Introduction
Phospholipase D (PLD) is a phospholipase that
participates in the catabolism of glycerol-based phospholipids.
There are six different isoforms of the phospholipase D subclass
(PLD1-6) and PLD1 and PLD2 are the best-studied isoforms. The
lipase activity of both these enzymes involves the preferential
hydrolysis of phosphatidylcholines (PCs), to release phosphatidic
acid (PA) and choline (Cho), crucial lipid mediators of cell
signaling pathways involved in cell proliferation, growth and
survival (1).
Cho is an essential nutrient for humans and it has a
wide range of vital physiological roles (2,3),
although higher levels of Cho have been associated with an
increased risk of melanoma and other cancer types (3–5).
However, as only small amounts of this molecule may be synthesized
de novo in humans, it must be obtained from other sources,
such as through the hydrolysis of the most abundant phospholipid,
PC, by the lipase activity of PLDs (3). PA is the other lipid mediator
produced by the lipase activity of PLDs, which may subsequently be
converted into essential second messengers such as lyso-PA by
phospholipase A2 (PLA2) or diacylglycerol
(DAG) by PA-phosphohydrolase. These products significantly expand
the range of the effects in which these enzymes may
participate.
Aside from the lipase activity of PLD2, a unique
feature of this isoform is that it also acts as a guanine
nucleotide exchange factor (GEF) (6,7).
Certain small GTPases regulate PLD2 activity through a complex
regulatory program of phosphorylation and dephosphorylation
switching involving S6K, Grb2, SoS, WASp and Rac2 (7). The GEF activity of PLD2 has been
associated with the migration of cancer cells, hypothesizing that
PLD2 constitutively activates Rac2, a GTPase known to participate
in cancer cell metastasis (8).
Furthermore, the GEF activity of PLD2 regulates the PA released by
hydrolysis of PC, adding a further layer of complexity to these
regulatory circuits (6,9,10).
It is known that both the expression and activity of
PLD1 and PLD2 are upregulated in several cancer types (1,10–18),
altering numerous signaling pathways involved in tumor progression
and metastasis. Indeed, it has been suggested that PLD2 has a
pivotal role in tumor cells, primarily in metastatic dissemination
(16,19). In fact, PLD2 activity promotes
proliferation and invasiveness of lymphomas (20) and breast cancer cells (12,21),
as well as representing a marker of poor prognosis in colorectal
and renal neoplasms (14–16). PLD2 has also been observed to be
overexpressed in melanoma along with enhanced lipase activity
(22,23), although there is no clear evidence
of its specific role in this pathology.
Melanoma is a cancer that arises from the
transformation of melanocytes and it mainly occurs in the skin. It
is the seventh most common tumor type in Europe and although the
incidence of most cancers is declining, the number of melanoma
cases is expected to continue to rise in western populations. While
early diagnosis guarantees a 5-year overall survival of 99% for
most melanomas, this rate falls to 25% in melanomas with distant
metastases (24). Although
numerous treatment options have been approved for metastatic tumors
in the past decade (25), there is
still no effective therapy for advanced melanoma. Thus, the aim of
the present study was to obtain novel insight into the contribution
of PLD2 in the development and progression of melanoma, assessing
its value as a potential novel biomarker or therapeutic target for
melanoma. It was indicated that an increase in the expression of
PLD2 and its lipase activity contribute to increased proliferation,
migration and invasion of melanoma cells.
Materials and methods
Commercial cell lines
The HEMn-LP, HEMn-MP and HEMn-DP melanocyte cell
lines were purchased from Thermo Fisher Scientific, Inc., while the
A375, G361, Sk-Mel-28, HT144, Hs294t, A2058, Sk-Mel-2, Sk-Mel-3,
WM-266-4 and VMM1 melanoma cell lines were obtained from the
American Type Culture Collection (ATCC). The Mel-Ho, Colo-800 and
RPMI-7951 melanoma cell lines were acquired from Innoprot S.L.
The cell lines used in the present study may be
classified into three different groups according to their origin:
Normal melanocytes (HEMn-LP, HEMn-MP and HEMn-DP), primary
melanomas (A375, G361, Sk-Mel-28, ME4405 and Mel-Ho) and metastatic
melanomas (HT144, Hs294t, A2058, Sk-Mel-2, Sk-Mel-3, WM-266-4,
VMM1, RPMI-7951 and Colo-800). Table
SI provides a brief description of these cell lines.
The Mel-Ho cell line was authenticated by
short-tandem repeat DNA profiling analysis (TH01: 7; D21S11: 29;
D5S818: 12; D13S317: 11; D7S820: 10,11; D16S539: 11; CSF1P0: 12;
AMEL: X; VWA: 14,18; TPOX: 8,10. The numbers after the loci name
indicate the number of repeats of the repeat unit for that locus in
the analysed cells' genome).
Cell culture
Melanocyte cell lines were cultured in medium 254
supplemented with human melanocyte growth supplement (Thermo Fisher
Scientific, Inc.). The A375, ME4405, Hs294t, RPMI-7951, A2058 and
HT144 lines were maintained in DMEM culture medium
(MilliporeSigma), while the Sk-Mel-28, WM-266-4 and Sk-Mel-2 lines
were cultured in Eagle's Minimum Essential Medium (ATCC). RPMI
1640-GlutaMAX™ (Thermo Fisher Scientific, Inc.) was used to culture
the Mel-Ho, Colo-800 and VMM1 cell lines, and McCoy's 5A was used
for G361 and Sk-Mel-3 cells (Thermo Fisher Scientific, Inc.). All
the culture media used for melanoma cell lines were supplemented
with 10% FBS (v/v; Cytiva), 100 UI/ml penicillin and 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc.). The cells were
cultured at 37°C in a humidified atmosphere with 5% CO2.
All of the cultured cell lines tested negative for mycoplasma
(Venor® GeM One Step Test Kit; Minerva Biolabs).
Western blot analysis
Cell protein extracts were obtained from cells
incubated for 15 min in RIPA buffer (Thermo Fisher Scientific,
Inc.) containing protease inhibitor cocktail-3 and a phosphatase
inhibitor (MilliporeSigma), and then centrifuged for 5 min at
15,000 × g at 4°C. The proteins were quantified with a BCA assay
(EMD Millipore) and equal amounts (40 µg) of each protein extract
were denatured for 30 min at 37°C (22) and separated by 7.5% SDS-PAGE
electrophoresis under reducing conditions. The proteins were then
wet-transferred to a nitrocellulose membrane (Cytiva) that was
probed overnight at 4°C with primary antibodies against
PLA2, PLC, PLD1, PLD2 and tubulin. Subsequently, the
membranes were incubated with HRP-conjugated secondary antibodies
for 2 h at room temperature. The details of the antibodies are
provided in Table I. The bands
were visualized by enhanced chemiluminescence and digital images of
the bands were obtained with a charge-couple device camera-based
imager, the Syngene G box imaging system (Syngene).
 | Table I.Antibodies for western blot
analysis. |
Table I.
Antibodies for western blot
analysis.
| Antibody | Company (cat.
no) | Source | Target species | Dilution |
|---|
| PLD2 | Abgent
(AO2358a) | Mouse | Human | 1/1500 |
| PLD1 | Cell Signaling
Technology, Inc. (3832) | Rabbit | Human | 1/1,000 |
|
PLA2 | Santa Cruz
Biotechnology, Inc. (Sc-376563) | Mouse | Human | 1/1,000 |
| PLC | Santa Cruz
Biotechnology, Inc. (Sc-5291) | Mouse | Human | 1/1,000 |
| α-Tubulin | MilliporeSigma
(T9026) | Mouse | Human | 1/3,000 |
| Mouse IgGκ-HRP | Santa Cruz
Biotechnology, Inc. (Sc-516102) | Goat | Mouse | 1/5,000 |
| Mouse IgG-HRP | SouthernBiotech
(1032–05) | Goat | Mouse | 1/8,000 |
| Rabbit IgG-HRP | Abcam
(Ab102279) | Goat | Rabbit | 1/10,000 |
Immunofluorescence staining
Cells were seeded on round coverslips, incubated
overnight, fixed in 4% paraformaldehyde and permeabilized with 0.1%
Triton X-100. The cells were incubated overnight at 4°C with
anti-human PLD2 primary antibody (1:200 dilution; Abgent), washed
and then incubated for 2 h at room temperature in the dark with an
Alexa-Fluor 594-conjugated secondary antibody (1:5,000 dilution;
cat. no. ab150080; Abcam). Finally, the cells were incubated with
Hoechst-33342 (Thermo Fisher Scientific, Inc.) for 5 min and the
coverslips were mounted with Fluoromount-G (MilliporeSigma) on a
microscope slide. The cells were visualized by generating 20
z-stacks on a Leica SP8 confocal fluorescence microscope (Leica
Microsystems GmbH) at an original magnification of ×63.
