7, SKBR3, MCF-10A and
RAW264.7 were purchased from the Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. MDA-MB-231, MCF-7
and SKBR3 cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (HyClone; Cytiva) and 100 U/ml penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). MCF-10A were cultured in DMEM/F12
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 5% horse
serum (Invitrogen; Thermo Fisher Scientific, Inc.), 10 µg/ml
insulin (Invitrogen; Thermo Fisher Scientific, Inc.), 20 ng/ml
epidermal growth factors (Invitrogen; Thermo Fisher Scientific,
Inc.), 100 ng/ml cholera toxin (Sigma-Aldrich; Merck KGaA) and 0.5
µg/ml hydrocortisone (Invitrogen; Thermo Fisher Scientific, Inc.).
RAW264.7 were cultured in α-minimum essential medium (α-MEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and 100
U/ml penicillin-streptomycin. All cells were placed at 37°C in a
humidified incubator containing 5% CO2.
Cell viability assay
A total of 35 BA derivatives were obtained and the
compound 20 was named SH-479. To obtain SH-479, a solution of BA
(2.28 g, 5 mmol) in tetrahydrofuran (10 ml) was added dropwise to a
stirring solution of 2-iodoxybenzoic acid (2.1 g, 7.5 mmol) in DMSO
(30 ml) at 23°C. The reaction mixture was stirred for 2 h at 23°C
and then diluted with ethyl acetate (AcOEt; 30 ml) and washed with
brine (50 ml). The organic layer was dried over anhydrous
Na2SO4 and concentrated in vacuo. The residue
was purified by flash chromatography (petroleum ether/AcOEt; 8/1
v/v) to give compound 1 (2.05 g, 90%) as a white solid (melting
point, 251–253°C) (23). The
concentrations used for BA are 0–25 µM. The concentrations used for
SH-479 are 0–10 µM. The durations of the treatments was 72 h. The
effect of BA and SH-479 on cell viability was determined using the
MTS method following the instructions from the Cell Titer 96
Aqueous One Solution Cell Proliferation assay (Promega
Corporation). Briefly, the cell density was adjusted to
105/ml. The cell suspension was inoculated into 96-well
plates at 100 µl/well and cultured overnight. Cells were treated
with BA (0–25 µM) and SH479 (0–10 µM) and cultured for 72 h. MTS
solution (20 µl) was added to each well and incubated for 4 h. The
absorbance was read at 490 nm on a VERSA max microplate reader
(Molecular Devices, LLC).
Live and dead assay
The cytotoxicity of SH-479 on MDA-MB-231 cells was
assessed using Live and Dead assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.) as previously described (29). Briefly, 2,500 cells were seeded into
96-well plates. After cell attachment, the cells were treated with
SH479 (0–10 µM) for 30 h. Cells were stained with Live and Dead
reagent (5 µM ethidium homodimer and 5 µM calcein-AM) and were
incubated at 37°C for 45 min. Live and dead cells were observed
under a fluorescence microscope (magnification, ×20).
F-actin assay
MDA-MB-231 were seeded into 96-well plates (5,000
cells/well) and treated with SH-479 (0–10 µM). After 3 days, cells
were fixed with 4% paraformaldehyde for 10 min at room temperature
and subsequently incubated for 5 min at room temperature with PBS
containing 0.1% Triton-X 100 and with rhodamine conjugated
phalloidin (cat. no. R415; 1:200 in PBS; Thermo Fisher Scientific,
Inc.) for 30 min in the dark at room temperature. F-actin staining
was observed under an inverted fluorescence microscope (Olympus
Corporation; magnification, ×40).
Western blotting
MDA-MB-231 cells were lysed on ice using RIPA buffer
(cat. no. R0278; Sigma-Aldrich; Merck KGaA). Protein concentration
was evaluated using the BCA method. Proteins (30 µg) were separated
by 10% SDS PAGE and transferred onto PVDF membranes. After blocking
for 1 h in 5% skimmed milk dissolved in PBS, membranes were
incubated with primary antibodies against cyclin E (cat. no. SC248
1:500 Santa Cruz Biotechnology, Inc.), PARP (cat. no. AY 0276;
1:1,000; Abways Technology, Inc.), cleaved PARP (cat. no. CY5035;
1:500; Abways Technology, Inc.) and β-actin (cat. no. A5441;
1:5,000; Sigma-Aldrich; Merck KGaA) at 4°C overnight. The membranes
were washed three times with PBST (0.1% Tween) and incubated with
infrared dye-labeled secondary antibody (LI-COR Biosciences) for 1
h at room temperature. The signal on the membrane was visualized
using LI-COR Odyssey System.
Cell-cycle analysis
The effect of SH-479 on cell cycle was determined by
flow cytometry. Briefly, cells were serum-starved for 24 h and
treated with 5 µM SH-479 for 12 h. Subsequently, cells were fixed
in 70% ethanol at 4°C overnight. Cells were stained with propidium
iodide (cat. no. R37108; Thermo Fisher Scientific, Inc.) for 30 min
at room temperature. Cell cycle analysis was conducted using a flow
cytometry (Becton, Dickinson and Company). The software used for
the analysis was FlowJo VX10.0 (FlowJo LLC).
Migration assay
The migration assay was performed in Boyden chambers
(Corning Inc.). MDA-MB-231 cells (80,000) were suspended in 100 µl
DMEM medium and treated with SH-479 (0–5 µM). The mixture was added
to the upper well of Boyden chambers. DMEM medium (600 µl)
supplemented with 2% FBS was added in the lower chamber. After 8 h
incubation, cells that have migrated to the lower chambers were
fixed with 4% paraformaldehyde and stained with 0.1% crystal violet
5 min at room temperature. Pictures of the cells were taken under
an inverted microscope (Olympus Corporation; magnification, ×20)
and quantification was performed using Image-Pro 6.0 software
(Media Cybernetics, Inc.).
