Introduction
Colon cancer is a common gastrointestinal tumor,
which ranks fourth in terms of incidence and third in terms of
mortality worldwide (1). The
incidence and mortality rates of colon cancer are rapidly
increasing worldwide, with the exception of a few developed
countries (1). To date, the common
and effective treatment for colon cancer includes radical excision
at the early stages, followed by chemotherapy and/or radiotherapy
for patients at advanced stages, which are invasive treatments
associated with adverse side effects (2). Recently, immune checkpoint molecules
programmed cell death-1 (PD-1) and programmed cell death ligand 1
(PD-L1) have been identified as promising targets for immunotherapy
in colon cancer (3).
PD-L1 is widely expressed in the human body, and
PD-L1 can be expressed by tumor cells and tumor stroma as a type I
transmembrane protein (4). As a
co-inhibitory molecule on T cells, PD-1 binds to PD-L1 on the
surface of tumor cells or stromal cells, which leads to the
apoptosis of activated T cells and causes immune escape (5). The expression of PD-L1 is significantly
elevated in colon cancer tissues (6,7), and
positive PD-L1 expression is an independent risk factor for poor
prognosis (8). Anti-PD-1 or
anti-PD-L1 therapy recovers the anti-tumor activity of immune
cells, which have been proven effective in various types of solid
tumors, including colon cancer with high PD-L1 expression and
microsatellite instability (9).
Nevertheless, the majority of patients with colon cancer are in
mismatch repair proficient or microsatellite stability subtypes,
and thus fail to respond to anti-PD-1 or anti-PD-L1 therapy
(10). Therefore, further
exploration of the regulatory mechanism of PD-1/PD-L1 is a required
for the application of immune checkpoint protein inhibitors in
colon cancer.
As a member of the interleukin (IL)-10 cytokine
family, IL-22 plays an important role in the occurrence and
development of colon cancer. IL-22 is mainly distributed in
cytoplasm and stroma, which is secreted by various immune cells and
binds to the IL-22 receptor complex that leads to the activation of
signal transducer and activator of transcription 3 (STAT3)
(11). IL-22 promotes the
proliferation, migration, chemotherapy resistance and stemness of
colon cancer by activating the STAT3 pathway (12–14). In
a previous study, it was found that IL-22 was involved in the
aerobic glycolysis of colon cancer through STAT3 phosphorylation
(15). STAT3 is an important
signaling mechanism that regulates PD-L1 expression in tumor cells
(16). Meanwhile, Seki et al
(17) reported that IL-22 was
associated with the regulation of PD-L1 expression in airway
epithelial cells via a STAT3-dependent mechanism. However, as an
activator of the STAT3 signaling pathway, the effect of IL-22 on
PD-L1 expression in colon cancer is still unclear. The aim of the
present study was to preliminary explore the association between
IL-22 and PD-L1 in vivo and in vitro, and briefly
elucidate the mechanism.
Materials and methods
Clinical samples
A total of 23 fresh tissue specimens were obtained
from patients who had received a pathological diagnosis of colon
cancer between August 2019 and November 2019. The patients included
13 males and 10 females, ranging between 43 and 75 years with a
mean age of 58.6 years. Tumor tissues and adjacent normal tissues
(2 cm away from the tumor) were collected with RNase-free
centrifuge tubes, and immediately placed into liquid nitrogen.
Written informed consent was obtained from all patients who
provided the samples for the present study. This study was approved
by the Ethics Committee of The First Affiliated Hospital of
Shandong First Medical University.
Cell culture
Two primary colon cancer cell lines (WRCA and JRCA)
were provided by Professor Weiping Zou (University of Michigan,
USA) (14). The colon cancer cell
line DLD-1 was obtained from the American Type Culture Collection.
Cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified incubator with 5%
CO2 atmosphere at 37°C. To test the role of STAT3 in the
expression of PD-L1 induced by IL-22, DLD-1 cells were stimulated
with IL-22 (10 ng/ml; PeproTech, Inc.) for 24 h after
pre-stimulation with Sttatic (5 µM; Selleck Chemicals) for 12 h.