PLD lipase activity
PLD activity in melanoma cells was assessed using a
protocol described previously (26). First, liposomes were prepared from
a short chain PC (8:0/8:0) (3.5 mM; Avanti Polar Lipids) in HEPES
(pH 7.8; 45 mM), phosphatidylinositol 4,5-biphosphate (1 µM; Avanti
Polar Lipids, Inc.) and 1.0 µCi [3H]-n-butanol (American
Radiolabeled Chemicals Inc.). Pellets of the cell lines studied
were resuspended in lysis buffer [5 mM HEPES (pH 7.8), 100 µM Na
orthovanadate, 0.4% Triton X-100, 2 µg/ml aprotinin and 5 µg/ml
leupeptin], sonicated on ice and the protein content of the
sonicates was measured with a BCA assay. The liposomes were
incubated for 20 min on a shaker with 50 µg of the cell sonicates
at 30°C to achieve transphosphatidylation of PLD. This reaction was
stopped by adding ice-cold chloroform/methanol (1:2) and the lipids
in the samples were isolated and resolved by thin-layer
chromatography. The amount of [3H]-phosphatidylbutanol
(PBut) that co-migrated with the PBut standards was measured in a
scintillation counter (PerkinElmer, Inc.).
PLD2 overexpression
Melanoma cells were seeded in 6-well plates and
grown until 60–70% confluence. They were then transfected by
incubation for 48 h with a transfection mixture of 1 µg of plasmid
DNA (cat. no. RC202042; Origene Technologies, Inc.),
TransIT®−2020 transfection reagent (Mirus Bio, LLC) and
Opti-MEM™ (Thermo Fisher Scientific, Inc.). Control cells were
transfected with empty plasmid (pc-DNA3.1-myc; Origene
Technologies, Inc.) following the same protocol and the
overexpression efficiency was confirmed using western blot
analysis.
PLD2 silencing
When the melanoma cells cultured in 6-well plates
reached 60–70% confluence, the culture medium was replaced with
silencing mixture containing 50 nM small interfering (si)RNA
(Ambion; Thermo Fisher Scientific, Inc.), Opti-MEM™ and
Lipofectamine RNAiMax (Thermo Fisher Scientific, Inc.), and the
cells were incubated for 48 h prior to performing the experiments.
For control transfections, a non-targeting siRNA molecule suitable
for negative controls (cat. no. AM4611; Ambion; Thermo Fisher
Scientific, Inc.) was used following the same procedure and the
correct silencing of the cells was confirmed using western blot
analysis.
Cell proliferation assay
The relative proliferation capacity of cells after
PLD2 silencing and overexpression was measured as the number of
viable cells in that condition after 24 h in culture, relative to
their control. Viable cells were quantified by XTT, which is based
on dehydrogenase enzymes that reduce the tetrazolium salt to a
highly coloured formazan dye. The amount of the formazan produced
is proportional to the number of viable cells. It was confirmed
that the treatments did not affect dehydrogenase enzyme activity
compared to the results obtained by XTT and those calculated by
trypan blue, so XTT is valid for measuring cell viability in the
present study. Melanoma cells were grown overnight in 96-well
plates in complete culture medium and then the medium was discarded
and low-FBS medium (0.5%) was added. After 2 h in the incubator at
37°C, the medium was replaced with complete culture medium and 24 h
later, XTT reagent (MilliporeSigma) was added to the cells. The
cell absorbance in each experimental condition was measured at 450
nm in a Synergy HT spectrophotometer (BioTek Instruments, Inc.) and
the proliferation rate of the cells was expressed as a ratio
relative to their corresponding transfection controls.
Cell migration and invasion assay
Cell migration was assessed using Boyden chambers
with 8-µm pores (Corning, Inc.). Melanoma cells were incubated for
2 h at 37°C in low-FBS medium (0.5%) and 400 µl of 5×104
cells/ml were seeded in the upper chambers in medium containing
0.5% FBS. For invasion, 400 µl of 7.5×105 cells/ml in
0.5% FBS medium were seeded in the upper chambers of
Matrigel®-coated Boyden chambers (Corning, Inc.).
Complete culture medium was added to the bottom well and after 20 h
of incubation at 37°C, the non-migrated/invaded cells were removed
by aspiration and wiping the upper side of the membrane with a
cotton bud, while the migrated/invaded cells were fixed with 4%
paraformaldehyde (Electron Microscopy Sciences, Inc.) and stained
with 0.2% crystal violet at room temperature for 8 min. Finally,
the filter was mounted and observed under a microscope at a
magnification of ×20. Images of six different fields were acquired
and the cells in each field were counted. The migratory/invasive
capacity of each cell line was calculated as a ratio relative to
their corresponding transfection control.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean of at least three experiments and the statistical
significance of the differences between the means was calculated
using one-way ANOVA and Bonferroni's post-hoc correction for the
comparison of more than two data sets, or Dunnett's
multiple-comparison test post-hoc correction for comparison of
multiple data sets with their control as indicated in each
experiment (GraphPad Prism 5.01; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
PLD2 protein expression is upregulated
in melanoma cell lines
As several phospholipases are overexpressed in a
variety of cancers (1,27) and they participate in their
malignant transition, the presence of this family of enzymes was
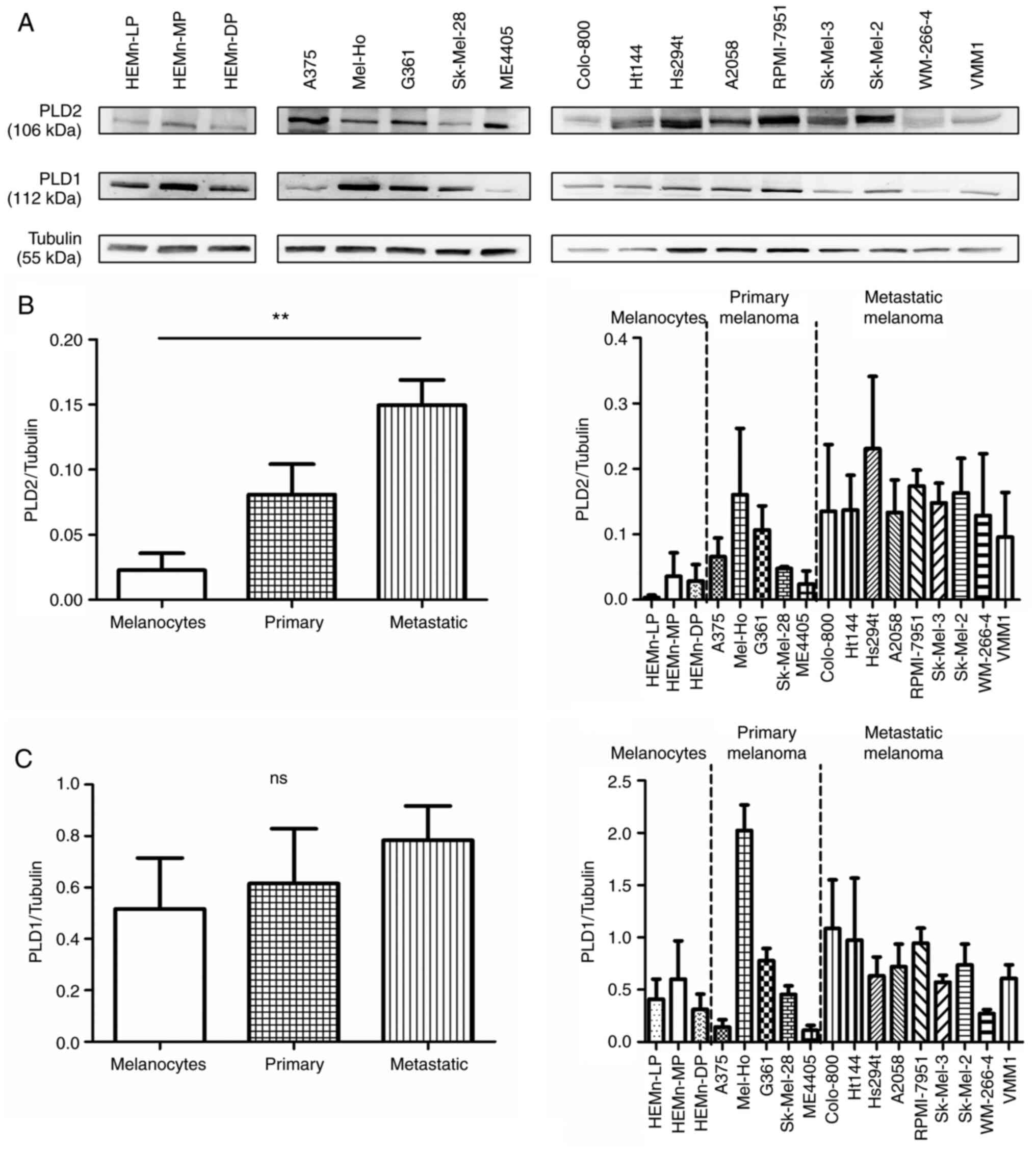
assessed in melanoma. The expression of PLD2, PLD1, PLA2
and PLC was observed to vary in western blots of 17 cell lines,
including skin melanocytes, as well as in primary and metastatic
melanoma cell lines. In particular, while PLD2 was weakly expressed
in skin melanocytes, it was expressed more strongly in melanoma
cells (Figs. 1A and B and S1), particularly metastatic cells,
although this increase in expression was cell line-specific.
