Introduction
Upregulation of human epidermal growth factor
receptor 2 (HER2) is very common in breast cancer, which makes it
an important target for systemic treatment (1). In the last decade, HER2-targeted
antibody-drug conjugates (ADCs) have been emerging agents for
HER2-positive breast cancer (2).
Trastuzumab emtansine (T-DM1) has been approved by the U.S. Food
and Drug Administration not only for metastatic or recurrent
disease, but also as a postoperative or adjuvant therapy for early
stages of the disease (2).
Disitamab vedotin (RC48) is a novel HER2-targeted ADC, the
cytotoxic payload of which is the microtubule inhibitor monomethyl
auristatin E (3). RC48 has been
granted marketing approval as later-line treatment for locally
advanced or metastatic HER2-positive gastric cancer by The National
Medical Products Administration (NMPA) of China. RC48 has also
exhibited promising efficacy in a phase I study for both
HER2-positive and HER2-low breast cancer (4,5). The
objective response rates (ORRs) were 31.4% (22/70) and 39.6%
(19/48) in the HER2-positive and HER2-low populations,
respectively. The median progression-free survival (PFS) times were
5.8 and 5.7 months in the HER2-positive and HER2-low populations,
respectively.
Immune checkpoint inhibitors (ICIs), such as
programmed cell death protein-1 (PD-1)/PD-1 ligand (PD-L1)
inhibitors, have changed the treatment landscapes of various
malignant tumors, which constitute the present mainstay of systemic
anti-cancer therapies along with cytotoxic agents and targeted
agents (6). PD-1/PD-L1 inhibitors
are widely recommended for a variety of solid tumors, and
pembrolizumab, with/without chemotherapy, is recommended for
HER2-negative or immune biomarker positive (for example,
microsatellite instability-high and mismatch repair deficient)
patients with breast cancer, according to current breast cancer
guidelines (7). Zimberelimab (also
referred to as GLS-010 and AB122), is a novel fully humanized
anti-PD-1 monoclonal antibody, which has exhibited marked antitumor
activities for several tumors in an early clinical trial (8). In a phase II study, zimberelimab
monotherapy was revealed to be associated with an ORR of 90.6% in
Chinese patients with relapsed or refractory classical Hodgkin's
lymphoma (9) and was thus approved
by the NMPA (10). In phase II
studies of previously treated advanced cervical cancer,
zimberelimab monotherapy was reported to be associated with a
higher ORR compared with pembrolizumab monotherapy (26.83 vs.
14.6%; non-head-to-head) in a PD-L1-positive [combined positive
score (CPS) ≥1] population (11,12).
To the best of our knowledge, it remains unclear
whether re-introduction of another ADC targeting the same molecule,
with the same type of cytotoxic payload, is effective for
ADC-treated HER2-positive breast cancer. In addition, there are few
reports addressing the efficacy and safety of ADC combined with
ICIs in recurrent or metastatic HER2-positive breast cancer. The
present report describes a 44-year-old female patient with
recurrent metastatic PD-L1-negative, HER2-positive breast cancer,
whose disease recurred in the chest wall during adjuvant treatment
with T-DM1 (Kadcyla®) within 6 months. The patient then
received combination treatment (inetetamab, pyrotinib and
vinorelbine), followed by anlotinib (tyrosine kinase inhibitor)
monotherapy. Due to poor tumor response, the patient was
subsequently switched to another ADC, RC48 in combination with
zimberelimab. The recurrent tumor lesions in the chest wall almost
disappeared after six cycles of combination therapy and remain in
remission at present.
Case report
In early March 2021, a 44-year-old, premenopausal
female patient was referred to Sanhuan Cancer Hospital (Beijing,
China) due to a suspected breast malignant tumor [Breast Imaging
Reporting and Data System (BI-RADS) ultrasound category 4], with
initial symptoms of a mass in their left breast, dermal edema (peau
d'orange) and enlarged left axillary lymph nodes. Later in March
2021, at the same hospital, magnetic resonance imaging (MRI)
confirmed that the left breast malignant tumor (multiple nodules;
maximum size, 4.9×1.9 cm; BI-RADS category 5) was metastatic to the
left supraclavicular, axillary and internal mammary lymph nodes.