Osteoclast differentiation assay
Since osteoclasts originate from
monocytes/macrophages in the bone marrow, monocytes were studied in
the present study. A total of 2×103 MDA-MB-231 cells and
5×103 RAW264.7 cells were co-cultured in 24-well plate
(30). Cells were cultured in α-MEM
medium containing 10% FBS and SH-479 (0–5 µM). After 7 days, cells
were fixed with 4% paraformaldehyde 5 min at room temperature and
incubated for 5 min with PBS containing 0.1% Triton-X 100 at room
temperature. Cells were subsequently stained with
tartrate-resistant acid phosphatase (TRAP) using Leukocyte acid
phosphatase kit (Sigma-Aldrich; Merck KGaA) for 5 min at 37°C. TRAP
positive cells were imaged under an inverted microscope (Olympus
Corporation; magnification, ×40) and counted using Image-Pro
software 6.0 (Media Cybernetics, Inc.).
Immunocytometric assay
National Institutes of Health guide for the care and
use of Laboratory animals has been followed for these experiments.
Animals experiments were approved by the Ethics Committee of East
China Normal University (ECNU; approval no. m20151002). The C57bl/6
wild type male mice (8 weeks; n=3) were purchased from the ECNU,
had free access to food pellets and tap water. They were maintained
under the standard 12-h dark/light cycle, with controlled
temperature (24±2°C) and relative humidity (55–60%). Mice were
euthanized by intraperitoneal injection of 3% pentobarbital sodium
(200 mg/kg) to collect bone marrow. Femurs and tibias were
collected from mice and the femoral head and ankle joint were
preserved in order to ensure the integrity of the bone marrow
cavity. The bone marrow cavities of the femurs and tibias were
opened, and the bone marrow was flushed with PBS solution
containing 1% FBS (31,32). The rinsing solution was collected and
centrifuged at 125 × g for 5 min at 4°C. After centrifugation,
cells collected were resuspended in PBS solution containing 1% FBS
and counted. Cells were then transferred into Eppendorf tube at the
concentration of 106/100 µl for later use and seeded in
a 6 cm plate and cultured in a cell incubator. Cells were treated
with 10 µM SH-479 for 48 h at 37°C in the 6 cm plate. After 48 h,
one million cells were incubated with 0.5 µl antibody (CD3-FITC,
cat. no. 100204; CD4-PerCP-Cy5.5, cat. no. 10043; CD8-APC, cat. no.
344722; CD11b-APC, cat. no. 101212; Ly6C-PerCP-Cy5.5, cat. no.
128012; Ly6G-PE, cat. no. 127608; BioLegend, Inc.) at 4°C for 30
min. Cells were then washed with 2% PBS, and T cells and MDSCs
(CD11b+) were detected by flow cytometry (Becton, Dickinson and
Company). Results were analyzed using FlowJo VX 10.0 software
(FlowJo LLC).
Statistical analysis
The data were expressed as the means ± standard
deviation. Differences between two groups were determined using
paired Student's t-test. Comparisons between multiple groups were
determined by one-way ANOVA followed by Dunnett's post hoc test.
All statistical analyses are performed using GraphPad Prism 5.0.
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibitory effect of BA and SH-479 on
the viability of breast cancer cells
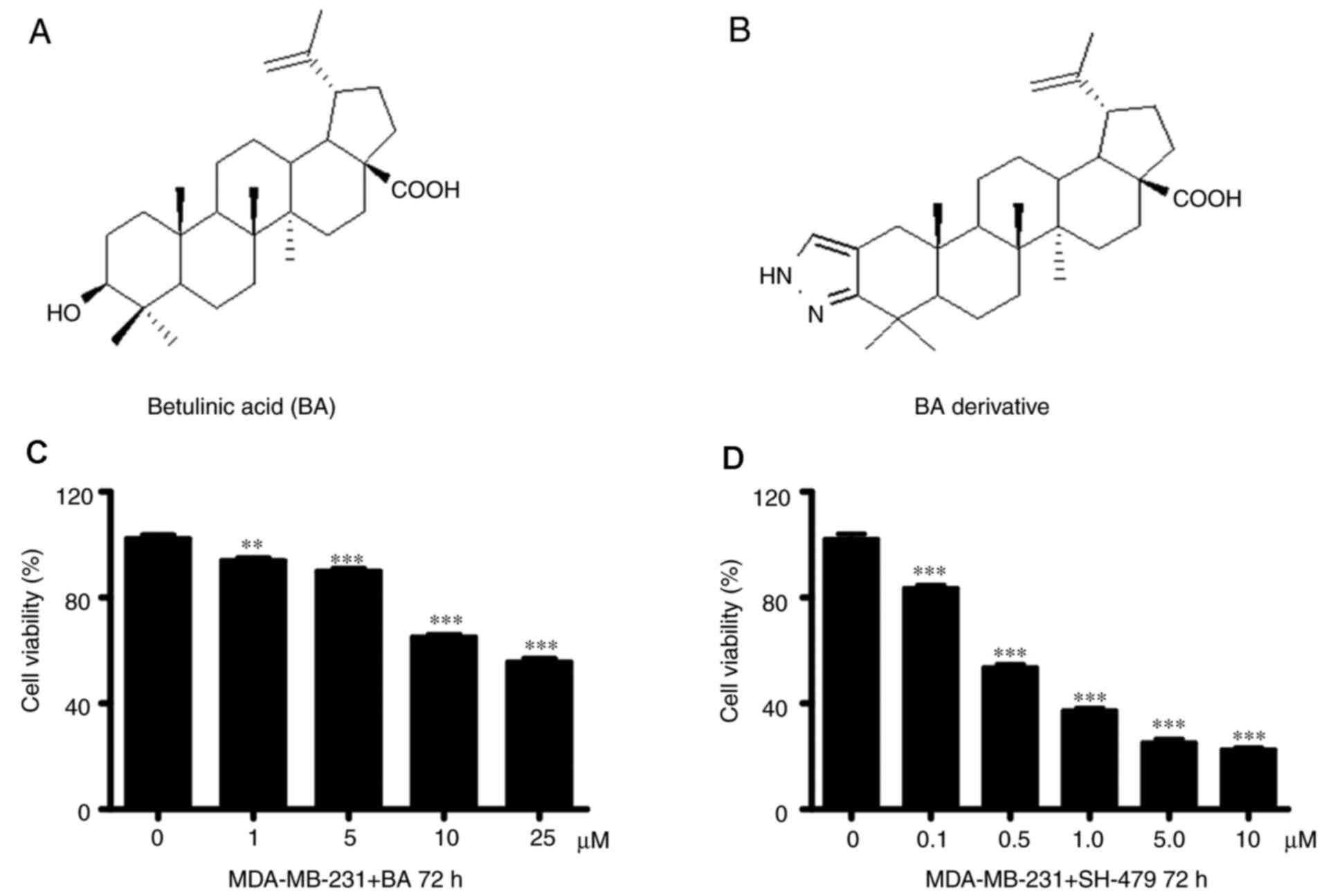
The effect of BA (Fig.