The optimal Sttatic concentration (5 µM) was selected from the
concentration gradient (0, 2.5, 5 and 10 µM).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from clinical tissues and
colon cancer cell DLD-1 with RNAiso Plus reagent (Takara Bio, Inc.)
and reverse transcribed into cDNA with PrimeScript™ RT Master Mix
kit (Takara Bio, Inc.), according to the manufacturer's
instructions. qPCR was performed using the SYBR green method
(Takara Bio, Inc.) on the StepOnePlus™ Real-time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Primer
sequences obtained from PrimerBank and were as follows: PD-L1
forward, GATACAAACTCAAAGAAGCAAAG and reverse,
CAAAATAAATAGGAAAAACTCAT; IL-22 forward, GCAGGCTTGACAAGTCCAACT and
reverse, GCCTCCTTAGCCAGCATGAA; β-actin forward, TGGCACCCAGCACAATGAA
and reverse CTAAGTCATAGTCCGCCTAGAAGC A. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 10
min, followed by 40 cycles of 95°C for 15 sec, 62°C for 20 sec and
72°C for 10 sec. The mRNA expression of PD-L1 and IL-22 were
normalized to the expression of β-actin. The relative expression of
mRNA was calculated using the2−∆∆Cq method (18).
Flow cytometry analysis
Cells were digested with pancreatin for single-cell
suspension and incubated with APC-A-conjugated mouse anti-human
PD-L1 antibody (cat. no. 329708; 1:100; BioLegend, Inc.) or isotype
control antibody (cat. no. 401210; 1:100; BioLegend, Inc.) for 30
min at 4°C. Cells were washed twice with PBS and resuspended to
detect PD-L1 expression on the surface of tumor cells using FACS
Aria II (BD Biosciences) and analyzed using FlowJo software
(version 7.6.1; FlowJo LLC).
Western blotting analysis
Protein was extracted from cells with RIPA lysis
buffer (Beyotime Institute of Biotechnology), and a BCA assay kit
(Beyotime Institute of Biotechnology) was used to determine the
protein concentration. Protein extracts (10 µg/lane) were separated
via 10% SDS-PAGE, and subsequently transferred to polyvinylidene
fluoride membranes. Membranes were blocked with 5% non-fat dry milk
for 1 h at room temperature, and then incubated overnight at 4°C
with the following primary antibodies: Anti-PD-L1 antibody (cat.
no. 13684T), anti-phosphorylated STAT3 (Tyr705) antibody (cat. no.
9145T), anti-STAT3 antibody (cat. no. 12640S) (all 1:1,000; Cell
Signaling Technology, Inc.) and anti-β-actin (cat. no. A3853;
1:4,000; Sigma-Aldrich; Merck KGaA). The membranes were incubated
with a horseradish peroxidase-conjugated secondary antibody for 1 h
at room temperature and visualized with electrochemiluminescence
(cat. no. A38555; Thermo Fisher Scientific, Inc.). Protein levels
were normalized to the level of β-actin.
Statistical analysis
A paired Student's t-test was performed to compare
data from clinical samples. ANOVA and Tukey's post hoc tests were
applied to identify significant differences between the indicated
cell groups. The correlation between IL-22 and PD-L1 mRNA
expression levels was calculated with a Pearson's correlation
analysis. The data are expressed as the mean ± standard deviation
(SD). P<0.05 was considered to indicate a statistically
significant difference.