Subsequently, the patient was pathologically diagnosed with grade
III invasive breast cancer by needle biopsy using hematoxylin and
eosin staining and a Ventana Benchmark XT system (Roche Tissue
Diagnostics), according to the manufacturer's instructions
(Fig. S1A and B) (13). Automated immunohistochemistry was
also performed on 4-µm whole-tissue sections using a Ventana
Benchmark XT system according to the manufacturer's instructions
(antibodies are listed in Table
SI). Histopathological examination was performed and images
captured using a light microscope (Nikon ECLIPSE 80i; Nikon
Corporation). The immunohistochemistry (IHC) scores were as follows
[according to the American Society of Clinical Oncology guidelines
(14,15)]: Estrogen receptor (ER)+, 40%
(Fig. S2A and B); progesterone
receptor (PR)- (Fig. S3A and B);
HER2 3+ (Fig. S4A and B); P53+,
95%; Ki-67 proliferation marker, 80%; and epidermal growth factor
receptor (EGFR) 2+. The tumor node metastasis (TNM) classification
was cT4N3M0, stage IIIc (American Joint Committee on Cancer, 8th
edition) (16).
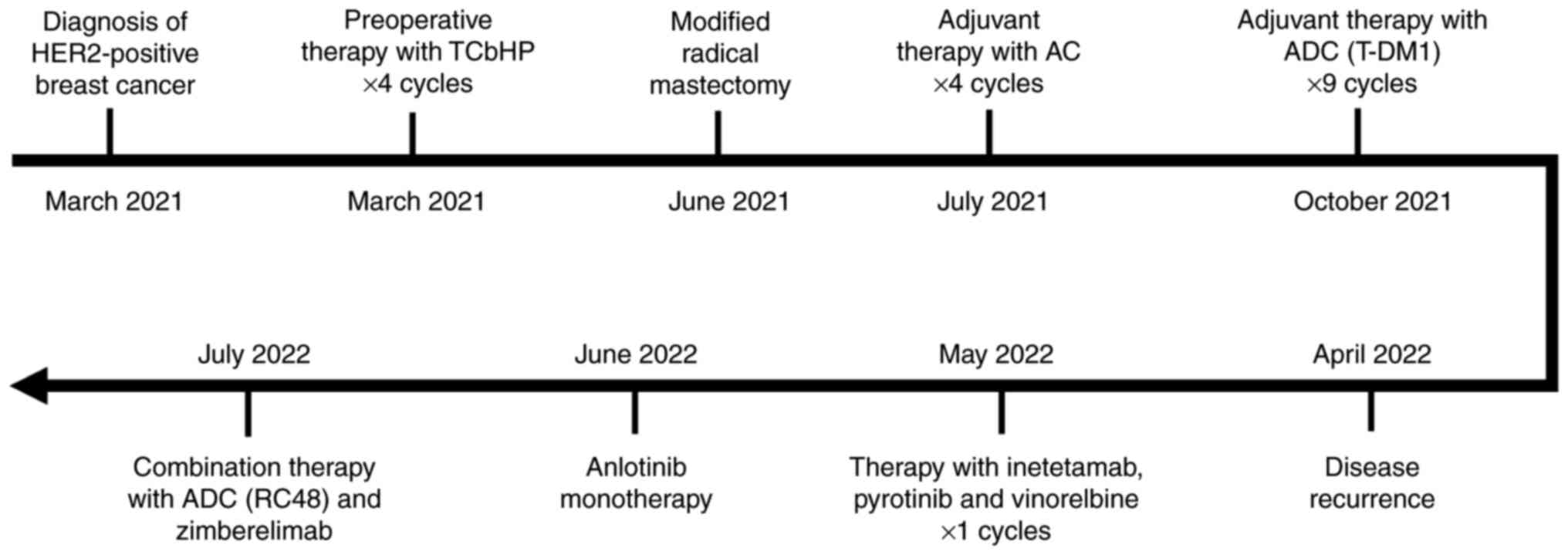
The patient received preoperative systemic treatment
with 270 mg paclitaxel liposome on day 2, 500 mg carboplatin on day
3, trastuzumab on day 1 (first cycle at 8 mg/kg and subsequent
cycles at 6 mg/kg) and pertuzumab on day 1 (first cycle at 840 mg
and subsequent cycles at 420 mg), intravenously (IV), every 3 weeks
(Q3W) (TCbHP) for four cycles (Fig.
