Introduction
Ovarian cancer is a malignant carcinoma that is
usually diagnosed at a late stage, by which time metastasis can be
observed at distant sites, including the lungs, liver and lymph
(1,2). Ovarian cancer usually develops from
the ovarian surface epithelium or from serous intra-epithelial
carcinoma (3). With the
advancement of medical technology, debulking surgery and
cis-platinum-based chemotherapy are the major therapeutics for
ovarian cancer. However, the outcome of debulking surgery is
dependent on surgical skill and the genetic features of the tumor
(4,5). Debulking surgery combined with
cis-platinum based chemotherapy can lead to remission in patients
with ovarian cancer; however, the majority of patients suffer from
cancer resistance, metastasis and relapse (6,7).
Following relapse, the outcome of chemotherapy declines
significantly, with rapid disease progression and drug resistance.
Furthermore, genetic alterations in cancer cells, which are widely
investigated for drug resistance and cancer metastasis, result in
drug inactivation, enhanced DNA repair mechanisms and changes in
the intracellular pathways (8).
However, cancer metastasis and relapse remain important obstacles
for ovarian cancer therapy, and novel and efficient therapeutics
for ovarian cancer are urgently required.
Epithelial-mesenchymal transition (EMT) plays key
roles in cancer proliferation and distant metastasis (9,10).
Various signaling pathways have been associated with extracellular
cues, such as TGF-β and play important roles in reprogramming gene
expression during EMT (11,12).
Furthermore, cancer stem cells (CSCs), which are characterized by
the markers, CD133 (also known as prominin-1) and octamer binding
transcription factor 4 (OCT4), exhibit a
CD44+/CD24−/low phenotype (13) and self-renewal ability. They also
constitute a minor proportion of neoplastic cells in the tumor
microenvironment, which are usually regarded as the
tumor-initiating cells (TICs) (14,15).
The proportion of TICs in tumors is an important source of
metastatic lesions in breast cancer. Current evidence has revealed
that cells, which undergo EMT, exhibit stem cell-resembling
characteristics (16,17). For example, Mani et al
(16) demonstrated that stem-like
cells isolated from either mouse or human mammary glands or mammary
carcinomas express EMT markers, illustrating an association between
EMT and the gain of epithelial stem cell properties. Wang et
al (18) revealed that
fusobacterium nucleatum produced CSC characteristics by activating
IL-6/STAT3 and eliciting EMT-resembling activation. Furthermore,
EMT induced cancer cell mesenchymal characteristics and promoted
cancer cells to gain stemness (19,20).
In addition, EMT induces invasion and dissemination of tumor cells,
and also assists CSCs to invade to distant organs, leading to
cancer distant metastasis (21,22).
Numerous studies have demonstrated that poor outcomes of ovarian
cancer therapies, i.e., metastasis and relapse, are usually due to
a small proportion of CSCs that have escaped from the primary
cancer lesion (23,24). Thus, CSC-target therapy is a
promising strategy to conquer ovarian cancer metastasis and
relapse.
Traditional Chinese Medicine has important roles and
has shown good therapeutic efficiency in the prevention of various
diseases. In recent years, numerous bioactive small molecular drugs
extracted from herbal medicine have been widely investigated in
diseases, such as cancer (25–28).
Emodin, which can be isolated from several Chinese herbs, including
Rheum palmatum L and Poly gonum cuspidatum, has been
reported to possess numerous bioactivities in regulating resistance
to oxidation and blood glucose levels, in addition to having
anti-bacterial properties (29–31).
The use of emodin in Traditional Chinese Medicine is ‘attack
stagnation, clear heat and dampness, purge fire, cool blood, remove
blood stasis and detoxification’ (32). In addition, according to recent
studies, emodin significantly inhibited colon cancer, prostate
cancer, breast cancer and skin cancer cells by triggering cancer
cell apoptosis (29,33,34).
However, the anti-proliferation and anti-metastasis effect of
emodin on ovarian cancer has been rarely studied. Therefore, the
antitumor effect of emodin in vitro and in vivo was
investigated in the present study. In addition, the corresponding
mechanism was also analyzed to provide evidence for the therapeutic
application of emodin in ovarian cancer.
Materials and methods
Cell lines and cell culture
The human ovarian cancer cell lines, SK-OV-3 and
A2780 were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.), and PA-1 were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.), containing 10% (FBS) (Gibco; Thermo Fisher
Scientific, Inc.), were purchased from American Type Culture
Collection. All the cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2. Emodin was purchased from
Sigma-Aldrich (Merck KGaA), dissolved in dimethyl sulfoxide (DMSO)
and stored at −20°C for further use. The antibodies against
N-cadherin (cat. no. 13116; 1:1,000), E-cadherin (cat. no. 14472,
dilution, 1:1,000), vimentin (cat. no. 5741; 1:1,000), CD133 (cat.
no. 64326; 1:1,000), OCT4 (cat. no. 2840; 1:1,000) and β-actin
(cat. no. 3700; 1:1,000) were purchased from Cell Signaling
Technology, Inc.
Cell proliferation assay
Briefly, suspensions of the SK-OV-3, A2780 and PA-1
cell lines (2,000-5,000 cells/100 µl) were added to 96-well
microplates. Approximately 12 h later, the cells were treated with
different concentrations (0, 2.5, 5, 10, 20, 40 and 80 µM) of
emodin for 24, 48 or 72 h. Then, the cell culture medium in each
well was replaced with 20 µl MTT solution (5 mg/ml) and the samples
were incubated at 37°C for a further 2-4 h. Subsequently, 150 µl
DMSO was added to each well to dissolve the formazan crystal
produced by the living cells. Finally, the optical density of each
well was measured using a Spectra MAX M5 microplate
spectrophotometer (Molecular Devices, LLC) at 570 nm. At least
three independent experiments were performed.
Colony formation assay
The clonogenic ability of the ovarian cancer cell
lines was assessed using the colony formation assay following
treatment with emodin. Briefly, the ovarian cancer cell lines
(400–600 cells/well) were seeded in 6-well plates and cultured for
a further 12 h. The cells were treated to various doses of emodin
(0, 5, 10, 20 and 40 µM) and cultured for a further 12 days. The
cell culture medium was replaced with fresh medium containing
corresponding concentrations of emodin every 3 days. Subsequently,
the culture medium was discarded, and the cell colonies were washed
three times with PBS, fixed with methanol for 15 min at 25°C and
stained with 0.5% crystal violet for 20 min at 25°C. Finally, the
number of cell colonies (>30 cells) was counted manually and
images were captured using an inverted microscope (Axiovert 200;
Zeiss AG).
Wound healing assay
The ovarian cancer cell lines were seeded in 6-well
plates and cultured for 12 h. When the monolayer of the cells
reached 80% confluence, the wound was created using a sterile 200
µl micropipette tip. Then, the cells were washed with fresh medium
and cultured in medium (with 0.5% FBS) and different concentrations
of emodin (0, 10, 20 and 40 µM) for 48 h. Next, the cell culture
medium was discarded, the cells were washed three times with PBS,
then images were captured using a light microscope (Axiovert 200;
Zeiss AG). The migration rates of the treated cells were quantified
using the following equation: Migration
rate=(1-W0/Wemodin)/(1-W0/Wctrl),
where W0 represents the width of the wound at 0 h;
Wctrl represents the width of the wound at 48 h in the
control group and Wemodin represents the width of the
wound at 48 h in emodin treatment group. The migration rate of the
control cells was regarded as 100%.