Results
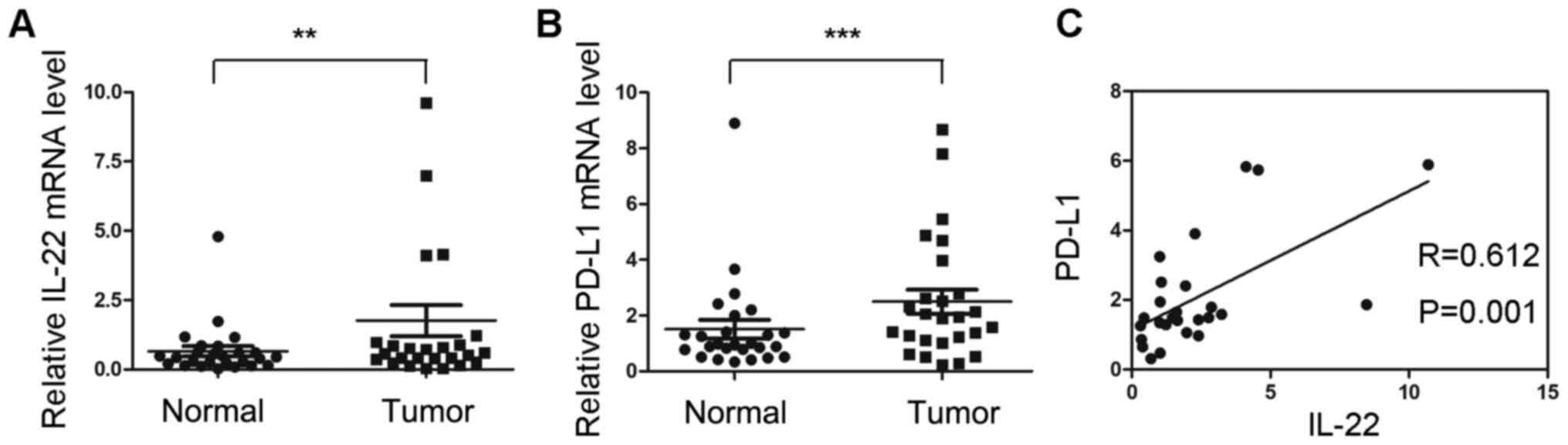
mRNA expression of PD-L1 is positively
correlated with IL-22 expression in colon cancer tissues
The mRNA expression of IL-22 and PD-L1 were
investigated in 23 colon cancer tissues and adjacent normal
tissues. The mRNA expression of IL-22 in tumor tissues was
significantly higher compared with that of normal tissues
(P<0.05; Fig. 1A). The relative
mRNA level of PD-L1 was significantly upregulated in tumor tissues
(P<0.05; Fig. 1B). Correlation
analysis indicated that the mRNA expression of IL-22 was positively
correlated with PD-L1 (r=0.612; P=0.001; Fig. 1C).
IL-22 promotes the expression of PD-L1
in colon cancer cells
In order to evaluate the effect of IL-22 on the
expression level of PD-L1 in colon cancer cells, primary colon
cancer cells and DLD-1 cells were treated with different
concentrations of IL-22 (0, 5, 10 and 50 ng/ml) for 24 h. The
protein expression of PD-L1 was significantly upregulated in colon
cancer cells in a dose-dependent manner (Fig. 2A and B). At the transcription level,
IL-22 promoted the mRNA expression of PD-L1 in DLD-1 cells in a
dose-dependent manner (Fig. 2C).
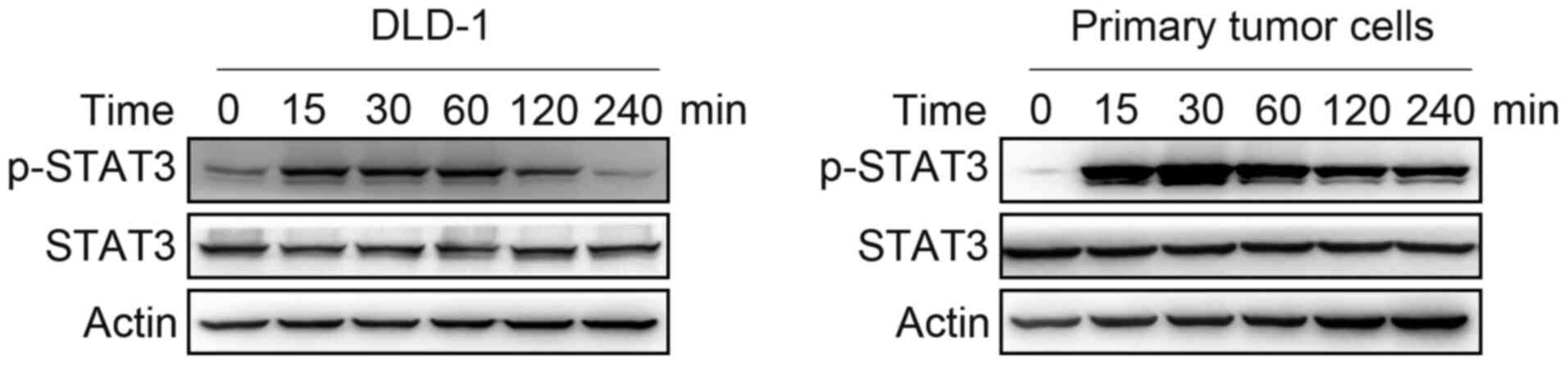
IL-22 activates STAT3 in colon cancer
cells
It is commonly known that STAT3 is an important
component of the signaling pathway induced by IL-22. Primary colon
cancer cells and DLD-1 cells were treated with IL-22 (10 ng/ml) for
15, 30, 60, 120 and 240 min. STAT3 phosphorylated was increased by
IL-22 in colon cancer cells up to 4 h (Fig. 3).