1). However, in June 2021, MRI indicated that the left breast
lesions and axillary nodes were progressively enlarged compared
with the previous imaging scan. The patient received the last dose
of trastuzumab and pertuzumab, and then underwent modified radical
mastectomy later in June 2021. The postoperative pathology
demonstrated that the tumor was 7.0×4.0×3.8 cm in size, with a
negative margin, positive axillary nodes (2/17), and lymphovascular
and nerve invasion. IHC indicated the following: ER 2+, 30%; PR-;
HER2 1+; Ki-67, 40%; P53+; and EGFR+. Between July and October
2021, the patient received adjuvant therapy with 20 mg doxorubicin
liposome on day 1, 40 mg on day 2 and 1,000 mg cyclophosphamide on
day 1, IV, Q3W (termed AC) for four cycles. From October 2021, the
patient was recommended to receive maintenance treatment with T-DM1
(200 mg; IV; Q3W) for nine cycles and underwent radiotherapy
simultaneously at another institute (details unavailable) (Fig. 1).
In April 2022, the patient complained of new nodules
in the left chest wall and following this, disease recurrence of
breast cancer was pathologically diagnosed by needle biopsy (TNM
classification: cT4N3M1) (Fig. S1C and
D). The IHC results were as follows: ER- (Fig. S2C and D); PR- (Fig. S4C and D); HER2 2+; Ki-67, 60%; and
PD-L1- (CPS=0) (anti-PD-L1 clone 22C3) (Fig. S5). Fluorescence in situ
hybridization (FISH), performed according to the manufacturer's
instructions (HER2 spectrum Orange/CEP 17 spectrum Green Probe;
PathVysion HER-2 DNA probe kit; cat. no. 02J01-030; Abbott
Molecular Diagnostics), indicated that there was no HER2
amplification, per the American Society of Clinical Oncology
guideline (Fig. S6) (15). In May 2022, the patient received
combination treatment with inetetamab (anti-HER2 antibody) plus
pyrotinib and vinorelbine. After the first cycle of therapy, the
patient discontinued the treatment because they wanted to join an
ongoing clinical trial and then received anlotinib monotherapy,