Transwell and Matrigel assays
For the analysis of the migratory effect of emodin,
1×105 SK-OV-3, A2780 and PA-1 cells, suspended in 100 µl
serum-free medium, were added to the upper chamber, while 600 µl
complete medium, containing 10% FBS, was added to the bottom
chamber. Different concentrations of emodin (0, 10, 20 and 40 µM)
were added to medium in the upper chambers. After migration for ~48
h at 25°C, non-migratory cells remaining in the upper chamber were
discarded using a cotton swab and the migrated cells located on the
bottom of membrane were washed, fixed with methanol for 15 min at
25°C, then stained with 0.5% crystal violet for 20 min at 25°C.
Images of the migrated cells were captured using a light microscope
and five random fields of view were counted to compare the
migration rate.
A Matrigel assay was performed to determine the
invasion ability of the cells treated with emodin. Briefly, the
upper surface of a 24-well Transwell plate (MilliporeSigma) was
pre-coated with ~70 µl Matrigel (BD Biosciences) diluted (1:4) in
serum-free cell culture medium. After Matrigel polymerization for 1
h at 37°C, 100 µl serum-free medium, containing 1×105
SK-OV-3, A2780 and PA-1 cells was added to upper chamber, while 600
µl the complete medium was added to the lower chamber, which served
as a chemoattractant. Different concentrations of emodin (0, 10, 20
and 40 µM) were added to both the upper and lower chambers. After
invasion for 48 h, the non-invasive cells remaining in the upper
chamber were discarded using a cotton swab and the invasive cells
on the bottom chamber were washed, fixed with methanol for 15 min
at 25°C, then stained with 0.5% crystal violet or 20 min at 25°C.
The images of the invasive cells were captured using a light
microscope and five random fields of view were selected to
calculate the invasive rate.
Western blot analysis
To investigate the effect of emodin on relevant
signaling pathways, the changes in the protein expression level of
key proteins in the SK-OV-3, A2780 and PA-1 cell lines were
evaluated using western blot analysis. After treatment with the
indicated concentrations of emodin (0, 10, 20 and 40 µM) for 48 h,
the SK-OV-3, A2780 and PA-1 cell lines were harvested and lysed
with RIPA buffer (Beyotime Institute of Biotechnology) to obtain
the total protein. Concentration of total protein was determined by
BCA method. An equal amount of protein (~30 ug), from the
differently treated samples were separated using SDS-PAGE (10%),
then transferred onto PVDF membranes (Amersham Bioscience; Cytiva).
The membranes were blocked with skimmed milk (5% in TBST buffer
(Tween-20, 0.5%) for 1 h at 37°C and incubated with the primary
antibodies overnight at 4°C, then incubated with the corresponding
secondary antibodies (horseradish peroxidase-conjugated goat
Anti-Rabbit IgG H&L; cat. No.: ab205718, dilution: 1:10,000,
Abcam) at 37°C for 2 h. Immunoreactive protein bands were
visualized and detected using an enhanced chemiluminescence kit
(MilliporeSigma). The β-actin antibody was used as a loading
control. Protein expression levels were determined by ImageJ (v
1.8.0, National Institutes of Health).
Mouse and tumor model
All the animal experiments were approved by the
Institutional Animal Care and Treatment Committee of Gannan Medical
University (Jiangxi, China) and were conducted according to the
approved guidelines. All mice (Balb/c nude; female; 6-8 weeks old;
weight, 18-20 g) used in the experiments were purchased from
Beijing HFK Bioscience Co. Ltd. The mice were kept in a
specific-pathogen-free condition facility, in an air-conditioned
room at 25±2°C, with a relative humidity of 40-70%, and a 12-h
light/dark cycle, with free access to food and water. A total of 18
female mice (6–8 weeks old; weight, 18-20 g) were subcutaneously
injected with 100 µl suspension (normal saline) of SK-OV-3 cells
(0.5×107 cells per mouse). Several days later, the
injected site (right flank) a successful mouse tumor model was
established if there were noticeable signs of growth. When the
tumor volume reached ~100 mm3, the mice were randomly
divided into three groups (vehicle, and 20 and 40 mg/kg emodin;
n=6). The mice were intraperitoneally administrated with 20 and 40
mg/kg emodin or vehicle once daily. The tumor volume and body
weight of the mice was measured and recorded every 3 days during
the treatment process, and the tumor volume was calculated as
follows: Volume (mm3)=LxW2/2, where L (mm)
represents the length of the tumor and W (mm) represents the width
of the tumor. All the mice were sacrificed with CO2 (70%
volume displacement rate) at the endpoint of the experiment, which
was defined by the tumor volume (~1,500 mm3) in the
control group. Tumor inhibitory rate
(%)=(V(emodin)-V(vehicle))/V(vehicle), where V represents tumor
volume. The tumors from each group were collected, weighted and
fixed with 4% paraformaldehyde for 48 h at 25°C for further
evaluation. In addition, the major organs (heart, liver, spleen,
the lungs and the kidneys) from the mice in each group were
collected, fixed in 4% paraformaldehyde for 24 h at 25°C, then
sectioned (3–5 µm) for hematoxylin and eosin staining. The sections
were stained with hematoxylin (1%) and eosin (1%) at 25°C for 2-5
min. Finally, sections were photographed by light microscope.
Immunohistochemistry (IHC)
The collected tumor tissue samples were fixed in 4%
paraformaldehyde for 24 h at 25°C, embedded in paraffin and sliced
into thin sections (3–5 µm). After dewaxing and rehydration, the
sections were incubated with 3% hydrogen peroxide at 25°C for 30
min to block the endogenous peroxidase activity, then treated with
5% bovine serum albumin (Thermo Fisher Scientific, Inc.) at 37°C
for 30 min to block non-specific binding. Subsequently, the
sections were incubated with primary antibodies against Ki67 (cat.
no. ab15580: 1:500), CD133 (cat. no. ab278053; 1:1,000), and
cleaved caspase3 (cat. no. ab32042; 1:1,000; all Abcam) overnight
at 4°C. The sections were washed with PBS 3 or 4 times, incubated
with the biotinylated secondary antibody (Abcam, ab64256, dilution:
1:1,000) at 37°C for 1 h, then treated with streptavidin
horseradish peroxidase at 37°C for 20-30 min. Images were captured
under a light microscope (Axiovert 200; Zeiss AG). Positive stained
cells were calculated by Image J (V1.8.0, National Institutes of
Health) from the average of four random fields of view.
Pulmonary metastasis tumor mouse
model
A total of 18 mice were intravenously injected with
a 100 µl suspension SK-OV-3 cells in saline (1×106 cells
per mouse) via the tail vein to produce an experimental lung
metastasis tumor mouse model. The mice were randomly divided into
three groups (vehicle, and 20 and 40 mg/kg emodin groups; n=6) 2
days following injection and were intraperitoneally injected with
20 or 40 mg/kg emodin, or vehicle once daily. The mice from the
experimental and control groups were euthanized on day 18, and
their lung tissues were isolated, weighted and collected for
observation of visible metastatic nodules. The dots on the lung
surface were counted manually and confirmed as ovarian cancer
metastases.
At the study endpoint, the lung tissues from each
group were collected and prepared as single-cell suspensions using
mechanic dispersion with surgical scissors and enzymatic method of
I-collagenase (1 mg/ml). Then, 1×106 freshly prepared
cells were suspended in 100 µl PBS and stained with different
combinations of FITC-CD24 (Biolegend, cat. no. 311103) and APC-CD44
(Biolegend, cat. no. 103011). The cells were analyzed using flow
cytometry (FACS Canto II, BD Biosciences) and the data were
analyzed using FlowJo software (FlowJo LLC, V7.6.1). The
proportions of CD44+/CD24− cells were
considered to be ovarian CSCs.
Statistical analysis
The data are presented as the mean ± standard
deviation of at least of three independent experiments. SPSS v16.0
(SPSS, Inc.) software was used to perform the statistical analyses.