STAT3 is involved in IL-22-induced
PD-L1 expression
STAT3 small-molecule inhibitor Stattic was employed
to assess the role of STAT3 activation in the regulation of PD-L1
expression of colon cancer cells by IL-22. After DLD-1 cells were
pretreated with different concentrations of Stattic (0, 2.5, 5 and
10 µM) for 12 h, IL-22 (10 ng/ml) was added to the culture medium
for 60 min. At a concentration of 5.0 µM, Stattic efficiently
inhibited STAT3 phosphorylation, as shown in Fig. 4A. Thus, DLD-1 cells were stimulated
with IL-22 (10 ng/ml) for 24 h after pre-stimulation on with
Sttatic (5 µM) for 12 h. IL-22 induced upregulation of PD-L1
expression was significantly attenuated (Fig. 4B).
Discussion
Tumor occurrence is often accompanied by the failure
of the immune surveillance system, namely immune escape of tumors.
Immune checkpoints act as a central mediator of immunosuppression
in the tumor microenvironment. PD-1 is an important immune
checkpoint. PD-L1 is the principal ligand of PD-1, which is not
only expressed on immune cells but also expressed on tumor cells
(19). Therefore, the present study
examined the mRNA expression of PD-L1 in colon cancer tissues. The
expression of PD-L1 was elevated in cancer tissues, which was in
line with the previous studies (6).
It is commonly known that a number of cytokines can induce PD-L1
expression on tumor cells, especially interferon-γ (20–22). In
the present study, it was found that the mRNA expression of PD-L1
was increased and positively correlated with IL-22 expression in
colon cancer tissues. Meanwhile, experiments in vitro
confirmed that exogenous IL-22 could induce an increase in the mRNA
and protein expression level of PD-L1 in colon cancer cells. To the
best of our knowledge, the present study is the first to
investigate the effect of IL-22 on the expression of PD-L1 in colon
cancer cells.
IL-22 is a unique cytokine that is produced by
immune cells, but only acts on non-lymphoid cells, epithelial cells
in particular (23). Interestingly,
IL-22 always plays a protective role on epithelial cells regardless
of whether they have gone bad. It has been demonstrated that IL-22
modulates the expression of numerous genes that encode proteins
involved in tissue protection and the remodeling of normal colon
epithelial cell (24). Furthermore,
IL-22 facilitates the migration of immune cells to attack the
pathogen by supporting the release of metalloproteinases (25). Numerous studies have indicated that
IL-22 is involved in the occurrence and development of colon cancer
via various different pathways. IL-22 could not only promote the
proliferation, migration and invasion of colon cancer cells
(12), but also maintain colon
cancer stemness (14). PD-L1 acts as
an immunosuppressor on colon cancer cells, which prevents
surveillance and elimination by immune cells. Hence, there are
theoretical foundations to support the notion that IL-22 promotes
the expression of PD-L1, which plays a role in facilitating the
development of colon cancer. However, the direct effect of IL-22 on
immune cells is absence, which will be the focus of the future
study.