Conversely, no clear variation was observed in PLD1 protein
expression between skin melanocytes and melanoma cells (Figs. 1A and C and S2). In fact, the expression of this
protein in the HEMn-MP cell line was similar to that in all the
primary melanomas except Mel-Ho. PLA2 was expressed more
strongly by skin melanocytes than by primary melanomas (Fig. S3), although HT144, Colo-800 and
RPMI-7951 metastatic melanomas had the highest levels of
PLA2, a trait that was cell line-specific as it was not
common to all the metastatic melanomas. PLC was expressed
relatively homogeneously in the cell lines studied and no marked
differences were observed (Fig.
S3). The enhanced presence of PLD2 in melanoma cells was
specific to this isoform, since the PLD1, PLA2 and PLC
proteins remained relatively constant in melanocytes and melanoma
cells.
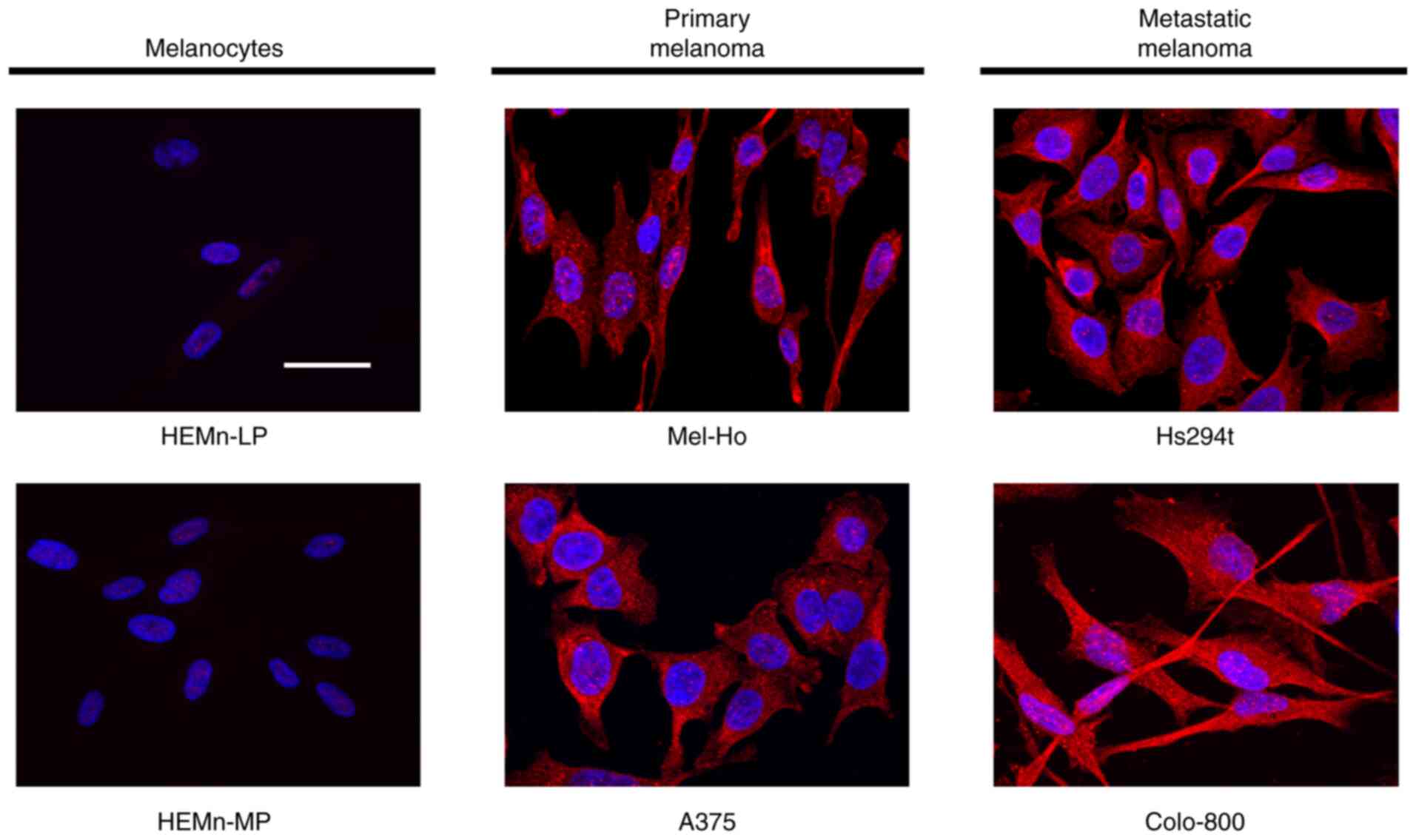
Immunofluorescence analyses confirmed that PLD2
expression was upregulated in the malignant cell lines studied
(Fig. 2). PLD2 was expressed more
intensely in primary melanomas (A375, Mel-Ho) and metastatic
melanomas (Hs294t, Colo-800), while its expression was faint in
skin melanocytes (HEMn-LP, HEMn-MP). By contrast,
immunofluorescence analysis of the other phospholipases
(PLA2, PLC and PLD1) did not indicate any consistent
differences between the cell lines studied (data not shown).
Indeed, both the western blot and immunofluorescence data
corroborated the enhanced expression of PLD2 in primary and
metastatic melanomas relative to normal skin melanocytes.