One-way ANOVA followed by Dunnett's or Tukey's post hoc test was
used for multi-group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Emodin inhibits the viability and
proliferation of ovarian cancer cell lines
The SK-OV-3, A2780 and PA-1 cell lines were used to
evaluate the antitumor effect of emodin on ovarian cancer. Firstly,
the time- and dose-dependent effect of emodin on these cells was
performed by treating the cells with different concentrations of
emodin (0, 2.5, 5, 10, 20, 40 and 80 µM) for various time points
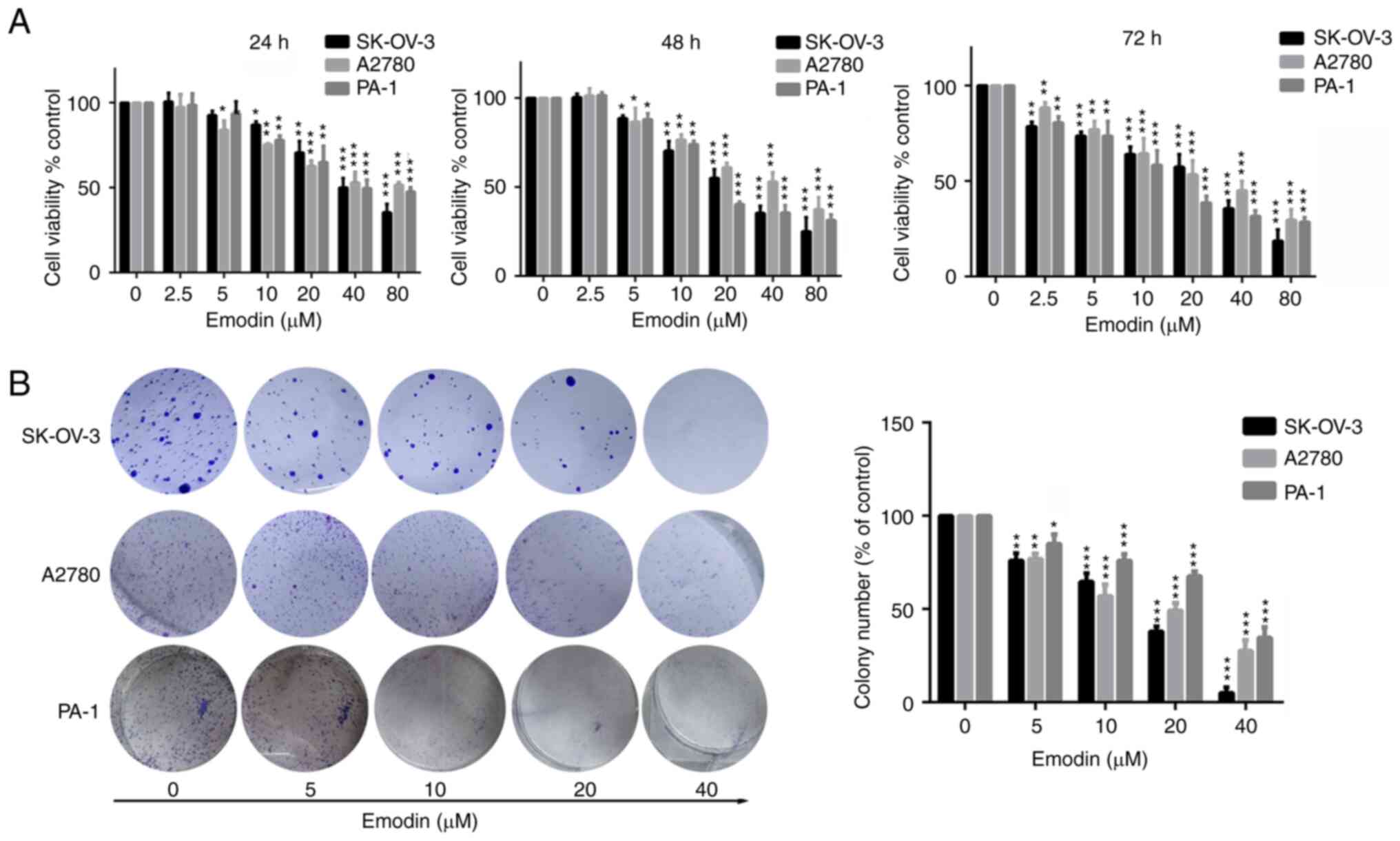
(24, 48 and 72 h). As shown in Fig.
1A, the SK-OV-3, A2780, and PA-1 cell lines treated with 10-20
µM emodin for 24 h exhibited weakened viability (P<0.05).
However, after treatment for 48 and 72 h, cell viability was
significantly (P<0.01) suppressed. In addition, treatment with
40 and 80 µM emodin significantly reduced the viability of the
SK-OV-3, A2780 and PA-1 cell lines at 24, 48 and 72 h. This
suggests that emodin exhibited a time- and dose-dependent (>2.5
µM emodin) effect in ovarian cancer cell lines. To further
investigate whether emodin could suppress the proliferation ability
of the ovarian cancer cell lines, a colony formation assay was
performed after the cells were treated with emodin. As shown in
Fig. 1B, the number of the
colonies in the SK-OV-3, A2780 and PA-1 cell lines treated with
emodin was significantly reduced in a dose-dependent manner. In
addition, emodin significantly inhibited the number of colonies in
the SK-OV-3, A2780 and PA-1 cell lines in comparison to the control
group. Taken together, these results suggest that emodin
significantly inhibited cell viability and colony formation in the
ovarian cancer cell lines.
Emodin inhibits ovarian cancer cell
migration and invasion by affecting EMT
A wound healing assay was performed using the
SK-OV-3, A2780 and PA-1 cell lines to investigate the in
vitro anti-migration ability of emodin in ovarian cancer cells.