The pro-tumorigenic potential of IL-22 is mostly
mediated by STAT3, a well-established oncogene that induces the
expression of a large number of genes involved in tumor development
(26–28). STAT3 is also an important regulator
of PD-L1 expression in tumors. A previous report indicated that
fibroblast growth factor receptor 2 induces the expression of PD-L1
via the JAK/STAT3 signaling pathway in human colon cancer cells to
increase the apoptosis of Jurkat T cells (29). A recent study reported that STAT3
inhibition activates an efficient immune response by decreasing
PD-L1 expression in colon cancer cells (30). The present study also confirmed that
STAT3 inhibition impaired IL-22-induced upregulation of PD-L1
expression in colon cancer cells. Of course, the regulatory
mechanism of PD-L1 expression calls for further study.
In brief, the present findings revealed that IL-22
promoted the expression of PD-L1 in colon cancer cells by
activating the STAT3 signaling pathway, which may attenuate
anti-tumor immunity and thus promote tumor development.
Acknowledgements
The authors would like to thank Professor Fan Xiang
(Department of Gastrointestinal Surgery, Union Hospital, Tongji
Medical College, Huazhong University of Science and Technology,
Wuhan, China) for their technical assistance.
Funding
This work was supported by the National Natural
Science Foundation (grant no. 81903044), Jinan Science and
Technology Innovation Development Program (grant no. 202019083) and
the Dean Foundation of The First Affiliated Hospital of Shandong
First Medical University (grant no. QYPY2019NSFC0805).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MT and LX contributed to the conception of the
project. JC and YL contributed towards project design. Acquisition
of data was by KX and YZ. XX, RH and QW conducted the molecular
experiments. HY and ZC analyzed and interpreted the data. XX and RH
wrote the original draft of the manuscript. JC and YL reviewed and
editing the manuscript. XX and YL confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Shandong First Medical University
(approval no. 2020S003). Written informed consent was obtained from
all patients who provided the samples for the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PD-L1
|
programmed cell death ligand 1
|
|
PD-1
|
programmed cell death 1
|
|
IL-22
|
interleukin-22
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Body A, Prenen H, Latham S, Lam M,
Tipping-Smith S, Raghunath A and Segelov E: The Role of Neoadjuvant
Chemotherapy in Locally Advanced Colon Cancer. Cancer Manag Res.
13:2567–2579. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yaghoubi N, Soltani A, Ghazvini K,
Hassanian SM and Hashemy SI: PD-1/ PD-L1 blockade as a novel
treatment for colorectal cancer. Biomed Pharmacother. 110:312–318.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and function of the PD-L1 checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Juneja VR, McGuire KA, Manguso RT, LaFleur
MW, Collins N, Haining WN, Freeman GJ and Sharpe AH: PD-L1 on tumor
cells is sufficient for immune evasion in immunogenic tumors and
inhibits CD8 T cell cytotoxicity. J Exp Med. 214:895–904. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masugi Y, Nishihara R, Yang J, Mima K, da
Silva A, Shi Y, Inamura K, Cao Y, Song M, Nowak JA, et al: Tumour
CD274 (PD-L1) expression and T cells in colorectal cancer. Gut.
66:1463–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pyo JS, Ko SH, Ko YS and Kim NY:
Clinicopathological significance of PD-L1 expression in colorectal
cancer: Impact of PD-L1 expression on pFOXO1 expression. Pathol Res
Pract. 216:1527642020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, He M, Zhou Y, Yang C, Wei S, Bian X,
Christopher O and Xie L: The prognostic and clinicopathological
roles of PD-L1 expression in colorectal cancer: A systematic review
and meta-analysis. Front Pharmacol. 10:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinicrope FA, Foster NR, Thibodeau SN,
Marsoni S, Monges G, Labianca R, Kim GP, Yothers G, Allegra C,
Moore MJ, et al: DNA mismatch repair status and colon cancer
recurrence and survival in clinical trials of 5-fluorouracil-based
adjuvant therapy. J Natl Cancer Inst. 103:863–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolk K, Witte E, Witte K, Warszawska K and
Sabat R: Biology of interleukin-22. Semin Immunopathol. 32:17–31.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang R, Wang H, Deng L, Hou J, Shi R, Yao
M, Gao Y, Yao A, Wang X, Yu L and Sun B: IL-22 is related to
development of human colon cancer by activation of STAT3. BMC
Cancer. 13:592013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun D, Lin Y, Hong J, Chen H, Nagarsheth
N, Peng D, Wei S, Huang E, Fang J, Kryczek I and Zou W: Th22 cells
control colon tumorigenesis through STAT3 and Polycomb Repression
complex 2 signaling. Oncoimmunology. 5:e10827042015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kryczek I, Lin Y, Nagarsheth N, Peng D,
Zhao L, Zhao E, Vatan L, Szeliga W, Dou Y, Owens S, et al:
IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3
transcription factor activation and induction of the
methyltransferase DOT1L. Immunity. 40:772–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Xiang F, Huang Y, Shi L, Hu C, Yang
Y, Wang D, He N, Tao K, Wu K and Wang G: Interleukin-22 promotes
aerobic glycolysis associated with tumor progression via targeting
hexokinase-2 in human colon cancer cells. Oncotarget.