1A) and the 35 BA derivatives on TNBC cell viability was
assessed. Because only SH-479 showed a significant effect, the
results from other BA derivatives were not presented. The results
demonstrated that SH-479 (Fig. 1B)
significantly inhibited the viability of MDA-MB-231 cells in a
concentration-dependent manner after 72 h compared with the control
group (Fig. 1D). However, the
viability of MDA-MB-231 cells was significantly inhibited by more
than 50% when higher concentration of BA was added (Fig. 1C).
SH-479 has an inhibitory effect on
TNBC cell viability
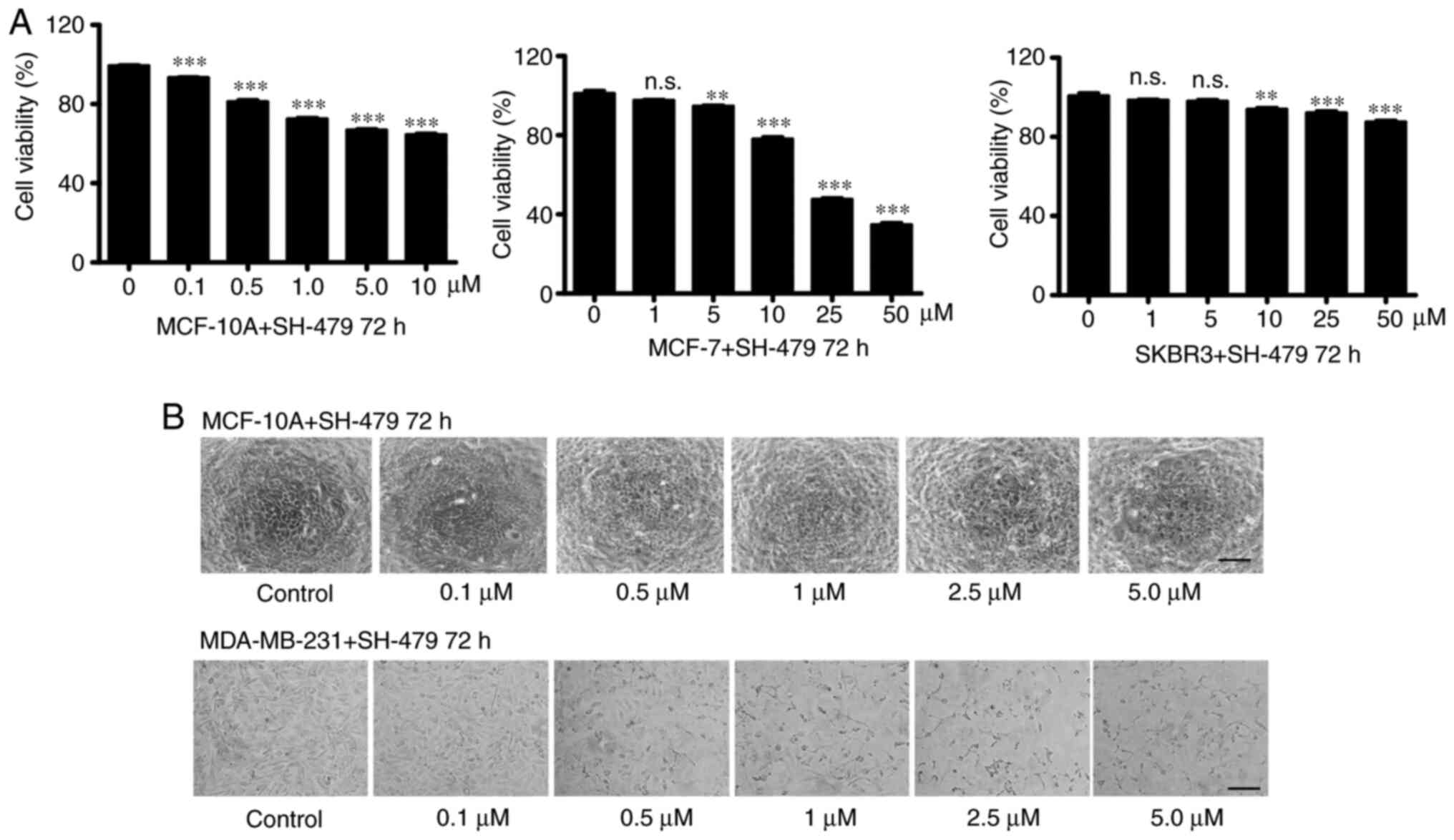
When using drugs to treat tumors, the common side
effects of drugs are toxicity to normal cells. The results showed
that only 30% of MCF-10A viability was inhibited after SH-479
treatment (0.1–10 µM; Fig. 2A).