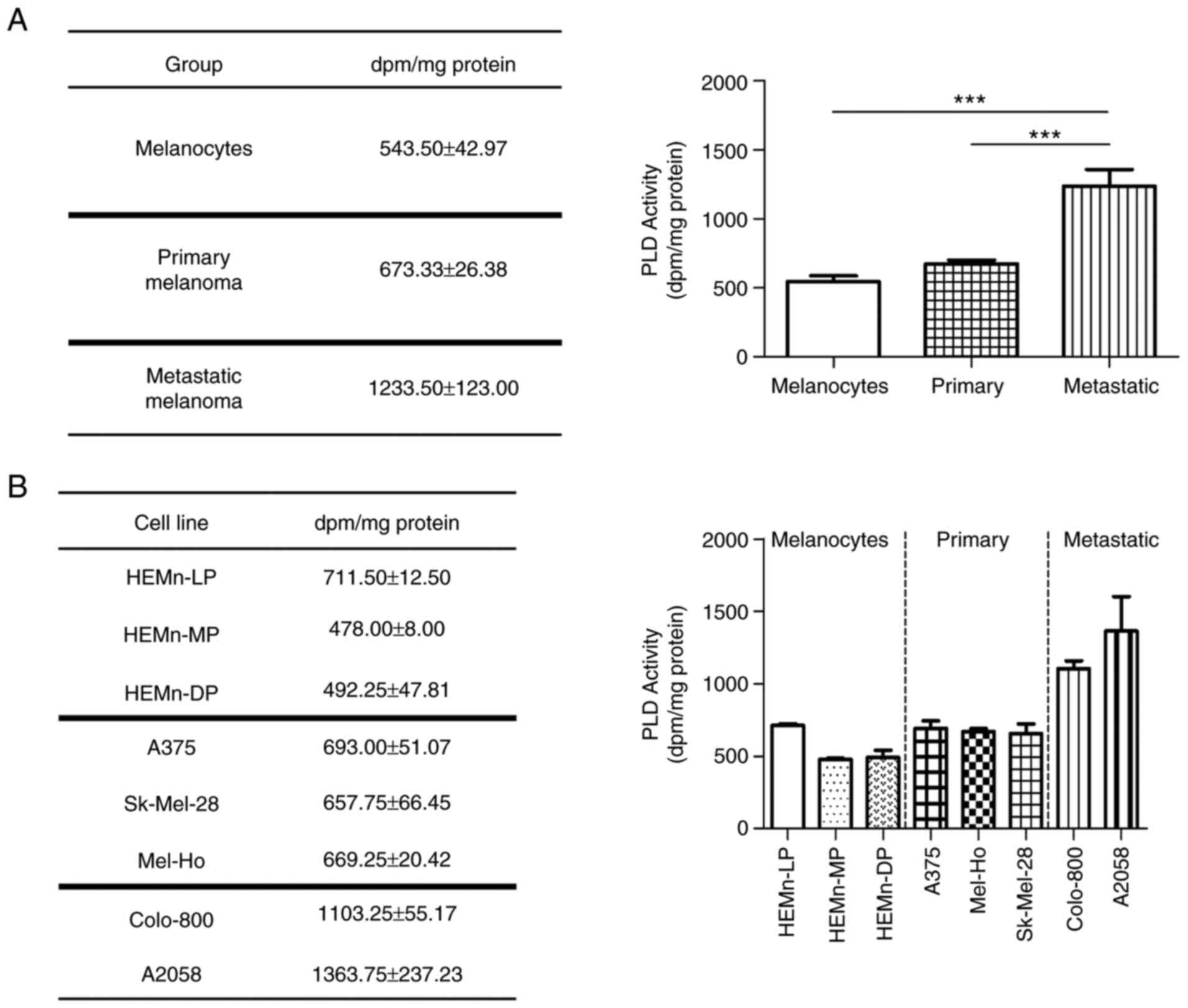
Enhanced PLD lipase activity in
melanoma cells
A comparative analysis of PLD activity was performed
in melanocytes, primary melanomas and metastatic melanomas
(Fig. 3). The results indicated
that melanoma cells exhibited enhanced PLD activity and that this
was associated with melanoma progression. Indeed, metastatic
melanoma cells had significantly more lipase activity, while
primary melanoma cell lines also had higher activity than skin
melanocytes. However, the activity in the HEMn-LP melanocyte cell
line was comparable to that in primary melanomas and therefore, the
differences were not statistically significant between both groups.
These results confirmed that, overall, the lipase activity of PLD
enzymes is increased in melanoma cells and raise the question of
whether they have a role in melanoma, particularly in the
metastatic process.
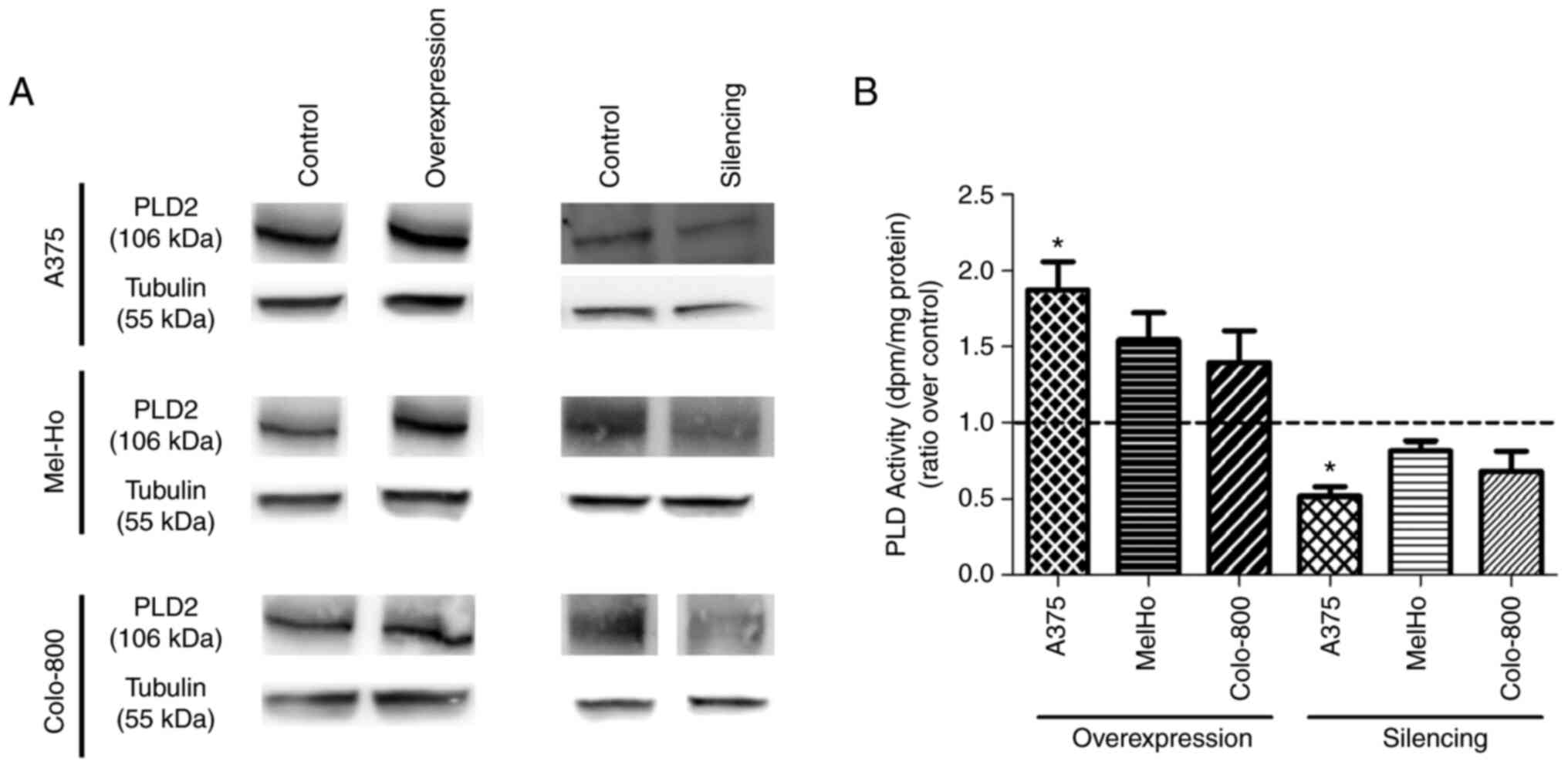
PLD2 overexpression and silencing in
melanoma cell lines
After demonstrating that aggressive melanoma cells
have stronger PLD2 expression and activity than skin melanocytes,
modifications to the amounts of this enzyme were performed to shed
light on the implication of PLD2 in specific carcinogenic events.
The effects of transient overexpression and silencing of PLD2 were
examined in primary melanoma cells (A375, Mel-Ho) or metastatic
melanoma cells (Colo-800), as confirmed in western blots (Figs. 4A and S4). Furthermore, these molecular
alterations were also manifested through enzymatic activity, as
lipase activity was altered in accordance with protein expression
in all cases, although only in the A375 line, the lipase activity
exhibited statistically significant differences compared to the
transfection controls. As indicated in Fig. 4B, PLD2 overexpression was
associated with an increase in lipase activity when the ratio
relative to the transfection control was considered: A375 cells,
1.87; Mel-Ho, 1.54; and Colo-800, 1.4. By contrast, PLD2-silenced
cells exhibited weaker lipase activity: A375, 0.52; Mel-Ho, 0.82;
and Colo-800, 0.68.