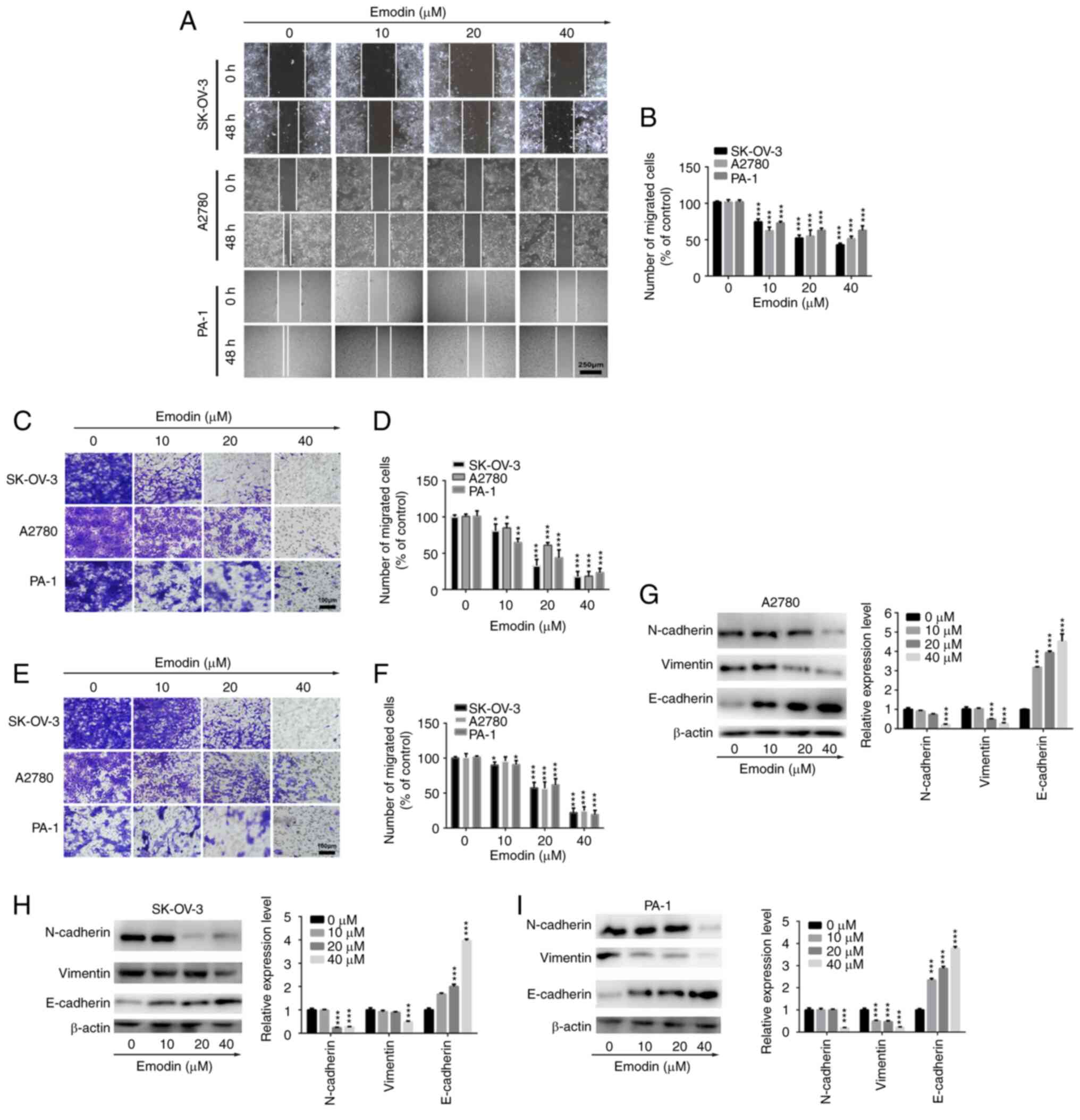
As displayed in Fig. 2A and B,
emodin significantly inhibited the wound healing rate of the
SK-OV-3 and A2780 cell lines in a dose-dependent manner, suggesting
an anti-migratory effect of emodin in ovarian cancer cells. In
addition, Transwell and Matrigel assays were performed to further
investigate the anti-migratory and anti-invasive abilities of
emodin in ovarian cancer cells. The results demonstrated that the
migration abilities of the SK-OV-3, A2780 and PA-1 cell lines were
significantly suppressed in the presence of emodin compared with
that in the control group (Fig. 2C and
D). As indicated in Fig. 2E and
F, treatment with different concentrations of emodin distinctly
inhibited the invasive abilities of the SK-OV-3, A2780 and PA-1
cell lines. Furthermore, as there is an association between cancer
cell migration and invasion, and EMT (35), it was investigated whether emodin
inhibited the migration and invasion abilities of the ovarian
cancer cells via EMT. As demonstrated in Fig. 2G-I, the protein expression levels
of N-cadherin and vimentin were decreased in the SK-OV-3, A2780 and
PA-1 cell lines following treatment with emodin, whereas the
protein expression levels of E-cadherin were increased, indicating
EMT was affected in the ovarian cancer cell lines. Taken together,
these results suggest that emodin suppressed the migration and
invasion abilities of the ovarian cancer cells by affecting
EMT.
Emodin significantly inhibits tumor
growth in a xenograft model of human ovarian cancer
To investigate whether the antitumor activity of
emodin in vivo was consistent with its anti-proliferation
effect in vitro, SK-OV-3 tumor-bearing mice were treated
with different doses of emodin (20 and 40 mg/kg) for 18 days
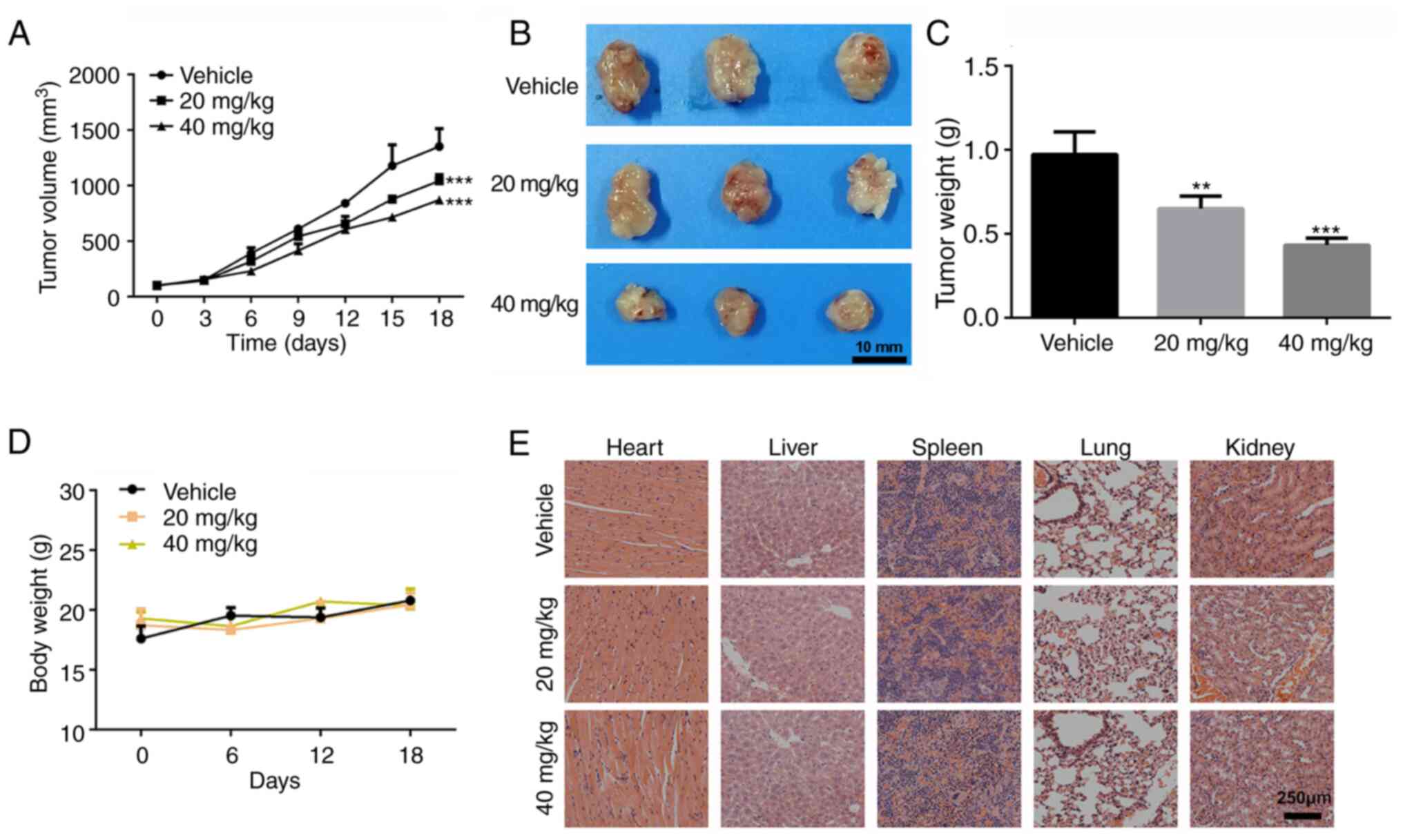
following injection of the cells. As shown in Fig. 3A, tumor growth was significantly
reduced following treatment with 20 and 40 mg/kg emodin compared
with that in the control group. Mice were sacrificed at the study
endpoint, and tumors from each group were isolated. The tumor size
and average weight in the 20 and 40 mg/kg treatment groups were
notably smaller compared with that in the control group (Fig. 3B and C). Furthermore, no adverse
effects, such as toxic death, skin ulceration and body weight loss
were observed, during the treatment of the SK-OV-3 tumor-bearing
mice with emodin. The body weight of the mice was not significantly
different between the three treatment groups (Fig. 3D). In addition, after pathological
evaluation of the major organs (heart, liver, spleen, the lungs and
the kidneys), no notable pathological changes were found in the
emodin treatment groups, suggesting that emodin is safe to use
in vivo (Fig. 3E).