8:25372–25383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Crabill GA, Pritchard TS, McMiller
TL, Wei P, Pardoll DM, Pan F and Topalian SL: Mechanisms regulating
PD-L1 expression on tumor and immune cells. J Immunother Cancer.
7:3052019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seki N, Kan-O K, Matsumoto K, Fukuyama S,
Hamano S, Tonai K, Ota K, Inoue H and Nakanishi Y: Interleukin-22
attenuates double-stranded RNA-induced upregulation of PD-L1 in
airway epithelial cells via a STAT3-dependent mechanism. Biochem
Biophys Res Comm. 494:242–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eppihimer MJ, Gunn J, Freeman GJ,
Greenfield EA, Chernova T, Erickson J and Leonard JP: Expression
and regulation of the PD-L1 immunoinhibitory molecule on
microvascular endothelial cells. Microcirculation. 9:133–145. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Yang L, Huang F, Zhang Q, Liu S,
Ma L and You Z: Inflammatory cytokines IL-17 and TNF-α up-regulate
PD-L1 expression in human prostate and colon cancer cells. Immunol
Lett. 184:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian J, Wang C, Wang B, Yang J, Wang Y,
Luo F, Xu J, Zhao C, Liu R and Chu Y: The IFN-γ/PD-L1 axis between
T cells and tumor microenvironment: Hints for glioma
anti-PD-1/PD-L1 therapy. J Neuroinflammation. 15:2902018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan LC, Li CW, Xia W, Hsu JM, Lee HH, Cha
JH, Wang HL, Yang WH, Yen EY, Chang WC, et al: IL-6/JAK1 pathway
drives PD-L1 Y112 phosphorylation to promote cancer immune evasion.
J Clin Invest. 129:3324–3338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hernandez P, Gronke K and Diefenbach A: A
catch-22: Interleukin-22 and cancer. Eur J Immunol. 48:15–31. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wolk K, Witte E, Wallace E, Döcke WD, Kunz
S, Asadullah K, Volk HD, Sterry W and Sabat R: IL-22 regulates the
expression of genes responsible for antimicrobial defense, cellular
differentiation, and mobility in keratinocytes: A potential role in
psoriasis. Eur J Immunol. 36:1309–1323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eyerich S, Eyerich K, Pennino D, Carbone
T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann
C, Behrendt H, et al: Th22 cells represent a distinct human T cell
subset involved in epidermal immunity and remodeling. J Clin
Invest. 119:3573–3585. 2009.PubMed/NCBI
|
|
26
|
Galoczova M, Coates P and Vojtesek B:
STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett.
23:122018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fathi N, Rashidi G, Khodadadi A, Shahi S
and Sharifi S: STAT3 and apoptosis challenges in cancer. Int J Biol
Macromol. 117:993–1001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li P, Huang T and Zou Q: FGFR2 promotes
expression of PD-L1 in colorectal cancer via the JAK/STAT3
signaling pathway. J Immunol. 202:3065–3075. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jahangiri A and Dadmanesh M: STAT3
inhibition reduced PD-L1 expression and enhanced antitumor immune
responses. J Cell Physiol. 235:9457–9463. 2020. View Article : Google Scholar : PubMed/NCBI
|