Furthermore, SH-479 did not significantly inhibit the viability of
the luminal type cell line MCF-7 and Her2 type cell line SKBR3 at
low concentration (Fig. 2A). In
addition, SH-479 had no effect on the morphology of the normal
breast tissue cells MCF-10 (Fig.
2B). However, when MDA-MB-231 cells were treated with SH-749,
cells seemed to shrink and their morphology changed in a
dose-dependent manner (Fig. 2B).
SH-479 alters microfilaments in breast
cancer cells
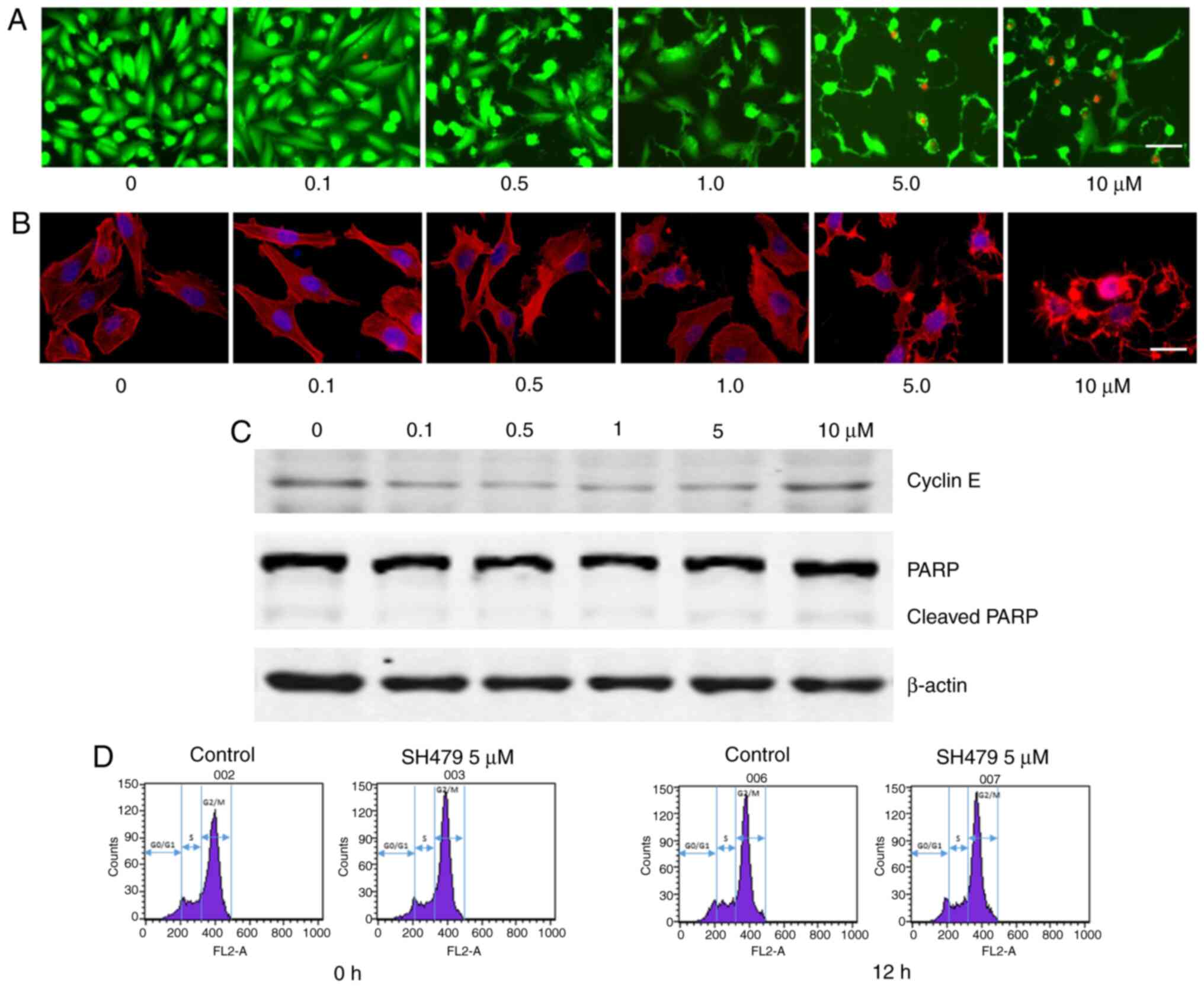
The morphological changes of cell shrinkage might be
caused by cell death or by structural changes within the cells. The
results demonstrated that after 30 h treatment with SH-479 (0.1–10
µM), MDA-MB-231 cells presented morphological shrinkage, although
no cell death was observed. Green fluorescence represents living
cells and red fluorescence represents dead cells (Fig. 3A). After 72 h treatment with SH-479
(0.1–10 µM), the microfilaments of MDA-MB-231 cells were altered
according to F-actin staining. The red fluorescence indicates the
cytoskeleton and the blue fluorescence indicates the nucleus
(Fig. 3B). Because SH-479 can
inhibit TNBC cell viability, cell cycle and apoptosis were
determined. The expression of cyclin E starts in the middle phase
of G1 phase and gradually decreases after reaching the peak in G1/S
phase. Cyclin E is therefore the key protein of G1 to S phase. The
results demonstrated that SH-479 had no effect on cell cycle
transition from G1 phase to S phase. There was no change in the
expression of proteins involved in cell cycle and apoptosis in
MDA-MB-231 cells treated with SH-479 (Fig. 3C). In order to further verify the
effect of SH-479 on MDA-MB-231 cell cycle, cell cycle was
determined by flow cytometry. The results demonstrated that after
12 h treatment with 5 µM SH-479, the cell cycle of MDA-MB-231 cells
was not modified (Fig. 3D).
