Introduction
Breast-conserving surgery followed by breast
irradiation has become the standard therapy for patients with early
breast cancer (EBC) (1). In
3-dimensional conformal radiotherapy (3DCRT), tangentially opposed
beams deliver radiation to the ipsilateral whole breast. However,
ipsilateral whole breast irradiation results in acute toxicities to
organs at risk (OAR), such as the ipsilateral lung and heart
(2). As such, considerable efforts
have been made to minimize the irradiation dose to adjacent normal
tissues to avoid acute and long-term adverse effects in patients
with breast cancer.
Recently, the paradigm of radiotherapy (RT) for
patients with EBC has been changing from conventional 3DCRT to
hypofractionated (HFX) intensity-modulated radiation therapy
(IMRT). HFX–IMRT, including volumetric modulated arc therapy
(VMAT), can improve the accuracy of radiation delivery. In
addition, HFX-RT can shorten the duration of RT, which is highly
beneficial to patients (3). HFX-RT
also has comparable results to conventional RT regarding local
control rate and skin toxicity (4–7).
Existing studies (4,5,8–11),
which include the treatment results of various RT techniques,
fields, or doses, demonstrate that despite the improvement in RT
technology, there are still concerns about the side effects on
adjacent normal organs. Therefore, the present study aimed to
analyze the acute and subacute RT toxicities of patients with EBC
who underwent breast-only HFX–VMAT at a single institution. It also
investigated the prognostic factors of radiation physics related to
radiation pneumonitis (RP) and dermatitis.
Materials and methods
Patients
The present study included 23 patients who underwent
breast-conserving surgery and HFX–VMAT between September 2021 and
February 2022 at Hanyang University Hanmaeum Changwon Hospital
(Changwon, South Korea). The inclusion criteria were as follows: i)
Histologically confirmed invasive breast cancer; ii) EBC with
pathologic tumor (T) staging of Tis to T2 and node-negative
staging, according to the 8th edition of the American Joint
Committee on Cancer (12); iii) no
prior RT to the thorax; and iv) the presence of follow-up chest
computed tomography (CT). The study was approved by the Korean
National Institute for Bioethics Policy (approval no.
P01-202207-01-025). Patient and tumor characteristics are given in
Table I.
 | Table I.Patient and tumor characteristics
(n=23). |
Table I.
Patient and tumor characteristics
(n=23).
| Characteristic | Value |
|---|
| Age,
yearsa | 50 (31–66) |
| Site, n (%) |
|
|
Right | 8 (34.8) |
| Left | 15 (65.2) |
| Pathological tumor
stage, n (%) |
|
| Tis | 4 (17.4) |
| T1 | 14 (60.9) |
| T2 | 5 (21.7) |
| Pathological nodal
stage, n (%) |
|
|
Yes | 2 (8.7) |
| No | 21 (91.3) |
| Neoadjuvant
chemotherapy, n (%) |
|
|
Yes | 2 (8.7) |
| No | 21 (91.3) |
| Adjuvant
chemotherapy, n (%) |
|
|
Yes | 3 (13) |
| No | 20 (87) |
| Hormone therapy, n
(%) |
|
|
Yes | 20 (87) |
| No | 3 (13) |
| Resection margin, n
(%) |
|
|
Negative | 11 (47.8) |
| Close
or positive | 12 (52.2) |
| Ipsilateral whole
breast target volume, cm3a | 418.07
(196.67-978.71) |
| Ipsilateral tumor
bed target volume, cm3a | 79.66
(38.48-277.56) |
Simulation and treatment planning
All patients underwent CT simulation in the supine
position and both arms were immobilized using a wing board. Free
breathing was facilitated during the simulation and each treatment.