which was permitted during the screening period per subject
inclusion criteria. Finally, the patient failed to be enrolled for
the clinical trial and was admitted with chest wall recurrence of
the left breast cancer in July 2022 (Fig. 1).
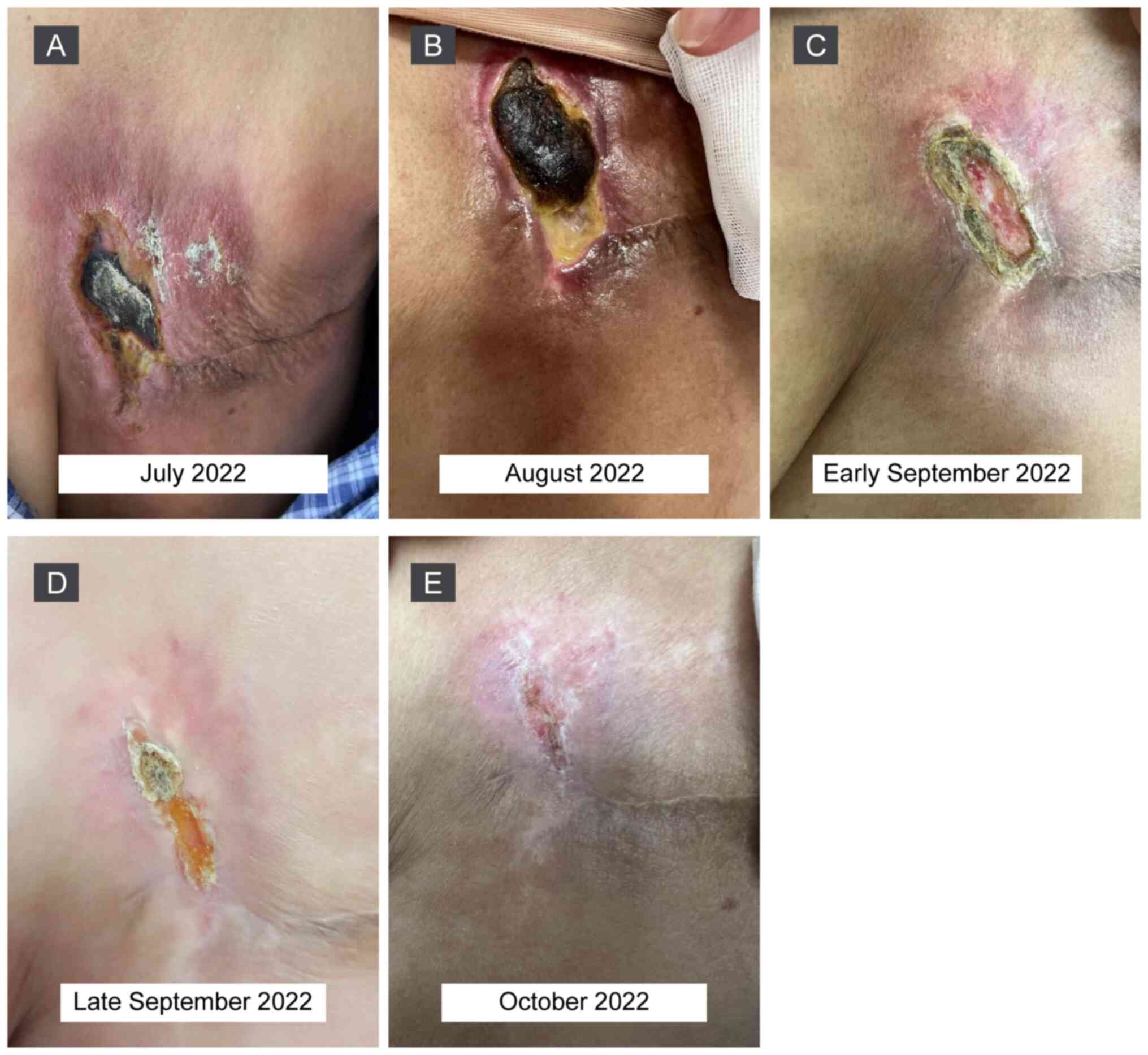
Physical examination demonstrated that the tumor
lesions were progressively enlarged and became confluent with the
surface of the left chest wall (Fig.
2A). Combination therapy with RC48 (120 mg; day 1) and
zimberelimab (240 mg; day 2), IV, every 2 weeks, was initiated.
After two cycles of treatment, the skin affected by the tumor
improved slightly (Fig. 2B) and
thus, the treatment regimen was continued. Notably, the tumor
lesions shrunk markedly after four cycles of treatment (Fig. 2C), healed gradually before the sixth
dose (Fig. 2D) and almost
disappeared after six cycles (Fig.
2E). The patient has remained in remission for >9 months as
confirmed using chest enhanced-CT scans by the latest follow-up in
March 2023 (Fig. S7) and has not
experienced any immune-related adverse events with good tolerance.
The next assessment of tumor response by imaging is planned to be
performed in June 2023.
Discussion
For recurrent metastatic HER2-positive breast
cancer, the standard systemic therapies are well established in
front-line settings and several choices of third-line treatment are
also currently available (7).
However, it remains a challenge to determine the regimens for
patients who respond poorly to several prior perioperative systemic
treatments, including chemotherapies and HER2-targeted therapy. In
addition, during the course of HER2-positive breast cancer, a
change in tumor expression of HER2 is associated with poor survival
outcome and makes it difficult to select an optimal systemic
therapy for either the adjuvant or recurrent metastatic setting
(17–21). In previous years, HER2-targeted ADCs
have become a novel option for recurrent/metastatic HER2-positive
and low-expressive breast cancer (5,22,23).