SH-479 inhibits breast cancer cell
migratory ability and breast cancer cell-induced osteoclast
differentiation
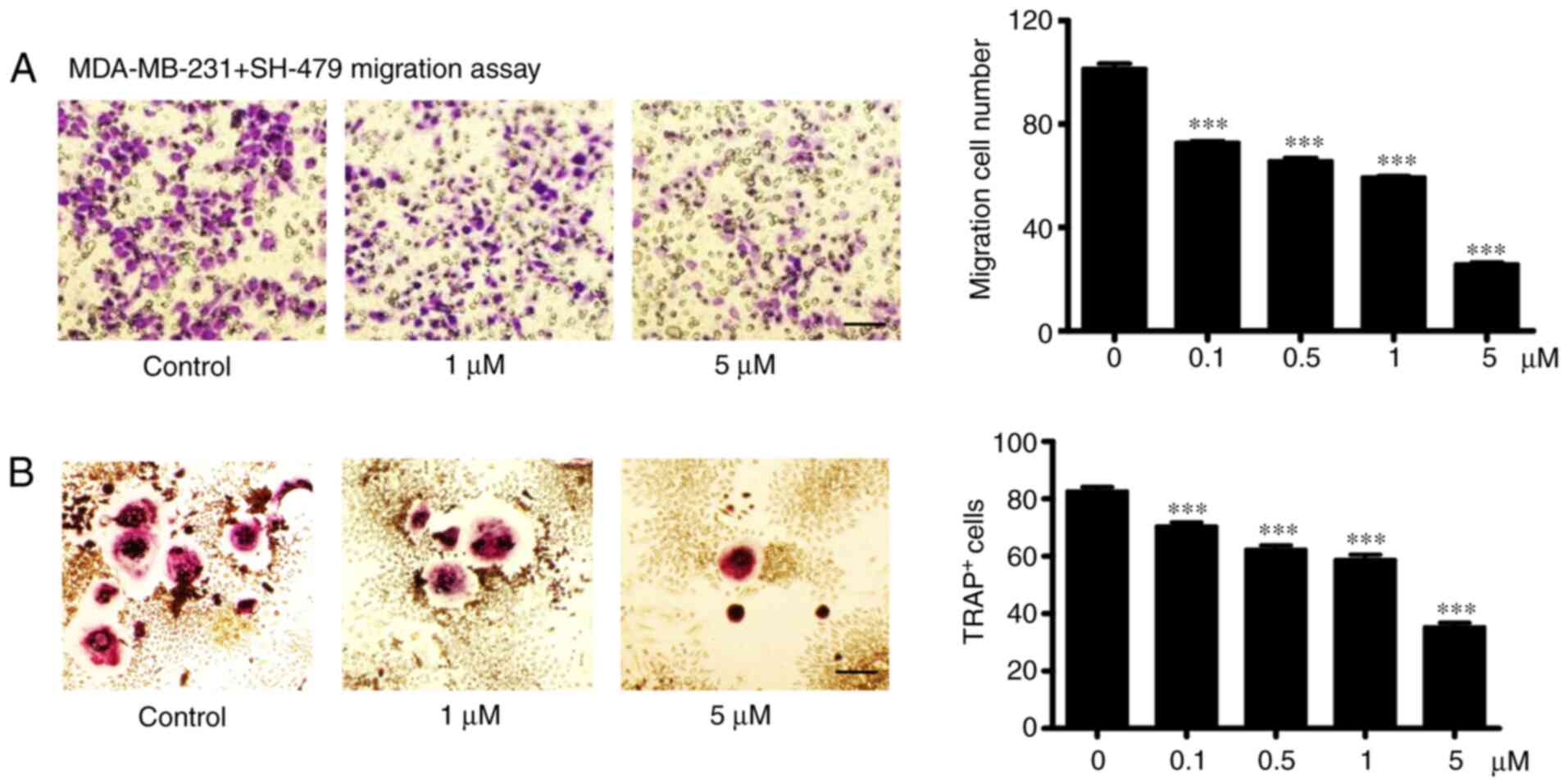
The incidence of bone metastases in breast cancer is
high, and TNBC usually leads to osteolytic bone metastasis. In the
present study, MDA-MB-231 cell treatment with SH-479 induced a
significant decrease in cell migratory ability in a dose-dependent
manner (Fig. 4A). Furthermore,
SH-479 treatment led to a decrease in the capacity of MDA-MB-231
cells to induce osteoclast differentiation in a dose-dependent
manner (Fig. 4B).
Effects of SH-479 on bone marrow
immune microenvironment
When bone metastasis occurs in patients with breast
cancer, immune cells in the bone marrow microenvironment play an
important role in the colonization and development of cancer cells.