In all cases, the prescribed dose to the ipsilateral whole breast
was 40.05 Gy in 15 fractions of 2.67 Gy, 5 days per week for 3
weeks. The dose for tumor bed boost was 10 Gy in 4 fractions for
clear resection margins and 12.5 Gy in 5 fractions for close or
positive resection margins, using 2.5 Gy per fraction for 1 week. A
total of 3 patients received electron-boost RT, while the remainder
received boost RT using VMAT. None of the patients received
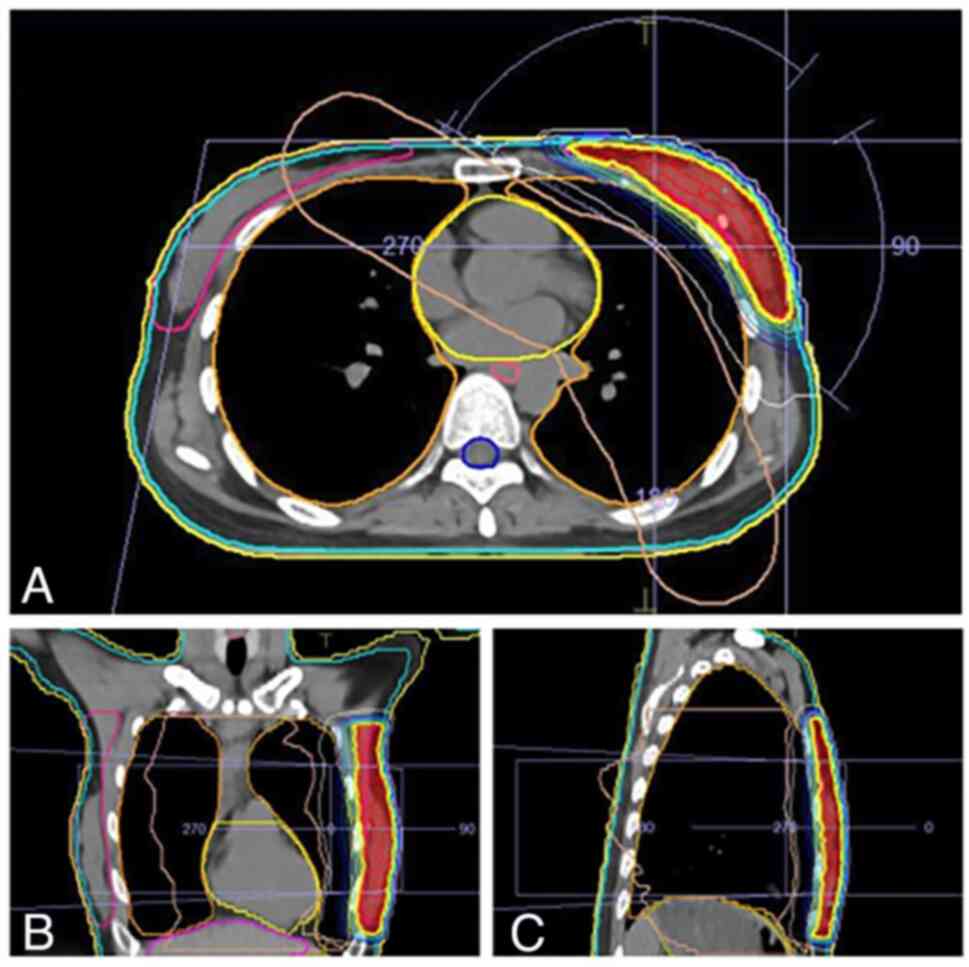
regional nodal irradiation. The VMAT plan in the current study
consisted of two partial arc beams that geometrically resembled the
breast. The arrangement of the beams was optimized to lower the OAR
doses while improving the planning target volume (PTV) coverage by
resembling the breast shape using tangential arcs. The axillary
nodal area was not included in the PTV. The VMAT treatment plans
were produced by the Monaco RTP system (Elekta Instrument AB
Stockholm) using an Elekta Versa HD treatment machine (Elekta
Instrument AB Stockholm) with 5 mm multileaf collimators (MLC) for
modeling. A total of two tangential photon arcs of 6 MV with arc
lengths of 240°, were applied to attain the prescribed dose
(Fig. 1). The optimization plan
produced by the Monaco RTP system provided >95% coverage of the
target isodose and minimized the OAR tolerance dose. The dynamic
MLC arc moved in the range of acute angles based on the angle of
incidence of the lungs. Thus, the radiation dose to the ipsilateral
lung was minimized. The dose for OAR was limited as follows: for
the ipsilateral lung, V5 (the percentage volume
receiving 5 Gy) <0%, V10 (the percentage volume
receiving 10 Gy) <35%, and V20 (the percentage volume
receiving 20 Gy) <20%, mean heart dose <Gy and mean dose for
contralateral breast <2 Gy.