However, there are very limited data regarding the efficacy of
combination therapy of ADCs and ICIs in heavily treated patients,
particularly in those who do not respond to ADC agents.
In the present case, the patient had breast cancer
with an initial tumor stage of T4N3M0, and their HER2 tumor
expression of at diagnosis and at time of recurrence was IHC (3+)
and IHC (2+)/FISH-negative, respectively. Therefore, preoperative
therapy with TCbHP was recommended for the patient, which is the
preferred regimen of preoperative therapy for this condition
according to breast cancer guidelines (24,25).
However, the tumor lesions were markedly enlarged after four cycles
of treatment with TCbHP and the patient had to undergo surgery due
to limited alternatives for preoperative treatment.
For HER2-positive breast cancer, as recommended by
Chinese guidelines (24), AC
followed by taxane, trastuzumab and pertuzumab (THP) is the
preferred regimen of adjuvant therapy for patients with positive
axillary lymph nodes. The patient completed post-surgical systemic
therapy with four cycles of the AC regimen. Considering the poor
tumor response to TCbHP treatment, T-DM1 instead of THP was used as
part of the post-surgical treatment following the AC regimen. T-DM1
is also a recommended option for adjuvant therapy in patients with
HER2-positive breast cancer according to the guidelines (24,25),
based on the KATHERINE phase III study demonstrating that T-DM1 is
a superior choice of adjuvant therapy for invasive HER2-positive
breast cancer compared with trastuzumab (26).
The cancer unexpectedly recurred in the chest wall
within 6 months of initiation with T-DM1 therapy. The protein
expression was ER (−), PR (−), HER2 IHC (2+) (FISH-negative) and
PD-L1 (−) (CPS=0) in recurrent lesions. Chinese guidelines
(24) recommend pyrotinib (a
HER2-targeted inhibitor) plus chemotherapy as a choice of salvage
treatment regimens for HER2-positve breast cancer if prior
treatment with trastuzumab has failed. In addition, inetetamab (an
anti-HER2 antibody) plus vinorelbine has been approved in China for
previously treated metastatic HER2-positive breast cancer. Hence,
combination therapy with pyrotinib, inetetamab and vinorelbine was
used as the first-line treatment for this patient.
However, the patient did not respond to the first
line systemic therapy for recurrent disease. The tumor appeared to
be resistant/refractory to multiple conventional chemotherapies,
anti-HER2 antibodies and molecules, T-DM1 and radiation. Treatment
with another ADC (RC48) in combination with zimberelimab was
attempted, although the PD-L1 expression in the tumor was negative.
RC48, instead of trastuzumab deruxtecan (T-DXd), was selected
because T-DXd is currently unavailable in the region. RC48 plus
zimberelimab was observed to exhibit marked antitumor activity for
recurrent disease and has been achieving satisfactory PFS in the
present case.
It was hypothesized that there were dual mechanisms
contributing to the antitumor effect of the combination therapy.
Compared with T-DM1, RC48 enhances antitumor activity through
significant bystander effects, although they target the same
molecule with the same class of cytotoxic agent (27). Preclinical data demonstrated that
RC48 was more effective than T-DM1 in a trastuzumab- and
lapatinib-resistant breast cancer model (28). Clinical data of RC48 also
demonstrated its promising efficacy in HER2-low breast cancer,
whereas T-DM1 did not exhibit its effect in these patients until
now (29). Furthermore, ICIs and
ADCs may have potential synergistic effects on tumor control by
blocking the upregulated immune inhibitory pathway (e.g.,
PD-1/PD-L1 interaction) upon treatment with ADCs. In
HER2-expressing breast tumor treated with ADCs, pre-clinical mouse
model studies revealed that the expression of PD-1 in
CD8+ T cells and of PD-L1 on tumor
cells/tumor-associated macrophages is upregulated and
tumor-infiltrating lymphocytes were also revealed to be increased
compared with in the vehicle control. Therefore, ADCs plus
anti-PD-1 antibodies were able to enhance the antitumor activities
and prolong the survival time versus ADC monotherapy in mouse
models (30,31). In addition, in a mouse model of
HER2-positive tumors, combination treatment with RC48 and anti-PD-1
antibody was shown to be more effective than RC48 monotherapy in
the tumor control (32). To the
best of our knowledge, regarding ADCs plus PD-1/PD-L1 inhibitors in
HER2-positive breast cancer, only one result from a phase II study
(KATE2) has been reported and there is a lack of phase III data at
present (33). The KATE2 study,
which compared the efficacy of atezolizumab or placebo plus T-DM1
in HER2-positive advanced breast cancer, failed to show any
meaningful clinical benefit on PFS; however, this may also be due
to study limitations (e.g., small sample size, unblinded before
target number of events reached) (34). Therefore, an ongoing phase III study
(KATE3; NCT04740918) was designed to investigate the clinical
benefit of this combination therapy (34). The combination of ADCs with PD-1
inhibitors appears to be a feasible approach for inoperable
HER2-positive or low-expressive breast cancer to improve the
efficacy and survival outcome; however, this is awaiting
confirmation by further large-population clinical trials (33).