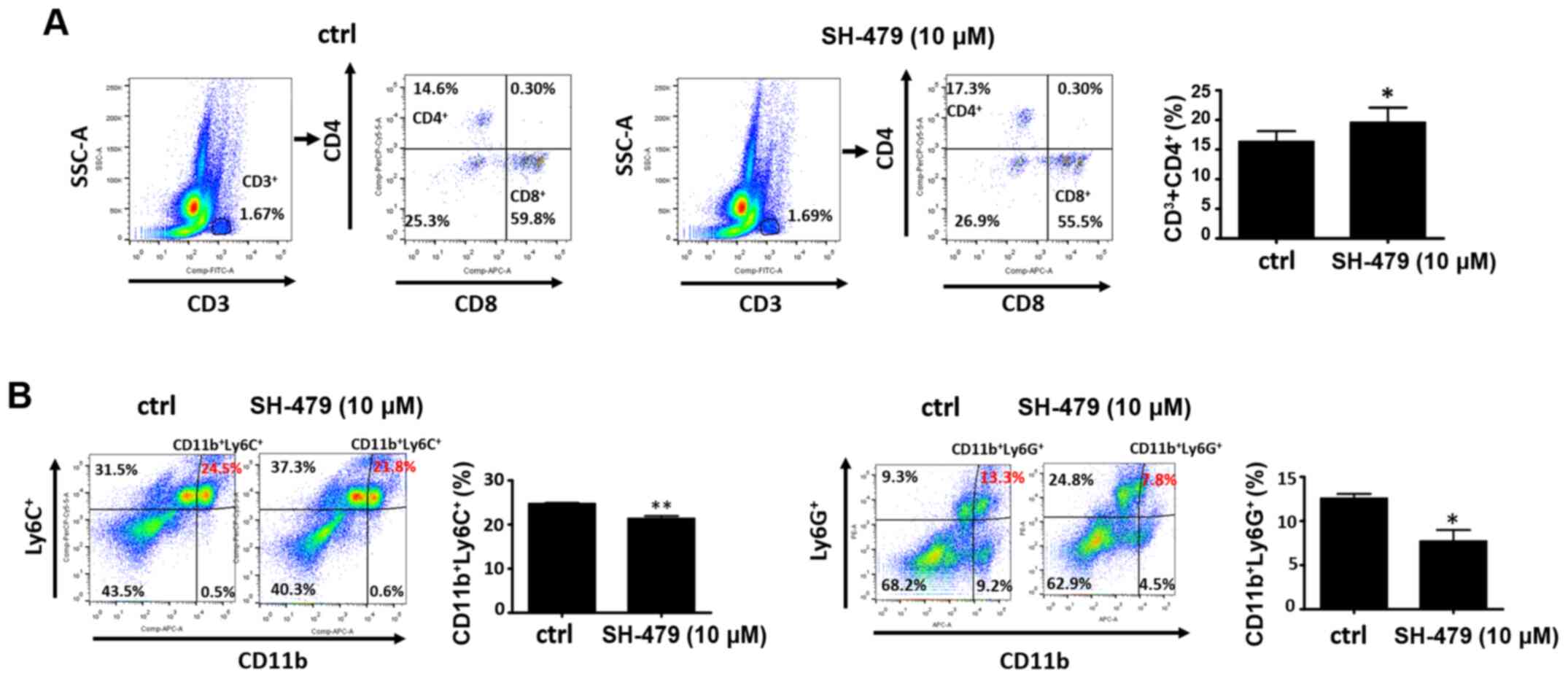
The results from the present study demonstrated that the number of
CD3+CD4+T lymphocytes in the bone marrow microenvironment was
significantly increased following treatment with SH-479 for 48 h
(Fig. 5A). Furthermore, the number
of MDSCs in the bone marrow microenvironment also serves a role in
breast cancer metastasis to bones, and it can indirectly promote
the development of breast cancer cells in the bone microenvironment
by inhibiting the function of immune cells. The results from the
present study demonstrated that SH-479 treatment decreased the
number of MDSCs in bone microenvironment (Fig. 5B).
Discussion
TNBC is one subtype of breast cancer that lacks
specific therapeutic targets. TNBC is characterized by a strong
invasiveness and a poor prognosis. In the present study, SH-479 had
small inhibitory effect on normal breast cell MCF-10A but had a
significant inhibitory effect on the viability of MDA-MB-231 cells,
which was not the case for the other types of breast cancer cells.
SH-479 had no effect on the morphology of the normal breast tissue
cells MCF-10, however, when MDA-MB-231 cells were treated with
SH-749, cells seemed to shrink.
At present, conventional chemotherapy remains the
main method of treatment for TNBC; however, drug resistance is
likely to occur. Combined immunotherapy may be interesting for the
treatment of TNBC (14).
Immunotherapy provides good results on numerous types of cancer,
including melanoma, lymphoma and prostate cancer (33). Due to the lack of therapeutic targets
for TNBC, it is difficult for traditional treatments to achieve
ideal therapeutic effect. Previous studies have reported that PD-L1
is highly expressed in TNBC and that it is associated with
increased T lymphocyte infiltration (34–36).
Regarding tumor treatment with immunotherapy, a class of MDSCs with
immunosuppressive effect deserves some attention. MDSCs in the
tumor microenvironment can reduce the innate immunity and adaptive
immunity by inhibiting the proliferation of T lymphocyte and NK
cells (37,38).
The present study demonstrated that SH-479 could
reduce the proportion of MDSC in the bone marrow, which may
stimulate the immune system to inhibit tumor growth. However, the
therapeutic effect of SH-479 on breast cancer bone metastasis
requires further in vivo investigation.
The role of BA in cancer is mainly due to the
induction of cell apoptosis and changes in cell cycle (39,40). In
the present study, the apoptotic proteins PARP and cleaved PARP
were detected by western blotting. No significant effect of SH-479
on PARP and cleaved PARP expression was observed. In addition, no
significant cell death was observed in MDA-MB-231 cells following
treatment with SH-479. The results from flow cytometry demonstrated
that SH-479 had no significant effect on cell cycle. Microfilaments
play an important role in tumor cell migration and are involved in
cell migratory ability, suggesting that they may serve a crucial
role in the occurrence of cancer metastasis (39,41). In
the present study, SH-479 treatment altered the microfilament
structure of MDA-MB-231 cells and inhibited the migratory ability
of MDA-MB-231 cells, suggesting that impaired migratory ability may
be related to microfilament interference in these cells. Cell
microfilaments play an important role in cell proliferation and
form contraction rings with myosin during mitosis (42–44).
Following treatment with SH-479, MDA-MB-231 cell proliferation was
inhibited, which may be related to cell microfilament interference.