Clinical and dosimetric analysis
Acute toxicities were defined during treatment and
within 6 months post-RT. Regular follow-up visits and chest CTs
were performed at 3 and 6 months post-RT. Lung and skin toxicities
were graded using the Common Terminology Criteria for Adverse
Events v.5.0 (13). The conformity
index (CI) and homogeneity index (HI) were analyzed as follows:
CI=VRI/TV (where VRI and TV are the reference
isodose volume and target volume, respectively), and
HI=Imax/RI (where Imax and RI are the maximum
isodose in the target and reference isodoses, respectively)
(14). The OAR dose and volume
related to RP were analyzed using the Mann-Whitney U test and
Fisher's exact test. Statistical analysis was performed using SPSS
v.19 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical analysis
Patient and tumor characteristics are summarized in
Table I. None of the patients had
underlying lung disease or a history of smoking. Among the 23
patients, eight had right-sided breast tumors and 15 had left-sided
breast tumors. A total of 12 patients (52.2%) received 12.5 Gy in 5
fractions due to close or positive resection margins. Three
patients received electron-boost RT; the remainder received boost
RT using VMAT. The median follow-up duration was 10.1 months
(range, 7.6-11.9). RP developed in seven patients (30.4%) at a
median of 3.8 months (range, 2.3-4.2) post-treatment. None of these
patients presented with RP-related symptoms; the diagnosis was
based on radiologic findings observed on follow-up chest CT. Among
the seven patients with RP, five had right-sided breast tumors, and
two had left-sided breast tumors (71.4 vs. 28.6%, P=0.026). Grade 1
erythema was observed in 19 patients (82.6%), and four presented
with grade 2 erythema (17.4%).
Dosimetric analysis
The RT characteristics are listed in Table II. In this study, the median
ipsilateral whole breast target volume was 418.1 cm3
(range, 196.7-978.7), and the median ipsilateral tumor bed target
volume was 79.7 cm3 (range, 38.5-277.6). In the
univariate analysis (Table III),
the mean target dose, D105% (the dose received by 105%
of the target volume), HI, mean lung dose, and ipsilateral lung
V20 and V30 for ipsilateral whole breast RT
were significantly associated with RP (P=0.039, 0.047, 0.018,
0.015, 0.018 and 0.003, respectively).
 | Table II.Radiotherapy characteristics
(n=23). |
Table II.
Radiotherapy characteristics
(n=23).
| A, Ipsilateral
whole breast target |
|---|
|
|---|
| Characteristic | Median (range) |
|---|
| Mean target dose,
Gy | 40.19
(39.35–40.91) |
|
D95%, % | 95.69
(95.01–98.31) |
|
D105%, % | 5 (0–5) |
| CI | 0.96
(0.95–0.98) |
| HI | 1.15
(1.10–1.18) |
| Mean lung dose,
Gy | 6.68
(4.08–8.56) |
| Lung
V5 Gy, % | 38.18
(22.44–49.98) |
| Lung
V10 Gy, % | 20.14
(12.56–33.14) |
| Lung
V20 Gy, % | 7.32
(3.01–13.31) |
| Lung
V30 Gy, % | 1.24
(0.03–5.03) |
| Mean heart dose,
Gy | 0.47
(0.05–1.12) |
| Heart
Dmax, Gy | 1.93
(0.28–4.19) |
|
| B, Ipsilateral
tumor bed target |
|
|
Characteristic | Median
(range) |
|
| Mean target dose,
Gy | 12.32
(9.85–13.12) |
|
D95%, % | 96.08
(92.56–99.65) |
|
D105%, % | 0 (0–99.27) |
|
D110%, % | 0 (0–98.19) |
| CI | 0.96
(0.93–1.00) |
| HI | 1.16
(1.09–1.53) |
| Mean lung dose,
Gy | 1.01
(0.11–1.88) |
| Lung V5
Gy, % | 1.34 (0–5.33) |
| Lung V10
Gy, % | 0 (0–0.05) |
| Mean heart dose,
Gy | 0.47
(0.05–1.12) |
| Heart
Dmax, Gy | 1.93
(0.28–4.19) |
 | Table III.Univariate analysis of factors for
radiation pneumonitis and dosimetry. |
Table III.