PLD2 enhances the proliferation,
migration and invasion of melanoma cells
The functional implications of the modifications to
PLD2 expression were analyzed by studying the differences in
proliferation, migration and invasion of transformed cells.
Although these changes were cell line-specific, there was a clear
trend towards an increase in proliferation, migration and invasion
of PLD2-overexpressing cells, while these processes were
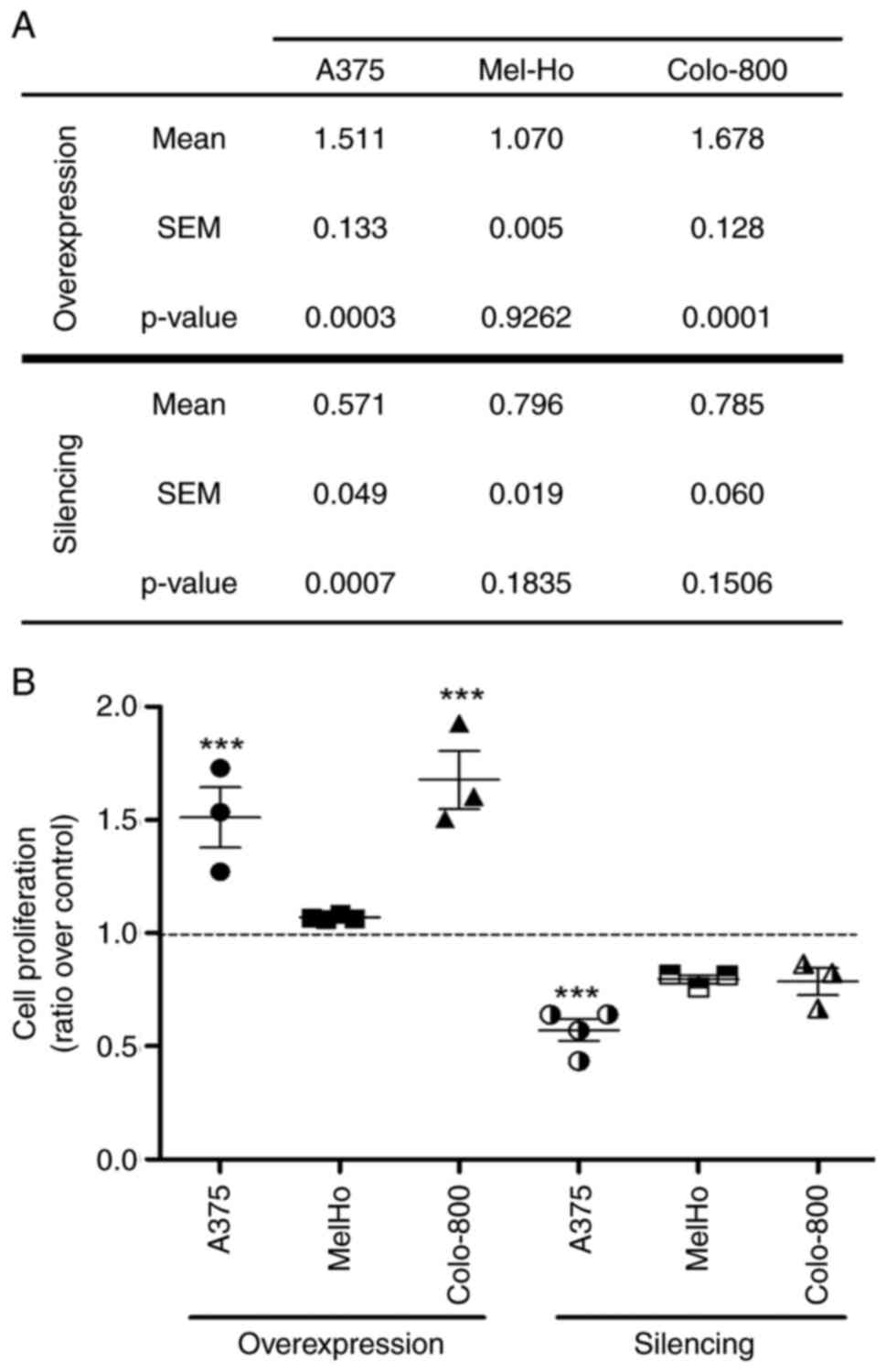
downregulated in PLD2-silenced cells (Figs. 5 and 6). Indeed, cell proliferation increased
markedly in A375 and Colo-800 cells overexpressing PLD2 but only
slightly in MelHo cells. Conversely, in cells that downregulated
PLD2, there was a consistent reduction in proliferation: A375, 0.55
(P<0,001); Mel-Ho, 0.8; and Colo-800, 0.79 (Fig. 5).
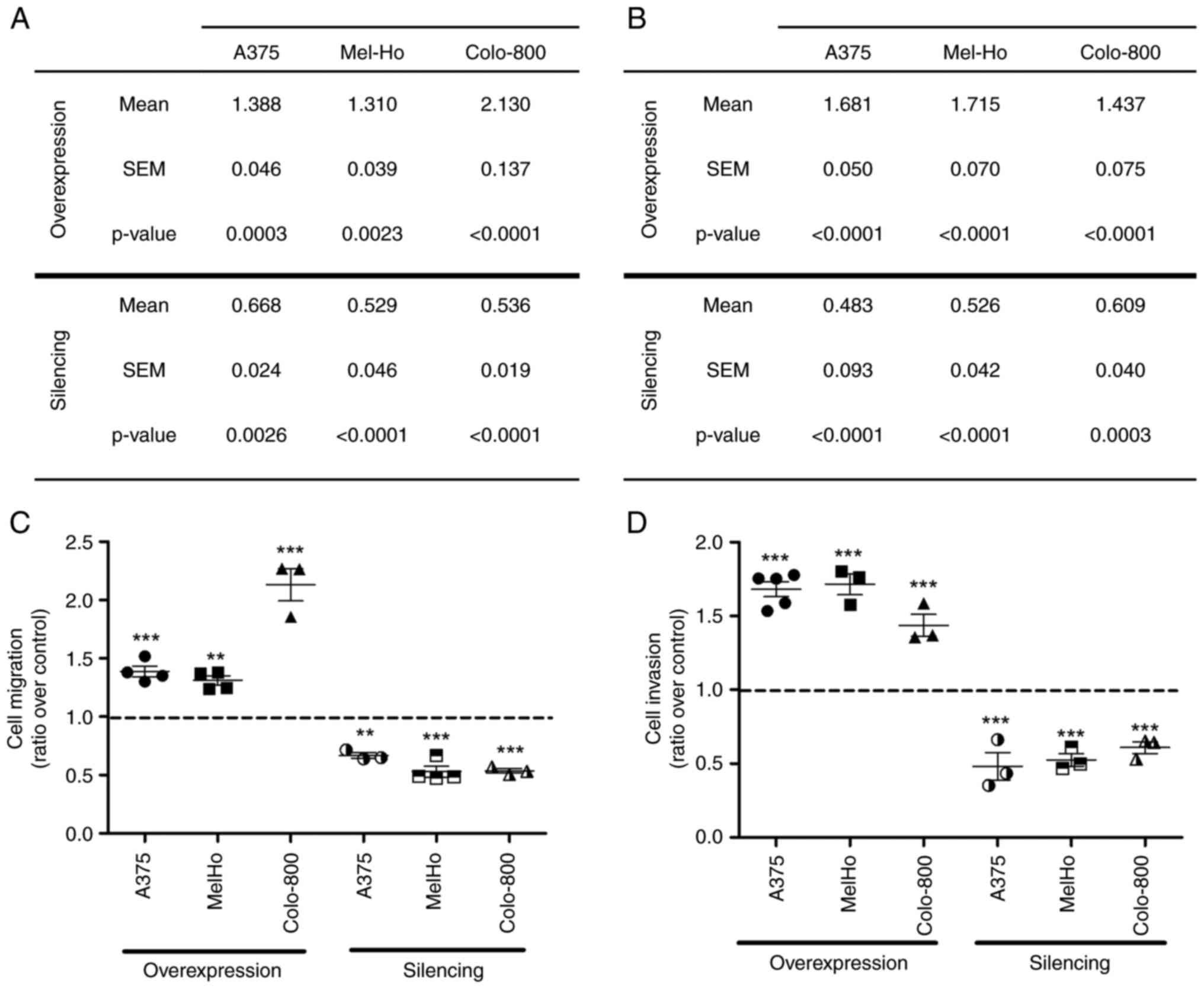
Melanoma cell migration and invasion were then
assessed and PLD2-overexpressing primary melanoma cells exhibited
moderate increments in cell migration: A375, 1.39; and Mel-Ho,
1.31. This effect was markedly enhanced in metastatic cells
(Colo-800, 2.13), whereas the results were more similar among the
silenced cell lines: A375, 0.66; Mel-Ho, 0.53; and Colo-800, 0.54
(Fig. 6A and B; images are
provided in Fig. S5). Finally,
increases in cell invasion in association with PLD2 overexpression
were observed in primary melanoma cells (A375, 1.68; Mel-Ho, 1.71)
relative to metastatic cells (Colo-800, 1.43). However, in the
silenced cells, the results were more similar among the three cell
lines, although primary melanoma cells were still more strongly
affected: A375, 0.48; Mel-Ho, 0.53; and Colo-800, 0.60 (Fig. 6C and D; images are provided in
Fig. S6). In conclusion, the
present results suggested that higher levels of PLD2 increased the
proliferation, migration and invasion of melanoma cells, while
silencing of this enzyme decreased those activities.