Because SH-479 is the ligand of the G protein-coupled bile acid
receptor 1, it plays a regulatory role through G-protein-coupled
receptors (GPCRs) (31). Previous
studies have reported that GPCR can regulate F-actin by regulating
Hippo signaling pathway and YAP/TAZ activity. The Hippo pathway is
highly conserved in mammals. MST1/2 (Hpo orthologs), Sav1, Lats1/2
(Wts orthologs) and Mob1 (MOBKL1A and MOBKL1B, Mats orthologs) form
a kinase cascade that phosphorylates and inhibits YAP/TAZ (Yki
orthologs). YAP/TAZ in conjunction with TEAD1-4 (Sd orthologs)
mediate major physiological functions of the Hippo pathway
(45–49). The hippo signaling pathway can be
used as a theoretical basis for further research. The activation of
osteoclasts plays an important role in the process of breast cancer
metastasis to bone. Tumor cells can activate osteoclasts directly
or indirectly by secreting cytokines. The growth factors released
by osteoclasts after absorbing bone tissue can promote the
proliferation of tumor cells and therefore lead to a vicious circle
(50). We previously demonstrated
that SH-479 could directly inhibit osteoclast differentiation
(23). The present study showed that
SH-479 had the ability to inhibit osteoclast differentiation
induced by tumor cells, suggesting that it may therefore have
potential therapeutic effects on breast cancer metastasis of the
bone.
In conclusion, the present study demonstrated that
SH-479 inhibited the activity of TNBC cells, the migratory ability
of TNBC cells and the differentiation of osteoclasts induced by
TNBCs. It was also demonstrated to enhance the immune
microenvironment of bone marrow. These findings indicate that
SH-479 may have a potential therapeutic significance for breast
cancer metastasis of the bone.
Acknowledgements
Not applicable.
Funding
This study was funded by the Science and Technology
Commission of Shanghai Municipality (grant no. 17411950301) and the
Science and Technology Commission of Shanghai Municipality (grant
no. 17ZR1439000).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX, JZ, ZL and HW designed the study and HW approved
the final version to be submitted. LT, SL, TH, WG and YX performed
experiments, acquired data and drafted the manuscript. ZW, MQ, XG,
XL and TW analyzed and interpretated the data. YX, XG, TW, TH, XL,
XG and WG revised the manuscript for intellectual content. All
authors read and approved the final manuscript. LT and HW confirmed
the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by the Animal Experiment
Ethics Committee of East China Normal University (approval no.
m20151002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beiki O, Hall P, Ekbom A and Moradi T:
Breast cancer incidence and case fatality among 4.7 million women
in relation to social and ethnic background: A population-based
cohort study. Breast Cancer Res. 14:R52012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buijs JT and van der Pluijm G: Osteotropic
cancers: From primary tumor to bone. Cancer Lett. 273:177–193.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang J and Xu Q: Differences of
osteoblastic bone metastases and osteolytic bone metastases in
clinical features and molecular characteristics. Clin Transl Oncol.
17:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuchuk I, Hutton B, Moretto P, Ng T,
Addison CL and Clemons M: Incidence, consequences and treatment of
bone metastases in breast cancer patients-Experience from a single
cancer centre. J Bone Oncol. 2:137–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harries M, Taylor A, Holmberg L, Agbaje O,
Garmo H, Kabilan S and Purushotham A: Incidence of bone metastases
and survival after a diagnosis of bone metastases in breast cancer
patients. Cancer Epidemiol. 38:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brenton JD, Carey LA, Ahmed AA and Caldas
C: Molecular classification and molecular forecasting of breast
cancer: Ready for clinical application? J Clin Oncol. 23:7350–7360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morris GJ, Naidu S, Topham AK, Guiles F,
Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, et al:
Differences in breast carcinoma characteristics in newly diagnosed
African-American and Caucasian patients: A single-institution
compilation compared with the National Cancer Institute's
Surveillance, Epidemiology, and End Results database. Cancer.
110:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katz H and Alsharedi M: Immunotherapy in
triple-negative breast cancer. Med Oncol. 35:132017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200.
2009.PubMed/NCBI
|
|
16
|
Chowdhury AR, Mandal S, Mittra B, Sharma
S, Mukhopadhyay S and Majumder HK: Betulinic acid, a potent
inhibitor of eukaryotic topoisomerase I: Identification of the
inhibitory step, the major functional group responsible and
development of more potent derivatives. Med Sci Monit.
8:BR254–BR265. 2002.PubMed/NCBI
|
|
17
|
Viji V, Helen A and Luxmi VR: Betulinic
acid inhibits endotoxin-stimulated phosphorylation cascade and
pro-inflammatory prostaglandin E(2) production in human peripheral
blood mononuclear cells. Br J Pharmacol. 162:1291–1303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Melo CL, Queiroz MG, Arruda Filho AC,
Rodrigues AM, de Sousa DF, Almeida JG, Pessoa OD, Silveira ER,
Menezes DB, Melo TS, et al: Betulinic acid, a natural pentacyclic
triterpenoid, prevents abdominal fat accumulation in mice fed a
high-fat diet. J Agric Food Chem. 57:8776–8781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Genet C, Strehle A, Schmidt C, Boudjelal
G, Lobstein A, Schoonjans K, Souchet M, Auwerx J, Saladin R and
Wagner A: Structure-activity relationship study of betulinic acid,
a novel and selective TGR5 agonist, and its synthetic derivatives:
Potential impact in diabetes. J Med Chem. 53:178–190. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Sá MS, Costa JF, Krettli AU, Zalis MG,
Maia GL, Sette IM, Câmara CA, Filho JM, Giulietti-Harley AM,
Ribeiro Dos Santos R, et al: Antimalarial activity of betulinic
acid and derivatives in vitro against Plasmodium falciparum and in
vivo in P. berghei-infected mice. Parasitol Res. 105:275–279. 2009.