Univariate analysis of factors for
radiation pneumonitis and dosimetry.
|
| Radiation
pneumonitis |
|
|---|
|
|
|
|
|---|
|
| No | Yes |
|
|---|
|
|
|
|
|
|---|
| Variable | Mean (SD) | Mean (SD) | P-value |
|---|
| Ipsilateral whole
breast target |
|
|
|
|
Volume | 443.04
(219.44) | 421.96
(151.66) | 0.871 |
| Mean
target dose, Gy | 40.24 (0.31) | 40.00 (0.36) | 0.039 |
|
D95%,
% | 95.97 (0.95) | 95.55 (0.63) | 0.154 |
|
D105%,
% | 3.26 (2.22) | 2.18 (2.64) | 0.047 |
|
CI | 0.96 (0.01) | 0.96 (0.01) | 0.871 |
|
HI | 1.15 (0.01) | 1.13 (0.02) | 0.018 |
| Mean
lung dose, Gy | 6.35 (1.11) | 7.14 (0.72) | 0.015 |
|
Ipsilateral lung V5 Gy, % | 36.63 (8.40) | 39.23 (3.27) | 0.974 |
|
Ipsilateral lung V10 Gy,
% | 20.08 (5.28) | 21.94 (2.99) | 0.118 |
|
Ipsilateral lung V20 Gy,% | 7.47 (2.42) | 9.60 (3.70) | 0.018 |
|
Ipsilateral lung V30 Gy,
% | 1.34 (1.24) | 3.27 (2.16) | 0.003 |
| Ipsilateral tumor
bed target |
|
|
|
|
Volume |
|
|
|
|
Mean target dose
(Gy) | 11.29 (1.32) | 12.00 (1.36) | 0.018 |
|
D95%
(%) | 96.14 (2.04) | 96.41 (1.55) | 0.341 |
|
D105%
(%) | 5.21 (15.19) | 14.91 (37.22) | 0.222 |
|
D110%
(%) | 0.88 (3.44) | 14.03 (37.11) | 0.922 |
|
CI | 0.96 (0.02) | 0.97 (0.01) | 0.341 |
|
HI | 1.14 (0.05) | 1.21 (0.14) | 0.198 |
| Mean lung dose,
Gy | 1.04 (0.47) | 0.83 (0.50) | 0.922 |
| Ipsilateral lung
V5 Gy, % | 1.85 (1.73) | 1.65 (1.55) | 0.123 |
| Ipsilateral lung
V10 Gy,% | 0.00 (0.01) | 0.01 (0.02) | 0.118 |
Discussion
The current study investigated the acute and
subacute toxicities of HFX–VMAT in patients with EBC. It showed
tolerable skin reactions of grade 2 or less and the occurrence of
asymptomatic RP (30.4%). The present study identified a correlation
of the following dosimetric factors with RP: Mean target dose,
D105%, HI, mean lung dose and ipsilateral lung
V20 and V30 for ipsilateral whole breast
RT.