The antitumor effect of emodin was also analyzed
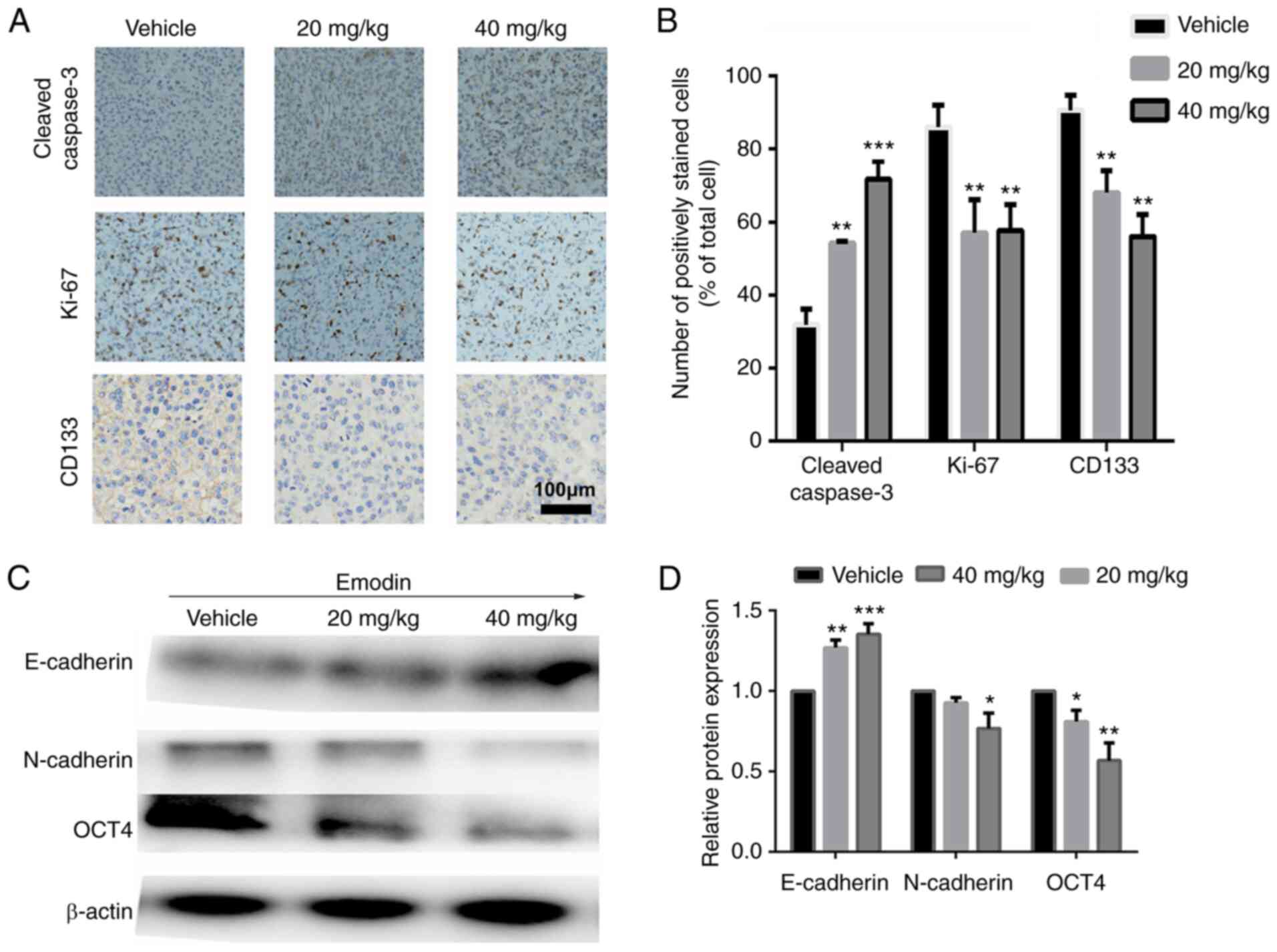
using IHC from the tumor sections of each group. As displayed in
Fig. 4A and B, increased
expression of cleaved-caspase 3 and decreased number of Ki-67
positive cells was observed in the emodin treatment groups.
Furthermore, compared with that in the control group, CD133, a key
marker of ovarian CSCs (36), was
significantly downregulated, suggesting a reduction in the
development of ovarian CSCs following treatment with emodin.
Subsequently, western blot analysis was used to
verify the anti-CSC effect of emodin. As demonstrated in Fig. 4C and D, the protein expression
level of E-cadherin was increased following treatment with emodin,
whereas the expression level of N-cadherin was decreased,
suggesting that emodin inhibited EMT. In addition, the stemness of
the tumors in the emodin treatment groups was significantly
suppressed, as evidenced by the decrease in protein expression
level of OCT4, which has been validated as a master regulator in
the maintenance of the cancer stem-like phenotype (37).
Emodin reduces pulmonary metastasis of
ovarian cancer cells by killing cancer stem cells
A pulmonary metastasis mouse model was established
using SK-OV-3 cells to evaluate the anti-metastasis properties of
emodin. Following treatment with emodin for 18 days, the mice were
sacrificed, the weight of the lung tissues was measured, and the
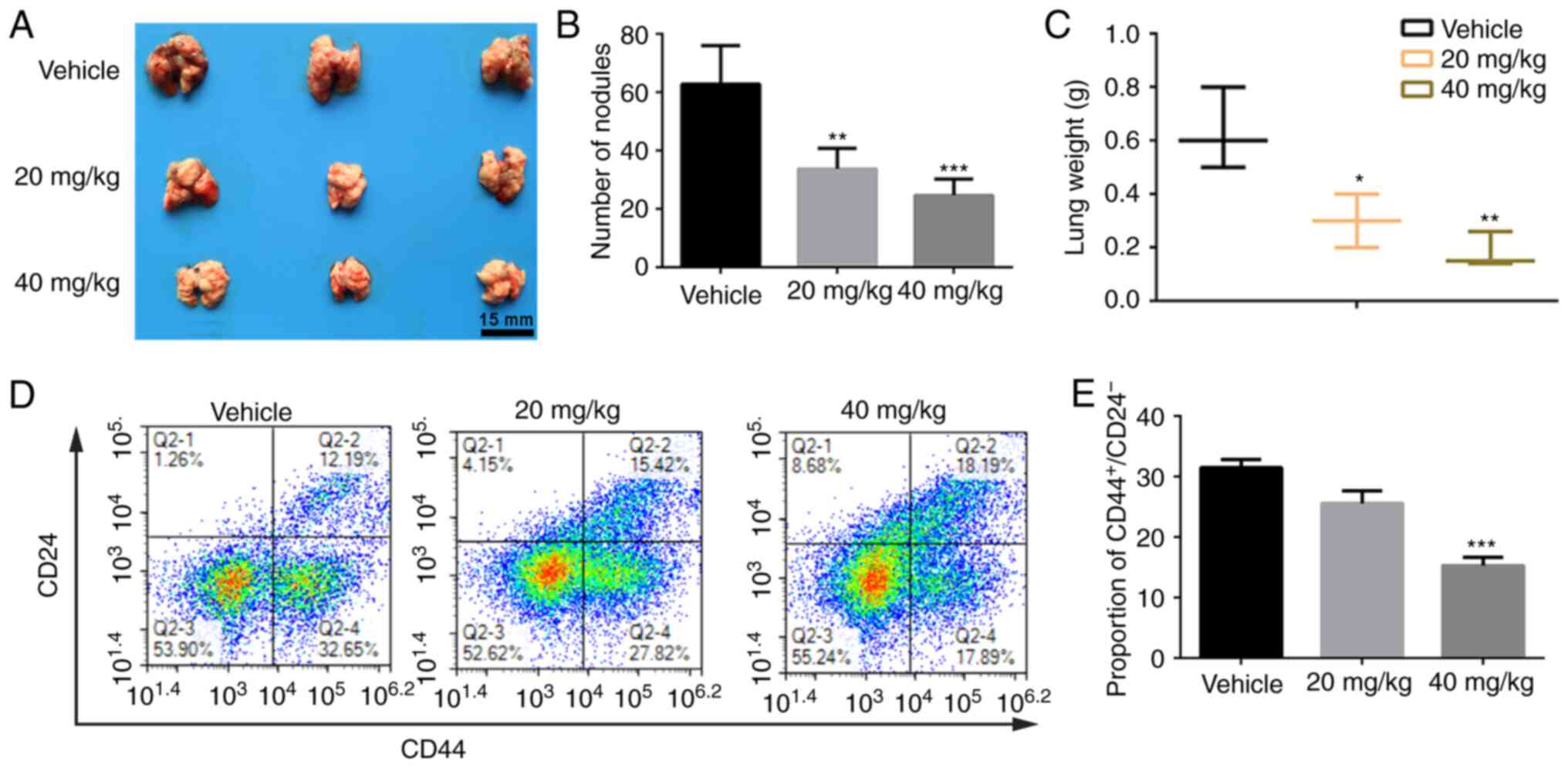
number of metastatic lung nodules were counted. As indicated in
Fig. 5A and B, compared with that
in the control group, the number of metastatic nodules on the lung
was markedly reduced in the emodin treatment groups. In addition,
the weight of the lungs in the emodin treatment groups was
significantly inhibited compared with that in the control
group.