Discussion
Phospholipases metabolize phospholipids, generating
bioactive molecules that influence cell fate by regulating distinct
cell activities, including events that favor tumorigenesis and
metastasis (11–13,28).
Deregulation of the expression and activity of phospholipases has
been implicated in different diseases, including cancer. Indeed,
the overexpression and enhanced activity of PLA2 serves as a
diagnostic marker of breast cancer and is correlated with the tumor
stage (29). Similarly, the
present analysis of the PLD2 protein demonstrated that it
accumulates at higher levels in metastatic melanoma than in normal
skin melanocytes, as indicated by the PLD2 expression analyzed by
western blot and immunofluorescence. However, the
immunohistochemical analysis performed was limited, as neither the
subcellular localization of PLD2 nor the changes in PLD2 expression
in the transfected cells was examined. The results prompted us to
analyze the activity of PLD, based on the notion that not only is
PLD expression enhanced in cancer tissues but its activity is also
increased (10,30). PLD activity was enhanced
particularly in murine melanoma cells, in association with an
acidic pH of the tumor microenvironment (31), consistent with the enhanced PLD
activity observed previously in melanoma cells relative to skin
melanocytes (22). This trait was
verified in the present study, as significantly stronger PLD
activity was observed in malignant cells. Furthermore, the present
study was the first, to the best of our knowledge, to indicate that
PLD activity in metastatic melanoma cells was substantially higher
than in primary melanoma cells.
One role of PLD2 is the hydrolysis of PC present in
cell membranes to release free Cho and a PA molecule. The molecules
liberated are known precursors and second messengers that support a
tumorigenic phenotype. Indeed, increased Cho levels have been
identified in cancer cells (3–5). Cho
is essential for the synthesis of PC and sphingomyelin lipids,
important structural and signaling lipids involved in generating
new cell membranes and reprogramming the metabolism of cancer
cells, thereby supporting their malignant transformation. However,
the effect of PLD activation is principally related to PA, a
ubiquitous second messenger that interacts with important signaling
molecules such as Akt and mTOR (13,23,32),
both in physiological and pathological processes (1,33).
These signaling pathways are constitutively activated in melanoma,
promoting cell proliferation, migration, survival, angiogenesis,
metabolism and chemoresistance. PLD-produced PA favors the
phosphorylation of these interactors in melanoma cells with
downregulated liver kinase B1 (23), thereby producing pro-tumorigenic
effects (1,33–40).
In addition, PA may also be dephosphorylated to yield DAG or
hydrolyzed to yield Lyso-PA, both of which are potent signaling
molecules known to favor carcinogenesis (32,41).
In particular, Lyso-PA supports the migration of melanoma cells
(42).
For the first time, to the best of our knowledge,
the present study demonstrated the influence of PLD2 expression and
activity on distinct carcinogenic events by overexpressing and
silencing this enzyme in primary and metastatic melanoma cells. The
results demonstrated that augmenting the activity and expression of
PLD2 promoted a pro-tumorigenic phenotype in melanoma, driving the
proliferation, invasion and migration of these cells, particularly
in metastatic cells. Conversely, downregulation of PLD2 inhibited
these activities. Similar results have been described in lymphoma
(20) and breast cancer cells
(12,19), and it was determined that PLD2
promoted metastatic activity by phosphorylating FAK and PAK, and by
activating mTOR, JAK3 and Akt (20,43).
Furthermore, PLD2 is present in the exosomes secreted by cancer
cells, favoring the metastatic spread of prostate cells (44) and the crosstalk of colon cancer
cells with their microenvironment (45). Thus, PLD has been associated with
cancer progression and metastasis in several tumor types (10–12,17,21,28,44–48),
to which melanoma may now be added. Concerning this point, the
survival of melanoma patients with high and low PLD2 expression
from The Cancer Genome Atlas PanCancer panel data were analysed
using the cBioPortal platform (49,50).
Although the results of this analysis should be interpreted with
caution, they indicate a significant difference in overall survival
between the two groups, where higher PLD2 expression (z score
>1) is associated with shorter survival (Fig. S7). These data support the
experimental results of the present study and reinforce the idea
that, in melanoma, high PLD2 expression may be associated with
malignant progression.
The association between PLD activity detected and
the functional processes studied in transfected cells is not
perfect, since the magnitude of this relationship differs between
cell lines and the type of process. For instance, in Mel-Ho and
Colo-800 cells overexpressing PLD2, similar levels of PLD activity
result in quite different rates of proliferation and migration. The
variability observed among them may be due to an activity of PLD2
other than its lipase activity. In fact, the GEF activity of PLD2
is important for cell motility and other functions (1,9,10).
Indeed, PLD2 is a GEF for the small GTPases Rho, Rac-2 and Arf6,
which fulfills an essential role in the actin rearrangements and
filopodia formation required for cell motility and migration
(6,16,51–53).
These activities are consistent with the present results, as it was
observed that the migratory and invasive capacities of melanoma
cells increase when PLD2 is overexpressed, particularly in
metastatic melanoma. In addition, the PLD activity determined
indicated the presence of enzymatic activity of both PLD1 and PLD2,
while in the functional study, only the protein levels of PLD2 were
modified. On the other hand, the fact that the products of PLD2
activity may be converted into essential second messengers means
that the range of effects of the enzyme is broad and its effect on
biological activities such as those studied may be cell
line-dependent.
Pharmacological inhibition or genetic downregulation
of PLD2 has been proposed as a potential treatment option for
certain cancer types. In breast cancer in particular, previous
studies have indicated that the use of a PLD2-specific inhibitor
markedly reduced the invasive capacity of cultured cell lines
(21) and it promoted cancer cell
apoptosis, while overexpression of PLD2 provided resistance to
chemotherapeutic agents (54).
Silencing of PLD2 or its pharmacological inhibition in a mouse
model repressed tumor progression, whereas overexpression of this
enzyme translated into an increase in tumorigenic potential
(12). In lymphoma, catalytic
inactivation of PLD2 reduced the formation of lung metastases
(20) and in colorectal cancer
cells, the pharmacological inhibition and silencing of PLD2
resulted in cancer cell autophagy (55).
In conclusion, PLD2 contributes to different aspects
of cancer development including the production of survival signals
(10,46,56–59),
proliferation (60–63), resistance to apoptosis (10,64),
angiogenesis (10,65), deregulated cellular energetics
(10), as well as matrix substrate
degradation, invadopodia formation, tumor-cell migration, invasion
and metastatic dissemination (10,19,31,43,46,47,66,67).