Traditionally, the radiation dose schedule for
ipsilateral whole breast consisted of 45–50.4 Gy in fractions of
1.8–2 Gy over 5 weeks. In radiobiological terms, the α/β ratio is
one of determinants of radiosensitivity, and breast cancer has a
relatively low α/β ratio of 4 Gy (8). Therefore, breast cancer responds more
sensitively to radiation therapy when the fraction size is larger
than conventional fraction sizes. The START trial (7), a long-term follow-up HFX-RT study,
showed the local control rate in breast cancer treatment with
HFX-RT to be equivalent, with fewer or no differences in side
effects to previous studies on conventional fractionation. Based on
these studies (7,15,16),
HFX-RT has been established as the standard treatment, with 40–40.5
Gy in 15–16 fractions for the ipsilateral whole breast (17). In 2018, the American Society for
Radiation Oncology (ASTRO) (18)
recommended an HFX regimen of 40 Gy in 15 fractions or 42.5 Gy in
16 fractions. The biologically effective doses in these regimens
(17,18), computed by the linear-quadratic
model and using α/β=4 for breast cancer, were equivalent to
44.8-48.9 Gy in fractions of 1.8 Gy.
RP, an acute inflammatory reaction with exudation in
the alveolar space, develops within 4–12 weeks of breast RT and can
result in changes in radiologic findings or lung function (19–23).
Symptomatic RP includes dry cough, dyspnea, chest discomfort, or
mild fever. A number of studies have reported a relationship
between the risk of RP and dosimetric parameters, including total
radiation dose, fractionation, mean lung dose and irradiated lung
volume, such as V5, V10, V20, and
V30 (22,24,25).
Advanced techniques, such as IMRT, VMAT and helical tomotherapy,
can improve dosimetry and reduce acute toxicities through the RT
planning process (26,27); thus, they are also useful in HFX-RT.
In the present study, irradiated lung volume was restricted to
reduce the risk of RP. The radiation dose constraints for the
ipsilateral lung in the current study fall within the ranges of
lung cancer RT guidelines (28).
Several studies have also tried to control the OAR dose, especially
to prevent RP (27–30) and showed an OAR dose constraint
range comparable to that of the current study.
Based on published data (26–32),
the current study applied the valid lung dosing constraints to plan
HFX–VMAT. For OAR, the doses were limited as follows: For
ipsilateral lung, V5 <50%, V10 <35% and
V20 <20%; mean heart dose <3 Gy; and mean
contralateral breast dose <2 Gy. It is widely reported that
limiting dosimetric parameters, such as V20 or
V30, contributes to reducing the risk of RP (27,29).
In the VMAT study with simultaneous integrated boost (32), which prescribed 40.5 and 48 Gy in 15
fractions to the ipsilateral whole breast and tumor bed boost,
respectively, certain dose limits were established. The mean dose
limits were defined as <10 Gy and V20 <10% for the
ipsilateral lung, while the mean heart dose was limited to <4
Gy. When planning RT, the current study restricted V5 of
the ipsilateral lung with a median value of 38.18%, which provided
evidence that a low radiation dose induces RP (30,33).
McKenzie et al (34)
prescribed 42.56 Gy in 16 fractions and set lung constraints at
30–35% for V17.5, which, in HFX regimens, is
radiobiologically equivalent to V20 in conventional
regimens. Following their HFX regimen, they reported a range of
24–36% of V17.5 and two cases of RP. In 2018, ASTRO
recommended that, in HFX regimens, the V16 of the
ipsilateral lungs be limited to 15–20% (18). In the current study, the
V20 of the ipsilateral lungs ranged from 3.01 to 13.31%,
falling within the ASTRO guidelines.
The primary limitations of the current study are the
small sample size and the focus on acute/subacute toxicity. The
factors that caused radiation pneumonitis included interstitial
lung disease, chronic obstructive pulmonary disease and diabetes
mellitus. The present study included only two cases with histories
of diabetes, due to its small sample size, and thus there was no
statistically significant analysis of diabetes and radiation
pneumonitis. Therefore, it is necessary to conduct a follow-up
study with a larger sample size. The present study also showed that
RP did not occur in the group with a high mean dose of breast
target. It is hypothesized that the inclination of clinicians to
prioritize lung dose reduction is reflected in the selection of RT
plans to satisfy the condition of target isodose coverage of more
than 95%. During the RT planning stage, the current study conducted
a comparison of multiple RT plans. Among these plans, it selected
one that achieved a target isodose coverage of over 95% while
further reducing the lung dose. However, rather than focusing just
on the tumor, it chose a plan with a relatively lower mean lung
dose. In the process of lowering the mean lung dose, the lung
constraint was given more weight, resulting in higher mean and
maximum target dose.