It is reported that poor survival and distant
metastasis in patients with ovarian cancer are caused by the
renewal of CSCs, and increased CSCs have been associated with tumor
recurrence or relapse, distant metastasis and chemoresistance
(38,39). As aforementioned, the protein
expression of the CSC markers, CD133 and OCT4 was significantly
decreased in the emodin treatment groups. Various studies have
indicated that the CD44+/CD24− population may
represent stem cell-like properties of ovarian cancer cells
(40–42). To further evaluate whether emodin
inhibited lung metastasis of ovarian cancer cells by destruction of
the ovarian CSCs, flow cytometry was used to measure the proportion
of ovarian CSCs (CD44+/CD24−) in metastatic
lung tissues following emodin treatment for 18 days. As shown in
Fig. 5D and E, the percentage of
CD44+/CD24− cells was 32.65±2.95% in the
control group, while treatment with 20 and 40 mg/kg emodin
significantly reduced the proportion of
CD44+/CD24− cells to 27.82±3.21 and
17.89±2.76%, respectively (P<0.001), indicating that emodin
reduces pulmonary metastasis of ovarian cancer cells by killing
cancer stem cells.
Discussion
Ovarian cancer has the worst prognosis among all
types of gynecological malignancies and patients are often only
diagnosed at an advanced stage (1). Even though chemotherapy combined with
surgery can result in remission in ovarian cancer patients,
resistance and distant metastasis remain an obstacle for ovarian
cancer therapy (43). Once the
patient has relapsed, the outcome of chemotherapy declines
significantly, with rapid tumor progression and drug resistance
(44). Therefore, the discovery of
novel potential drug candidates to prevent tumor metastasis is
urgently required.
CSCs have a key role in tumor initiation, invasion,
metastasis and therapeutic resistance, as well as in local
recurrence following curative resection (45). Therefore, the elimination of CSCs
in patients with ovarian cancer is considered to represent an
effective strategy for the treatment of this highly refractory
malignancy. Furthermore, EMT is an important component of cancer
proliferation and distant metastasis (46). The crosstalk between CSC and EMT
has been shown to increase cancer cell mesenchymal characteristics
on the CSCs and promote cancer cells to gain stemness (19,20).
However, there are currently limited agents that preferentially
inhibit ovarian CSCs by regulating EMT.
Emodin, which is derived from natural plants, has
been reported to exhibit therapeutic effects in several diseases
such as hyperlipidemia, anti-viral and anti-liver fibrosis
(47–49). Various in vitro studies have
demonstrated its effectiveness on the promotion of apoptosis or the
inhibition of proliferation in lung, breast and cervical cancer
cells (50–52). In the present study, it was found
that emodin could reduce the viability of ovarian cancer cells at
low concentrations. The anti-proliferation activity of emodin
against ovarian cancer cells was verified with MTT and colony
formation assays.
Emodin was also found to exhibit an inhibitory
effect on migration and invasion in ovarian cancer cells by
inhibiting EMT, as evidenced by the decrease in the protein
expression level of N-cadherin and vimentin, and the increase in
the protein expression level of E-cadherin following treatment with
emodin. It has previously been reported that emodin inhibited
pancreatic cancer EMT and invasion by increase the expression level
of microRNA-1271 (53). It has
been demonstrated that emodin inhibited colon cancer cell invasion
and migration by suppressing EMT via the WNT/β-catenin pathway
(54). Emodin could also suppress
the proliferation and invasion of colorectal cancer cells by
inhibiting VEGFR2 protein expression (55). Therefore, the antitumor effects of
emodin were analyzed using a subcutaneous xenograft SK-OV-3 tumor
mouse model. The results of the animal experiments suggested that
the tumor growth rate and tumor weight were significantly inhibited
by the administration of emodin (40 mg/kg), with an inhibitory rate
of ~45%. The effects of emodin (20 mg/kg) on tumor growth rate and
tumor weight were lower than that of 40 mg/kg. Mechanistic analysis
demonstrated that emodin reduced the proliferative ability of the
tumors and decreased EMT. In addition, decreased protein expression
levels of CD133 and Oct4 were observed in the tumor tissues
following treatment with emodin, which indicates that emodin could
inhibit the stemness of ovarian cancer cells. Various studies have
demonstrated that inhibition of EMT results in impairment of
stemness during the reprogramming of somatic cells (56,57).
Liu et al (51) reported
that emodin reduced breast cancer lung metastasis by suppressing
macrophage-induced breast cancer cell EMT and cancer stem cell
formation. It has also been reported that fusobacterium nucleatum
produced cancer stem cell characteristics by activating IL-6/STAT3
and eliciting EMT-resembling activation (18). However, there is not enough
evidence to demonstrate that downregulation of CD133 and OCT4 could
directly lead to impairment of stemness of tumor cells. CD133 and
OCT4 are only markers of tumor stem cells and emodin was only found
to markedly inhibit EMT activity and impair the stemness of tumor
cells from the decrease in the expression level of CD133 and Oct4.
In future experiments, CD133 and Oct4 expression will be knocked
down or overexpressed using transfection with small inhibiting
RNA/overexpression plasmid to further investigate the function of
CD133 and Oct4 in the formation of cancer stem cells.
Ovarian cancer, which starts as a local tumor lesion
can metastasize to distant organs, including the lymph nodes, the
lungs and the breasts (58). The
metastatic process of cancer cells from the primary tumor to the
distant site is complex, and requires migration from local lesions
to blood vessels (59). As emodin
reduced the migratory and invasion ability of the ovarian cancer
cell lines, the anti-metastasis effect of emodin was investigated
using a pulmonary metastasis tumor mouse model. The results showed
that administration of emodin, at 20 and 40 mg/kg, significantly
inhibited lung metastasis of ovarian cancer, which was consistent
with the in vitro experiments. Furthermore, poor outcomes of
ovarian cancer therapies are usually caused by a small proportion
of CSCs, that have escaped from the primary cancer lesion (60). Thus, elimination of CSCs in the
tumor microenvironment could be a promising strategy to conquer
ovarian cancer metastasis. In the present study, it was
demonstrated that emodin could reduce the proportion of ovarian CSC
in metastatic lung tissues, suggesting that emodin suppressed
pulmonary metastasis of ovarian cancer.