View Article : Google Scholar
|
|
21
|
Aisha AF, Ismail Z, Abu-Salah KM, Siddiqui
JM, Ghafar G and Abdul Majid AM: Syzygium campanulatum korth
methanolic extract inhibits angiogenesis and tumor growth in nude
mice. BMC Complement Altern Med. 13:1682013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fulda S: Betulinic acid: A natural product
with anticancer activity. Mol Nutr Food Res. 53:140–146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Li Z, Luo J, Yang F, Liu T, Liu M,
Qiu WW and Tang J: Synthesis and biological evaluation of
heterocyclic ring-fused betulinic acid derivatives as novel
inhibitors of osteoclast differentiation and bone resorption. J Med
Chem. 55:3122–3134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sawant A, Hensel JA, Chanda D, Harris BA,
Siegal GP, Maheshwari A and Ponnazhagan S: Depletion of
plasmacytoid dendritic cells inhibits tumor growth and prevents
bone metastasis of breast cancer cells. J Immunol. 189:4258–4265.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan W, Zhang W, Strasner A, Grivennikov S,
Cheng JQ, Hoffman RM and Karin M: Tumour-infiltrating regulatory T
cells stimulate mammary cancer metastasis through RANKL-RANK
signalling. Nature. 470:548–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sawant A and Ponnazhagan S:
Myeloid-derived suppressor cells as osteoclast progenitors: A novel
target for controlling osteolytic bone metastasis. Cancer Res.
73:4606–4610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sung B, Pandey MK, Ahn KS, Yi T,
Chaturvedi MM, Liu M and Aggarwal BB: Anacardic acid (6-nonadecyl
salicylic acid), an inhibitor of histone acetyltransferase,
suppresses expression of nuclear factor-kappaB-regulated gene
products involved in cell survival, proliferation, invasion, and
inflammation through inhibition of the inhibitory subunit of
nuclear factor-kappaBalpha kinase, leading to potentiation of
apoptosis. Blood. 111:4880–4891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou T, Lou Y, Li S, Zhao C, Ji Y, Wang D,
Tang L, Zhou M, Xu W, Qian M, et al: Kadsurenone is a useful and
promising treatment strategy for breast cancer bone metastases by
blocking the PAF/PTAFR signaling pathway. Oncol Lett. 16:2255–2262.
2018.PubMed/NCBI
|
|
31
|
Li Z, Huang J, Wang F, Li W, Wu X, Zhao C,
Zhao J, Wei H, Wu Z, Qian M, et al: Dual targeting of Bile Acid
Receptor-1 (TGR5) and Farnesoid X Receptor (FXR) prevents
estrogen-dependent bone loss in mice. J Bone Miner Res. 34:765–776.
2019.PubMed/NCBI
|
|
32
|
van der Heide V, Möhnle P, Rink J, Briegel
J and Kreth S: Down-regulation of MicroRNA-31 in CD4+ T cells
contributes to immunosuppression in human sepsis by promoting TH2
skewing. Anesthesiology. 124:908–922. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pusztai L, Karn T, Safonov A, Abu-Khalaf
MM and Bianchini G: New strategies in breast cancer: Immunotherapy.
Clin Cancer Res. 22:2105–2110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McArthur HL and Page DB: Immunotherapy for
the treatment of breast cancer: Checkpoint blockade, cancer
vaccines, and future directions in combination immunotherapy. Clin
Adv Hematol Oncol. 14:922–933. 2016.PubMed/NCBI
|
|
36
|
Schalper KA, Velcheti V, Carvajal D,
Wimberly H, Brown J, Pusztai L and Rimm DL: In situ tumor PD-L1
mRNA expression is associated with increased TILs and better
outcome in breast carcinomas. Clin Cancer Res. 20:2773–2782. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sinha P, Clements VK and Ostrand-Rosenberg
S: Reduction of myeloid-derived suppressor cells and induction of
M1 macrophages facilitate the rejection of established metastatic
disease. J Immunol. 174:636–645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suzuki E, Kapoor V, Jassar AS, Kaiser LR
and Albelda SM: Gemcitabine selectively eliminates splenic
Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and
enhances antitumor immune activity. Clin Cancer Res. 11:6713–6721.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mullauer FB, Kessler JH and Medema JP:
Betulinic acid, a natural compound with potent anticancer effects.
Anticancer Drugs. 21:215–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Petrie RJ and Yamada KM: At the leading
edge of three-dimensional cell migration. J Cell Sci.
125:5917–5926. 2012.PubMed/NCBI
|
|
42
|
Akhshi TK, Wernike D and Piekny A:
Microtubules and actin crosstalk in cell migration and division.
Cytoskeleton (Hoboken). 71:1–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goode BL and Eck MJ: Mechanism and
function of formins in the control of actin assembly. Annu Rev
Biochem. 76:593–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kovar DR: Molecular details of
formin-mediated actin assembly. Curr Opin Cell Biol. 18:11–17.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aragona M, Panciera T, Manfrin A, Giulitti
S, Michielin F, Elvassore N, Dupont S and Piccolo S: A mechanical
checkpoint controls multicellular growth through YAP/TAZ regulation
by actin-processing factors. Cell. 154:1047–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu FX and Guan KL: The Hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Xiao Y, Dong Q, Ouyang W and Qin
Q: Neferine in the Lotus Plumule Potentiates the Antitumor Effect
of Imatinib in Primary Chronic Myeloid Leukemia Cells In Vitro. J
Food Sci. 84:904–910. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yoneda T, Michigami T, Yi B, Williams PJ,
Niewolna M and Hiraga T: Use of bisphosphonates for the treatment
of bone metastasis in experimental animal models. Cancer Treat Rev.
25:293–299. 1999. View Article : Google Scholar : PubMed/NCBI
|