However, the strengths of the current study are that
it was conducted at a single institution and focused on a single
radiation technique, HFX–VMAT. The VMAT technique is widely used to
treat various cancers, and the HFX-RT schedule has been applied to
breast cancer treatment (35,36).
Thus, the focus of the current study on HFX–VMAT is novel and
provides convincing data for the literature. The data on long-term
follow-up after HFX-RT in EBC is lacking, especially for IMRT,
including VMAT, despite reports of late toxicity, such as fibrosis
and secondary malignancy (7,26,27,32,37).
Further research would be helpful in identifying the long-term
treatment outcomes after HFX-RT in EBC.
In conclusion, the current study demonstrated
tolerable acute and subacute toxicities following HFX–VMAT and the
correlation between its dosimetric parameters with RP in EBC
patients. It suggested that HFX–VMAT can be an effective and safe
treatment option for EBC and that RT planning must be considered to
reduce the incidence of RP. However, a longer follow-up is
necessary to determine the long-term outcomes following HFX-RT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MC and JL confirm the authenticity of all the raw
data. MC and JL designed the study and performed the analysis and
interpretation of data. JL drafted the manuscript. MC conceived the
review and revised the manuscript. Both authors have read and
approved the manuscript.
Ethics approval and consent to
participate
The study was approved by the Korean National
Institute for Bioethics Policy (approval no.
P01-202207-01-025).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al:
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rudat V, Nour A, Hammoud M and Ghaida SA:
Better compliance with hypofractionation vs. conventional
fractionation in adjuvant breast cancer radiotherapy: Results of a
single, institutional, retrospective study: Results of a single,
institutional, retrospective study. Strahlenther Onkol.
193:375–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yarnold J, Ashton A, Bliss J, Homewood J,
Harper C, Hanson J, Haviland J, Bentzen S and Owen R: Fractionation
sensitivity and dose response of late adverse effects in the breast
after radiotherapy for early breast cancer: Long-term results of a
randomised trial. Radiother Oncol. 75:9–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
START Trialists' Group, . Bentzen SM,
Agrawal RK, Aird EGA, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss
JM, Brown J, Dewar JA, et al: The UK Standardisation of Breast
Radiotherapy (START) Trial B of radiotherapy hypofractionation for
treatment of early breast cancer: A randomised trial. Lancet.
371:1098–1107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hopwood P, Haviland JS, Sumo G, Mills J,
Bliss JM and Yarnold JR; START Trial Management Group, : Comparison
of patient-reported breast, arm, and shoulder symptoms and body
image after radiotherapy for early breast cancer: 5-year follow-up
in the randomised Standardisation of Breast Radiotherapy (START)
trials. Lancet Oncol. 11:231–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haviland JS, Owen JR, Dewar JA, Agrawal
RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA,
Magee BJ, et al: The UK standardisation of breast radiotherapy
(START) trials of radiotherapy hypofractionation for treatment of
early breast cancer: 10-year follow-up results of two randomised
controlled trials. Lancet Oncol. 14:1086–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Owen JR, Ashton A, Bliss JM, Homewood J,
Harper C, Hanson J, Haviland J, Bentzen SM and Yarnold JR: Effect
of radiotherapy fraction size on tumour control in patients with
early-stage breast cancer after local tumour excision: Long-term
results of a randomised trial. Lancet Oncol. 7:467–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
START Trialists' Group, . Bentzen SM,
Agrawal RK, Aird EGA, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown
J, Dewar JA, Dobbs HJ, et al: The UK Standardisation of Breast
Radiotherapy (START) Trial A of radiotherapy hypofractionation for
treatment of early breast cancer: A randomised trial. Lancet Oncol.