In summary, the present study provided important
information regarding the antitumor activities of emodin in ovarian
cancer. Emodin exhibited antitumor and anti-metastasis effects on
ovarian cancer by inhibiting EMT and ovarian CSC formation. The
results suggest that emodin could serve as a potential drug for
treating ovarian cancer and metastasis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HML and HYC conceived and designed the study. HML
acquired and analyzed the data. HML, HMC and JY interpreted the
data and wrote the manuscript. HML and HYC confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All the animal experiments in this study were
performed according to the National Institutes of Health guidelines
and were approved by the Institutional Animal Care and Use
Committee of Gannan Medical University (Jiangxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kleppe M, Wang T, Van Gorp T, Slangen BF,
Kruse AJ and Kruitwagen RF: Lymph node metastasis in stages I and
II ovarian cancer: A review. Gynecol Oncol. 123:610–614. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pakneshan S, Safarpour D, Tavassoli F and
Jabbari B: Brain metastasis from ovarian cancer: A systematic
review. J Neurooncol. 119:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottevanger PB: Ovarian cancer stem cells
more questions than answers. Semin Cancer Biol. 44:67–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crosbie EJ, Flaum N, Harkness EF, Clayton
RD, Holland C, Martin-Hirsch P, Wood N, Keating P, Woodward ER,
Lalloo F, et al: Specialist oncological surgery for removal of the
ovaries and fallopian tubes in BRCA1 and BRCA2 pathogenic variant
carriers may reduce primary peritoneal cancer risk to very low
levels. Int J Cancer. 148:1155–1163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng H and Gao YN: Primary debulking
surgery or neoadjuvant chemotherapy followed by interval debulking
surgery for patients with advanced ovarian cancer. Chin J Cancer
Res. 24:304–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ducoulombier S, Golfier F, Colomban O,
Benayoun D, Bolze PA, Tod M and You B: Modeling CA-125 during
neoadjuvant chemotherapy for predicting optimal cytoreduction and
relapse risk in ovarian cancer. Anticancer Res. 37:6879–6886.
2017.PubMed/NCBI
|
|
7
|
Wang Y, Herrstedt J, Havsteen H, DePoint
Christensen R, Mirza MR, Lund B, Maenpaa J and Kristensen G: A
multicenter, non-randomized, phase II study of docetaxel and
carboplatin administered every 3 weeks as second line chemotherapy
in patients with first relapse of platinum sensitive epithelial
ovarian, peritoneal or fallopian tube cancer. BMC Cancer.
14:9372014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Chen F, Xu Q, Han L, Xu J, Gao L,
Sun X, Li Y, Li Y, Qian M and Sun Y: Revisiting ovarian cancer
microenvironment: A friend or a foe? Protein Cell. 9:674–692. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo Q, Wu T, Wu W, Chen G, Luo X, Jiang L,
Tao H, Rong M, Kang S and Deng M: The functional role of
voltage-gated sodium channel Nav1.5 in metastatic breast cancer.
Front Pharmacol. 11:11112020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Wang J, Yan H, Zhang K and Liu Y:
Upregulation of USP11 promotes epithelialtomesenchymal transition
by deubiquitinating Snail in ovarian cancer. Oncol Rep.
41:1739–1748. 2019.PubMed/NCBI
|
|
11
|
Dai G, Sun B, Gong T, Pan Z, Meng Q and Ju
W: Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of
colorectal cancer cells by suppressing TGF-beta/Smad signaling.
Phytomedicine. 56:126–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oh E, Hong J and Yun CO: Regulatory T
cells induce metastasis by increasing Tgf-β and enhancing the
epithelial-mesenchymal transition. Cells. 8:13872019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Schaijik B, Davis PF, Wickremesekera
AC, Tan ST and Itinteang T: Subcellular localisation of the stem
cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: A review.
J Clin Pathol. 71:88–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu W, Lei C, Yu Y, Liu S, Li T, Lin F, Fan
X, Shen Y, Ding M, Tang Y, et al: EGFR/Notch antagonists enhance
the response to inhibitors of the PI3K-Akt pathway by decreasing
tumor-initiating cell frequency. Clin Cancer Res. 25:2835–2847.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu S, Fu W, Li T, Yuan Q, Wang F, Lv G, Lv
Y, Fan X, Shen Y, Lin F, et al: Antagonism of EGFR and Notch limits
resistance to EGFR inhibitors and radiation by decreasing
tumor-initiating cell frequency. Sci Transl Med. 9:eaag03392017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morel AP, Lievre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Yu C, Yue C and Liu X:
Fusobacterium nucleatum produces cancer stem cell characteristics
via EMT-resembling variations. Int J Clin Exp Pathol. 13:1819–1828.
2020.PubMed/NCBI
|
|
19
|
Čipak Gašparović A, Milković L, Dandachi
N, Stanzer S, Pezdirc I, Vrančić J, Šitić S, Suppan C and Balic M:
Chronic oxidative stress promotes molecular changes associated with
epithelial mesenchymal transition, NRF2, and breast cancer stem
cell phenotype. Antioxidants (Basel). 8:6332019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Navas T, Kinders RJ, Lawrence SM,
Ferry-Galow KV, Borgel S, Hollingshead MG, Srivastava AK, Alcoser
SY, Makhlouf HR, Chuaqui R, et al: Clinical evolution of
epithelial-mesenchymal transition in human carcinomas. Cancer Res.
80:304–318. 2020.PubMed/NCBI
|
|
21
|
Hoca M, Becer E, Kabadayi H, Yucecan S and
Vatansever HS: The effect of resveratrol and quercetin on
epithelial-mesenchymal transition in pancreatic cancer stem cell.
Nutr Cancer. 72:1231–1242. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou JM, Hu SQ, Jiang H, Chen YL, Feng JH,
Chen ZQ and Wen KM: OCT4B1 promoted EMT and regulated the
self-renewal of CSCs in CRC: Effects associated with the balance of
miR-8064/PLK1. Mol Ther Oncolytics. 15:7–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kenda Suster N and Virant-Klun I: Presence
and role of stem cells in ovarian cancer. World J Stem Cells.
11:383–397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, He M, Cui L, Gao M, Zhang M, Yue
F, Shi T, Yang X, Pan Y, Zheng X, et al: Chemotherapy exacerbates
ovarian cancer cell migration and cancer stem cell-like
characteristics through GLI1. Br J Cancer. 122:1638–1648. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song L, Chen X, Mi L, Liu C, Zhu S, Yang
T, Luo X, Zhang Q, Lu H and Liang X: Icariin-induced inhibition of
SIRT6/NF-κB triggers redox mediated apoptosis and enhances
anti-tumor immunity in triple-negative breast cancer. Cancer Sci.
111:4242–4256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, Wang J, Chen K, Pang H, Li X, Zhu
J, Ma Y, Qiu T, Li W, Xie J and Zhang J: Caprylic acid (C8:0)
promotes bone metastasis of prostate cancer by dysregulated
adipo-osteogenic balance in bone marrow. Cancer Sci. 111:3600–3612.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng A, Liang X, Zhu S, Liu C, Luo X,
Zhang Q and Song L: Baicalin, a potent inhibitor of NF-κB signaling
pathway, enhances chemosensitivity of breast cancer cells to
docetaxel and inhibits tumor growth and metastasis both in vitro
and in vivo. Front Pharmacol. 11:8792020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Ruan Q, Zhai Y, Lu D, Li C, Fu Y,
Zheng Z, Song Y and Guo J: Baicalein inhibits non-small-cell lung
cancer invasion and metastasis by reducing ezrin tension in
inflammation microenvironment. Cancer Sci. 111:3802–3812. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui Y, Chen LJ, Huang T, Ying JQ and Li J:
The pharmacology, toxicology and therapeutic potential of
anthraquinone derivative emodin. Chin J Nat Med. 18:425–435.