However, in melanoma, a specific role for PLD2 has yet to be
described in tumorigenesis. The present study was the first to
report increased PLD activity in metastatic melanoma cells relative
to primary melanoma cells, as well as the influence of changes in
PLD2 levels and activity in several properties of metastatic
melanoma. In the light of the data on PLD2 from other cancers and
the present in vitro results in melanoma, it is suggested
that PLD2 may be a promising therapeutic target and biomarker for
melanoma tumors, which merits further study.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Julian
Gomez-Cambronero from the Department of Biochemistry and Molecular
Biology of Wright State University (Dayton, OH, USA) for his
support with the PLD lipase activity measurements and PLD2
transfection in cultured cells.
Funding
This study was supported by grants from the University of the
Basque Country/EHU (grant no. GIU17/066) and Ministerio de Economía
y Competividad MINECO-ONCOFINDER of the Spanish Government (grant
no. RTC.2015-3693-1).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contribution
APV performed the experiments described in the
study, collected the data and drafted the manuscript. MDB and AA
guided the design and execution of the study and revised the
manuscript. BO provided advice on lipid biology and function and
analyzed the results of certain experiments. KNS participated in
the execution of PLD activity assays and PLD2 transfection assays.
EA and GBG obtained the financial support and performed parts of
the statistical analysis. APV and AA confirm the authenticity of
all the raw data. All authors read, reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to
declare.
Glossary
Abbreviations
Abbreviations:
|
Cho
|
choline
|
|
DAG
|
diacylglycerol
|
|
GEF
|
guanine nucleotide exchange factor
|
|
PA
|
phosphatidic acid
|
|
PBut
|
phosphatidylbutanol
|
|
PC
|
phosphatidylcholine
|
|
PLD
|
phospholipase D
|
References
|
1
|
Bowling FZ, Frohman MA and Airola MV:
Structure and regulation of human phospholipase D. Adv Biol.
79:1007832021.PubMed/NCBI
|
|
2
|
Zeisel SH and da Costa KA: Choline: An
essential nutrient for public health. Nutr Rev. 67:615–623. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Onono FO and Morris AJ: Phospholipase d
and choline metabolism. In: Lipid signaling in human diseases.
Gomez-Cambronero J and Frohman MA: 259. Springer International
Publishing; Cham: pp. 205–218. 2019
|
|
4
|
Kosmopoulou M, Giannopoulou AF, Iliou A,
Benaki D, Panagiotakis A, Velentzas AD, Konstantakou EG,
Papassideri IS, Mikros E, Stravopodis DJ and Gikas E: Human
melanoma-cell metabolic profiling: Identification of novel
biomarkers indicating metastasis. Int J Mol Sci. 21:24362020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glunde K, Bhujwalla ZM and Ronen SM:
Choline metabolism in malignant transformation. Nat Rev Cancer.
11:835–848. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahankali M, Henkels KM and
Gomez-Cambronero J: A GEF-to-phospholipase molecular switch caused
by phosphatidic acid, Rac and JAK tyrosine kinase that explains
leukocyte cell migration. J Cell Sci. 126:1416–1428.
2013.PubMed/NCBI
|
|
7
|
Gomez-Cambronero J, Morris AJ and Henkels
KM: PLD protein-protein interactions with signaling molecules and
modulation by PA. Methods Enzymol. 583:327–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henkels KM, Mahankali M and
Gomez-Cambronero J: Increased cell growth due to a new lipase-GEF
(Phospholipase D2) fastly acting on Ras. Cell Signal. 25:198–205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomez-Cambronero J: Phospholipase D in
cell signaling: From a myriad of cell functions to cancer growth
and metastasis. J Biol Chem. 289:22557–22566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gomez-Cambronero J: Phosphatidic acid,
phospholipase D and tumorigenesis. Adv Biol Regul. 54:197–206.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown HA, Thomas PG and Lindsley CW:
Targeting phospholipase D in cancer, infection and
neurodegenerative disorders. Nat Rev Drug Discov. 16:351–367. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henkels KM, Boivin GP, Dudley ES,
Berberich SJ and Gomez-Cambronero J: Phospholipase D (PLD) drives
cell invasion, tumor growth and metastasis in a human breast cancer
xenograph model. Oncogene. 32:5551–5562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao Y, Wang X, Li H, Fan J, Qian X, Li H
and Xu Y: Phospholipase D as a key modulator of cancer progression.
Biol Rev Camb Philos Soc. 95:911–935. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oshimoto H, Okamura S, Yoshida M and Mori
M: Increased activity and expression of phospholipase D2 in human
colorectal cancer. Oncol Res. 14:31–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito M, Iwadate M, Higashimoto M, Ono K,
Takebayashi Y and Takenoshita S: Expression of phospholipase D2 in
human colorectal carcinoma. Oncol Rep. 18:1329–1334.
2007.PubMed/NCBI
|
|
16
|
Kandori S, Kojima T, Matsuoka T, Yoshino
T, Sugiyama A, Nakamura E, Shimazui T, Funakoshi Y, Kanaho Y and
Nishiyama H: Phospholipase D2 promotes disease progression of renal
cell carcinoma through the induction of angiogenin. Cancer Sci.
109:1865–1875. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDermott MI, Wang Y, Wakelam MJO and
Bankaitis VA: Mammalian phospholipase D: Function, and
therapeutics. Prog Lipid Res. 78:1010182020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noble AR, Hogg K, Suman R, Berney DM,
Bourgoin S, Maitland NJ and Rumsby MG: Phospholipase D2 in prostate
cancer: Protein expression changes with Gleason score. Br J Cancer.
121:1016–1026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henkels KM, Farkaly T, Mahankali M, Segall
JE and Gomez-Cambronero J: Cell invasion of highly metastatic MTLn3
cancer cells is dependent on phospholipase D2 (PLD2) and janus
kinase 3 (JAK3). J Mol Biol. 408:850–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knoepp SM, Chahal MS, Xie Y, Zhang Z,
Brauner DJ, Hallman MA, Robinson SA, Han S, Imai M, Tomlinson S and
Meier KE: Effects of active and inactive phospholipase D2 on signal
transduction, adhesion, migration, invasion, and metastasis in EL4
lymphoma cells. Mol Pharmacol. 74:574–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scott SA, Selvy PE, Buck JR, Cho HP,
Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW and
Brown HA: Design of isoform-selective phospholipase D inhibitors
that modulate cancer cell invasiveness. Nat Chem Biol. 5:108–117.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riebeling C, Müller C and Geilen C:
Expression and regulation of phospholipase D isoenzymes in human
melanoma cells and primary melanocytes. Melanoma Res. 13:555–562.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dogliotti G, Kullmann L, Dhumale P, Thiele
C, Panichkina O, Mendl G, Houben R, Haferkamp S, Püschel AW and
Krahn MP: Membrane-binding and activation of LKB1 by phosphatidic
acid is essential for development and tumour suppression. Nat
Commun. 8:157472017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
American Cancer Society, . Cancer Facts
& Figures 2020. American Cancer Society; Atlanta, GA: 2020
|
|
25
|
Guterres AN, Herlyn M and Villanueva J:
Melanoma. eLS. John Wiley & Sons; Hoboken, NJ: pp. 1–10.
2018
|
|
26
|
Gomez-Cambronero J, Horwitz J and Sha'afi
RI: Measurements of phospholipases A2, C, and D
(PLA2, PLC, and PLD): In vitro microassays, analysis of
enzyme isoforms, and intact-cell assays. Methods Mol Biol.
218:155–176. 2003.PubMed/NCBI
|
|
27
|
Henderson F, Johnston HR, Badrock AP,
Jones EA, Forster D, Nagaraju RT, Evangelou C, Kamarashev J, Green
M, Fairclough M, et al: Enhanced fatty acid scavenging and
glycerophospholipid metabolism accompany melanocyte neoplasia
progression in zebrafish. Cancer Res. 79:2136–2151. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JB, Lee CS, Jang JH, Ghim J, Kim YJ,
You S, Hwang D, Suh PG and Ryu SH: Phospholipase signalling
networks in cancer. Nat Rev Cancer. 12:782–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu J, Zhao X, Wang J, Liu C, Sun Y, Cai H
and Liu J: Plasma phospholipase A2 activity may serve as a novel
diagnostic biomarker for the diagnosis of breast cancer. Oncol
Lett. 15:5236–5242. 2018.PubMed/NCBI
|
|
30
|
Gomez-Cambronero J, Fite K and Miller TE:
How miRs and mRNA deadenylases could post-transcriptionally
regulate expression of tumor-promoting protein PLD. Adv Biol Regul.