9:331–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Attar MA, Bahadur YA, Constantinescu CT
and Eltaher MM: Lung dose analysis in loco-regional
hypofractionated radiotherapy of breast cancer. Saudi Med J.
37:631–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Linares I, Tovar MI, Zurita M, Guerrero R,
Expósito M and Del Moral R: Hypofractionated breast radiation:
Shorter scheme, lower toxicity. Clin Breast Cancer. 16:262–268.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Byrd DR, Brookland RK, Washington MK,
Gershenwald JE, Compton CC, Hess KR, Sullivan DC and Jessup JM:
Part XI Breast. AJCC Cancer Staging Manual. Amin MB, Edge SB and
Greene FL: 8th edition. Springer International Publishing;
Heidelberg, Germany: pp. 589–636. 2017
|
|
13
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdfJune
1–2023
|
|
14
|
Petrova D, Smickovska S and Lazarevska E:
Conformity index and homogeneity index of the postoperative whole
breast radiotherapy. Open Access Maced J Med Sci. 5:736–739. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whelan TJ, Pignol JP, Levine MN, Julian
JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H,
et al: Long-term results of hypofractionated radiation therapy for
breast cancer. N Engl J Med. 362:513–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Offersen BV, Alsner J, Nielsen HM,
Jakobsen EH, Nielsen MH, Krause M, Stenbygaard L, Mjaaland I,
Schreiber A, Kasti UM, et al: Hypofractionated versus standard
fractionated radiotherapy in patients with early breast cancer or
ductal carcinoma in situ in a randomized phase III trial: The DBCG
HYPO trial. J Clin Oncol. 38:3615–3625. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Comprehensive Cancer Network
(NCCN). NCCN Guidelines, . Breast Cancer. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419June
21–2022
|
|
18
|
Smith BD, Bellon JR, Blitzblau R, Freedman
G, Haffty B, Hahn C, Halberg F, Hoffman K, Horst K, Moran J, et al:
Radiation therapy for the whole breast: Executive summary of an
American society for radiation oncology (ASTRO) evidence-based
guideline. Pract Radiat Oncol. 8:145–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krengli M, Sacco M, Loi G, Masini L,
Ferrante D, Gambaro G, Ronco M, Magnani C and Carriero A: Pulmonary
changes after radiotherapy for conservative treatment of breast
cancer: a prospective study. Int J Radiat Oncol Biol Phys.
70:1460–1467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verbanck S, Hanon S, Schuermans D, Van
Parijs H, Vinh-Hung V, Miedema G, Verellen D, Storme G, Fontaine C,
Lamote J, et al: Mild lung restriction in breast cancer patients
after hypofractionated and conventional radiation therapy: A 3-year
follow-up. Int J Radiat Oncol Biol Phys. 95:937–945. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanania AN, Mainwaring W, Ghebre YT,
Hanania NA and Ludwig M: Radiation-induced lung injury: Assessment
and management. Chest. 156:150–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehnati P, Ghorbanipoor M, Mohammadzadeh
M, Motlagh BN and Mesbahi A: Predicting the risk of radiation
pneumonitis and pulmonary function changes after breast cancer
radiotherapy. J Biomed Phys Eng. 11:459–464. 2021.PubMed/NCBI
|
|
23
|
Konkol M, Śniatała P and Milecki P:
Radiation-induced lung injury-what do we know in the era of modern
radiotherapy? Rep Pract Oncol Radiother. 27:552–565.