2020.PubMed/NCBI
|
|
30
|
Li Q, Gao J, Pang X, Chen A and Wang Y:
Molecular mechanisms of action of emodin: As an anti-cardiovascular
disease drug. Front Pharmacol. 11:5596072020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo N, Fang J, Wei L, Sahebkar A, Little
PJ, Xu S, Luo C and Li G: Emodin in atherosclerosis prevention:
Pharmacological actions and therapeutic potential. Eur J Pharmacol.
890:1736172020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Zhang M, Hu G, Zhang Z and Song
R: Elevated system exposures of baicalin after combinatory oral
administration of rhein and baicalin: Mainly related to breast
cancer resistance protein (ABCG2), not
UDP-glucuronosyltransferases. J Ethnopharmacol. 250:1125282020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song K, Lv T, Chen Y, Diao Y, Yao Q and
Wang Y: Emodin inhibits TGF-β2 by activating the FOXD3/miR199a axis
in ovarian cancer cells in vitro. Oncol Rep. 39:2063–2070.
2018.PubMed/NCBI
|
|
35
|
Dhamija S and Diederichs S: From junk to
master regulators of invasion: lncRNA functions in migration, EMT
and metastasis. Int J Cancer. 139:269–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caspa Gokulan R and Devaraj H: Stem cell
markers CXCR-4 and CD133 predict aggressive phenotype and their
double positivity indicates poor prognosis of oral squamous cell
carcinoma. Cancers (Basel). 13:58952021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu HL, Tang HT, Yang HL, Deng TT, Xu YP,
Xu SQ, Peng L, Wang Z, Fang Q, Kuang XY and Li QS: Oct4 regulates
the transition of cancer stem-like cells to tumor endothelial-like
cells in human liver cancer. Front Cell Dev Biol. 8:5633162020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopez de Andres J, Grinan-Lison C, Jimenez
G and Marchal JA: Cancer stem cell secretome in the tumor
microenvironment: A key point for an effective personalized cancer
treatment. J Hematol Oncol. 13:1362020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu J, Su Q, Gao M, Liang Q, Li J and Chen
X: Differential expression and effects of Peroxiredoxin-6 on drug
resistance and cancer stem cell-like properties in non-small cell
lung cancer. Onco Targets Ther. 12:10477–10486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He QZ, Luo XZ, Wang K, Zhou Q, Ao H, Yang
Y, Li SX, Li Y, Zhu HT and Duan T: Isolation and characterization
of cancer stem cells from high-grade serous ovarian carcinomas.
Cell Physiol Biochem. 33:173–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang R, Zhu L and Zhang Y: XIST lost
induces ovarian cancer stem cells to acquire taxol resistance via a
KMT2C-dependent way. Cancer Cell Int. 20:4362020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang W, Gao Y, Hai J, Yang J and Duan S:
HER2 decreases drug sensitivity of ovarian cancer cells via
inducing stem cell-like property in an NFκB-dependent way. Biosci
Rep. 39:BSR201808292019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Corrado G, Salutari V, Palluzzi E,
Distefano MG, Scambia G and Ferrandina G: Optimizing treatment in
recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther.
17:1147–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Karadimitris A, Chaidos A, Caputo V,
Goudevenou K, Ponnusamy K and Xiao X: Myeloma propagating cells,
drug resistance and relapse. Stem Cells. 33:3205–3211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Clarke MF: Clinical and therapeutic
implications of cancer stem cells. N Engl J Med. 380:2237–2245.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saxena K, Jolly MK and Balamurugan K:
Hypoxia, partial EMT and collective migration: Emerging culprits in
metastasis. Transl Oncol. 13:1008452020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He L, Wang C, Zhang Y, Guo C, Wan Y and Li
Y: Effect of emodin on hyperlipidemia and hepatic lipid metabolism
in zebrafish larvae fed a high-cholesterol diet. Chem Biodivers.
e202100675. 2021.doi: 10.1002/cbdv.202100675 (Epub ahead of print).
View Article : Google Scholar
|
|
48
|
Horvat M, Avbelj M, Duran-Alonso MB,
Banjanac M, Petkovic H and Iskra J: Antiviral activities of
halogenated emodin derivatives against human coronavirus NL63.
Molecules. 26:68252021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Y, Wu R, Cai FF, Zhou WJ, Lu YY,
Zhang H, Chen QL, Sun MY and Su SB: Development of a novel
anti-liver fibrosis formula with luteolin, licochalcone A,
aloe-emodin and acacetin by network pharmacology and
transcriptomics analysis. Pharm Biol. 59:1594–1606. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao R, Wu X, Huang Z, Wang B, Li F, Xu H
and Ran L: Anti-tumor effect of aloe-emodin on cervical cancer
cells was associated with human papillomavirus E6/E7 and glucose
metabolism. Onco Targets Ther. 12:3713–3721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Q, Hodge J, Wang J, Wang Y, Wang L,
Singh U, Li Y, Yao Y, Wang D, Ai W, et al: Emodin reduces breast
cancer lung metastasis by suppressing Macrophage-induced breast
cancer cell Epithelial-mesenchymal transition and cancer stem cell
formation. Theranostics. 10:8365–8381. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tong H, Huang Z, Chen H, Zhou B, Liao Y
and Wang Z: Emodin reverses gemcitabine resistance of pancreatic
cancer cell lines through inhibition of IKKβ/NF-κB signaling
pathway. Onco Targets Ther. 13:9839–9848. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li N, Wang C, Zhang P and You S: Emodin
inhibits pancreatic cancer EMT and invasion by upregulating
microRNA1271. Mol Med Rep. 18:3366–3374. 2018.PubMed/NCBI
|
|
54
|
Gu J, Cui CF, Yang L, Wang L and Jiang XH:
Emodin inhibits colon cancer cell invasion and migration by
suppressing epithelial-mesenchymal transition via the
Wnt/beta-catenin pathway. Oncol Res. 27:193–202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dai G, Ding K, Cao Q, Xu T, He F, Liu S
and Ju W: Emodin suppresses growth and invasion of colorectal
cancer cells by inhibiting VEGFR2. Eur J Pharmacol. 859:1725252019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu SC, Huang CM, Bamodu OA, Lin CS, Liu
BL, Tzeng YM, Tsai JT, Lee WH and Chen TM: Ovatodiolide suppresses
nasopharyngeal cancer by targeting stem cell-like population,
inducing apoptosis, inhibiting EMT and dysregulating JAK/STAT
signaling pathway. Phytomedicine. 56:269–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tanabe S, Quader S, Cabral H and Ono R:
Interplay of EMT and CSC in cancer and the potential therapeutic
strategies. Front Pharmacol. 11:9042020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Luo Z, Wang Q, Lau WB, Lau B, Xu L, Zhao
L, Yang H, Feng M, Xuan Y, Yang Y, et al: Tumor microenvironment:
The culprit for ovarian cancer metastasis? Cancer Lett.
377:174–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bockhorn M, Jain RK and Munn LL: Active
versus passive mechanisms in metastasis: Do cancer cells crawl into
vessels, or are they pushed? Lancet Oncol. 8:444–448. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nowicki A, Kulus M, Wieczorkiewicz M,
Pieńkowski W, Stefańska K, Skupin-Mrugalska P, Bryl R, Mozdziak P,
Kempisty B and Piotrowska-Kempisty H: Ovarian cancer and cancer
stem cells-cellular and molecular characteristics, signaling
pathways, and usefulness as a diagnostic tool in medicine and
oncology. Cancers (Basel). 13:41782021. View Article : Google Scholar : PubMed/NCBI
|