68:107–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato Y, Lambert CA, Colige AC, Mineur P,
Noël A, Frankenne F, Foidart JM, Baba M, Hata RI, Miyazaki K and
Tsukuda M: Acidic extracellular pH induces matrix
metalloproteinase-9 expression in mouse metastatic melanoma cells
through the phospholipase D-mitogen-activated Protein Kinase
Signaling. J Biol Chem. 280:10938–10944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jenkins GM and Frohman MA: Phospholipase
D: A lipid centric review. Cell Mol Life Sci. 62:2305–2316. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gomez-Cambronero J: New concepts in
phospholipase D signaling in inflammation and cancer.
ScientificWorldJournal. 10:1356–1369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon MS, Rosenberger CL, Wu C, Truong N,
Sweedler JV and Chen J: Rapid mitogenic regulation of the mTORC1
inhibitor, DEPTOR, by phosphatidic acid. Mol Cell. 58:549–556.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin H, Gui Y, Du G, Frohman MA and Zheng
XL: Dependence of Phospholipase D1 multi-monoubiquitination on its
enzymatic activity and palmitoylation. J Biol Chem.
285:13580–13588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jang YH and Min DS: The hydrophobic amino
acids involved in the interdomain association of phospholipase D1
regulate the shuttling of phospholipase D1 from vesicular
organelles into the nucleus. Exp Mol Med. 44:571–577. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Donaldson JG: Phospholipase D in
endocytosis and endosomal recycling pathways. Biochim Biophys Acta.
1791:845–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dall'Armi C, Hurtado-Lorenzo A, Tian H,
Morel E, Nezu A, Chan RB, Yu WH, Robinson KS, Yeku O, Small SA, et
al: The phospholipase D1 pathway modulates macroautophagy. Nat
Commun. 1:1422010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh NK, Hansen DE III,
Kundumani-Sridharan V and Rao GN: Both Kdr and Flt1 play a vital
role in hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal
neovascularization. Blood. 121:1911–1923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Su Y and Wang X: Phosphatidic
Acid-Mediated Signaling. Lipid-mediated Protein Signaling.
Capelluto DGS: Springer Netherlands; Dordrecht: pp. 159–176. 2013,
View Article : Google Scholar
|
|
41
|
Frohman MA: The phospholipase D
superfamily as therapeutic targets. Trends Pharmacol Sci.
36:137–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee S and Lynch KR: Brown recluse spider
(Loxosceles reclusa) venom phospholipase D (PLD) generates
lysophosphatidic acid (LPA). Biochem J. 391:317–323. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Z, Zhang F, He J, Wu P, Tay LWR, Cai
M, Nian W, Weng Y, Qin L, Chang JT, et al: Binding of
PLD2-generated phosphatidic acid to KIF5B promotes MT1-MMP surface
trafficking and lung metastasis of mouse breast cancer cells. Dev
Cell. 43:186–197.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Borel M, Lollo G, Magne D, Buchet R,
Brizuela L and Mebarek S: Prostate cancer-derived exosomes promote
osteoblast differentiation and activity through phospholipase D2.
Biochim Biophys Acta Mol Basis Dis. 1866:1659192020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Muñoz-Galván S, Lucena-Cacace A, Perez M,
Otero-Albiol D, Gomez-Cambronero J and Carnero A: Tumor
cell-secreted PLD increases tumor stemness by senescence-mediated
communication with microenvironment. Oncogene. 38:1309–1323. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng Y, Rodrik V, Toschi A, Shi M, Hui L,
Shen Y and Foster DA: Phospholipase D couples survival and
migration signals in stress response of human cancer cells. J Biol
Chem. 281:15862–15868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Williger BT, Ho WT and Exton JH:
Phospholipase D mediates matrix metalloproteinase-9 secretion in
phorbol ester-stimulated human fibrosarcoma cells. J Biol Chem.
274:735–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Noble AR, Maitland NJ, Berney DM and
Rumsby MG: Phospholipase D inhibitors reduce human prostate cancer
cell proliferation and colony formation. Br J Cancer. 118:189–199.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Powner DJ and Wakelam MJO: The regulation
of phospholipase D by inositol phospholipids and small GTPases.
FEBS Lett. 531:62–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Santy LC and Casanova JE: Activation of
ARF6 by ARNO stimulates epithelial cell migration through
downstream activation of both Rac1 and phospholipase D. J Cell
Biol. 154:599–610. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chae YC, Kim JH, Kim KL, Kim HW, Lee HY,
Heo WD, Meyer T, Suh PG and Ryu SH: Phospholipase D activity
regulates integrin-mediated cell spreading and migration by
inducing gtp-rac translocation to the plasma membrane. Mol Biol
Cell. 19:3111–3123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hwang WC, Kang DW, Kang Y, Jang Y, Kim JA
and Min DS: Inhibition of phospholipase D2 augments histone
deacetylase inhibitor-induced cell death in breast cancer cells.
Biol Res. 53:342020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hwang WC, Kim MK, Song JH, Choi KY and Min
DS: Inhibition of phospholipase D2 induces autophagy in colorectal
cancer cells. Exp Mol Med. 46:e1242014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rodrik V, Zheng Y, Harrow F, Chen Y and
Foster DA: Survival signals generated by estrogen and phospholipase
D in MCF-7 breast cancer cells are dependent on myc. Mol Cell Biol.
25:7917–7925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hui L, Rodrik V, Pielak RM, Knirr S, Zheng
Y and Foster DA: MTOR-dependent suppression of protein phosphatase
2a is critical for phospholipase D survival signals in human breast
cancer cells. J Biol Chem. 280:35829–35835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hui L, Zheng Y, Yan Y, Bargonetti J and
Foster DA: Mutant p53 in MDA-MB-231 breast cancer cells is
stabilized by elevated phospholipase D activity and contributes to
survival signals generated by phospholipase D. Oncogene.
25:7305–7310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen Y, Rodrik V and Foster DA:
Alternative phospholipase D/mTOR survival signal in human breast
cancer cells. Oncogene. 24:672–679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Moolenaar WH, Kruijer W, Tilly BC, Verlaan
I, Bierman AJ and de Laat SW: Growth factor-like action of
phosphatidic acid. Nature. 323:171–173. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rizzo MA, Shome K, Vasudevan C, Stolz DB,
Sung TC, Frohman MA, Watkins SC and Romero G: Phospholipase D and
its product, phosphatidic acid, mediate agonist-dependent Raf-1
translocation to the plasma membrane and the activation of the
mitogen-activated protein kinase pathway. J Biol Chem.
274:1131–1139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bruntz RC, Lindsley CW and Brown HA:
Phospholipase D signaling pathways and phosphatidic acid as
therapeutic targets in cancer. Pharmacol Rev. 66:1033–1079. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao C, Du G, Skowronek K, Frohman MA and
Bar-Sagi D: Phospholipase D2-generated phosphatidic acid couples
EGFR stimulation to Ras activation by Sos. Nat Cell Biol.
9:707–712. 2007. View Article : Google Scholar
|
|
64
|
Chen Y, Zheng Y and Foster DA:
Phospholipase D confers rapamycin resistance in human breast cancer
cells. Oncogene. 22:3937–3942. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ghim J, Moon JS, Lee CS, Lee J, Song P,
Lee A, Jang JH, Kim D, Yoon JH, Koh YJ, et al: Endothelial deletion
of phospholipase d2 reduces hypoxic response and pathological
angiogenesis. Arterioscler Thromb Vasc Biol. 34:1697–1703. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Park MH, Ahn BH, Hong YK and Min DS:
Overexpression of phospholipase D enhances matrix
metalloproteinase-2 expression and glioma cell invasion via protein
kinase C and protein kinase A/NF-κB/Sp1-mediated signaling
pathways. Carcinogenesis. 30:356–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kang DW, Park MH, Lee YJ, Kim HS, Lindsley
CW, Brown HA and Min DS: Autoregulation of phospholipase D activity
is coupled to selective induction of phospholipase D1 expression to
promote invasion of breast cancer cells. Int J Cancer. 128:805–816.
2011. View Article : Google Scholar : PubMed/NCBI
|