2022.PubMed/NCBI
|
|
24
|
Marks LB, Bentzen SM, Deasy JO, Kong FMS,
Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV,
Timmerman RD, et al: Radiation dose-volume effects in the lung. Int
J Radiat Oncol Biol Phys. 76:S70–S76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McKenzie E, Razvi Y, Wronski M, Zhang L,
Bosnic S, Vesprini D, Paszat L, Rakovitch E, Drost L, Yee C, et al:
Trends and correlates of mean lung dose in patients receiving
breast radiotherapy in a single institution from 2014 to 2018. Clin
Oncol (R Coll Radiol). 32:647–655. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dicuonzo S, Leonardi MC, Raimondi S,
Corrao G, Bagnardi V, Gerardi MA, Morra A, Zerella MA, Zaffaroni M,
Pansini F, et al: Acute and intermediate toxicity of 3-week
radiotherapy with simultaneous integrated boost using TomoDirect:
Prospective series of 287 early breast cancer patients. Clin Transl
Oncol. 23:1415–1428. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jagsi R, Griffith KA, Moran JM, Matuszak
MM, Marsh R, Grubb M, Abu-Isa E, Dilworth JT, Dominello MM,
Heimburger D, et al: Comparative effectiveness analysis of
3D-conformal radiation therapy versus intensity modulated radiation
therapy (IMRT) in a prospective multicenter cohort of patients with
breast cancer. Int J Radiat Oncol Biol Phys. 112:643–653. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
National Comprehensive Cancer Network
(NCCN). NCCN Guidelines, . Non-Small Cell Lung Cancer. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450September
26–2022
|
|
29
|
Goldman UB, Wennberg B, Svane G, Bylund H
and Lind P: Reduction of radiation pneumonitis by V20-constraints
in breast cancer. Radiat Oncol. 5:992010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doi Y, Nakao M, Miura H, Ozawa S, Kenjo M
and Nagata Y: Hybrid volumetric-modulated arc therapy for
postoperative breast cancer including regional lymph nodes: the
advantage of dosimetric data and safety of toxicities. J Radiat
Res. 61:747–754. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee BM, Chang JS, Kim SY, Keum KC, Suh CO
and Kim YB: Hypofractionated radiotherapy dose scheme and
application of new techniques are associated to a lower incidence
of radiation pneumonitis in breast cancer patients. Front Oncol.
10:1242020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franceschini D, Fogliata A, Spoto R,
Dominici L, Lo Faro L, Franzese C, Comito T, Lobefalo F, Reggiori
G, Cozzi L, et al: Long term results of a phase II trial of
hypofractionated adjuvant radiotherapy for early-stage breast
cancer with volumetric modulated arc therapy and simultaneous
integrated boost. Radiother Oncol. 164:50–56. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang S, Liao Z, Wei X, Liu HH, Tucker SL,
Hu CS, Mohan R, Cox JD and Komaki R: Analysis of clinical and
dosimetric factors associated with treatment-related pneumonitis
(TRP) in patients with non-small-cell lung cancer (NSCLC) treated
with concurrent chemotherapy and three-dimensional conformal
radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 66:1399–1407.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McKenzie E, Razvi Y, Bosnic S, Wronski M,
Karam I, Vesprini D, Rakovitch E, Soliman H, Wong G and Chow E:
Case series of radiation pneumonitis in breast cancer. J Med
Imaging Radiat Sci. 53:167–174. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Otsuka K, Otsuka M, Itaya T, Matsumoto A,
Sato R, Sagara Y, Oga M and Asayama Y: Risk factors for rectal
bleeding after volumetric-modulated arc radiotherapy of prostate
cancer. Rep Pract Oncol Radiother. 28:15–23. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu K, Xu X, Li X, Wang J, Zhu L, Chen X,
Wang B, Zhang M, Xia B and Ma S: Radiation pneumonitis in lung
cancer treated with volumetric modulated arc therapy. J Thorac Dis.
10:6531–6539. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Najas GF, Stuart SR, Marta GN, Teixeira
LAB, de Carvalho Gico V, Serante AR, Mauro GP, Lima MC and de
Andrade Carvalho H: Hypofractionated radiotherapy in breast cancer:
A 10-year single institution experience. Rep Pract Oncol Radiother.
26:920–927. 2021. View Article : Google Scholar : PubMed/NCBI
|