In the present case, there were three limitations in
the diagnosis and anticancer treatment. Firstly, with the exception
of PD-L1, other established biomarkers for immunotherapy (e.g.,
tumor mutation burden, microsatellite instability and mismatch
repair deficiency) were not examined before initiation with
zimberelimab. Secondly, adjuvant endocrine therapy for
ER-positive/HER2-positive breast cancer was not implemented.
Finally, there was a change in HER2 status between the primary and
recurrent disease, but it has not been clarified whether the
treatment regimen should be adjusted according to the discordance
at present.
In conclusion, the present report describes the
successful re-introduction of a HER2-targeted ADC combined with a
PD-1 inhibitor in a patient with recurrent HER2-positive breast
cancer whose disease progressed upon treatment with similar ADCs.
The findings of this case suggest the potential clinical benefit of
combination therapy with ICIs and ADCs in previously unsuccessfully
treated patients with breast cancer showing HER2 upregulation, even
after ADC failure. However, this should be further investigated in
prospective clinical trials.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Yaxi Wang
(Department of Pathology, National Cancer Center/National Clinical
Research Center for Cancer/Cancer Hospital, Chinese Academy of
Medical Sciences and Peking Union Medical College) for providing
the pathological images. They would also like to thank Mr. Debin Du
(Department of Imaging, Sanhuan Cancer Hospital) for providing the
CT scan images.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DS contributed to conceptualization, data collection
and interpretation, revision and supervision for the case report.
SMF acquired the data and conceptualized the manuscript. LXH
drafted and revised the manuscript and contributed to the
interpretation of data. DS and SMF confirmed the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siddiqui T, Rani P, Ashraf T and Ellahi A:
Enhertu (Fam-trastuzumab-deruxtecan-nxki)-Revolutionizing treatment
paradigm for HER2-low breast cancer. Ann Med Surg (Lond).
82:1046652022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferraro E, Drago JZ and Modi S:
Implementing antibody-drug conjugates (ADCs) in HER2-positive
breast cancer: State of the art and future directions. Breast
Cancer Res. 23:84–94. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi F, Liu Y, Zhou X, Shen P, Xue R and
Zhang M: Disitamab vedotin: A novel antibody-drug conjugates for
cancer therapy. Drug Deliv. 29:1335–1344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu B, Wang J, Fang J, Chen X, Han Y, Li Q,
Zhang P, Yuan P, Ma F, Luo Y, et al: Abstract PD4-06: Early
clinical development of RC48-ADC in patients with HER2 positive
metastatic breast cancer. Cancer Res. 80:PD4–06-PD04-06. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Liu Y, Zhang Q, Feng J, Fang J,
Chen X, Han Y, Li Q, Zhang P, Yuan P, et al: RC48-ADC, a
HER2-targeting antibody-drug conjugate, in patients with
HER2-positive and HER2-low expressing advanced or metastatic breast
cancer: A pooled analysis of two studies. J Clin Oncol. 39:1022.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Q, Qian W, Sun X and Jiang S:
Small-molecule inhibitors, immune checkpoint inhibitors, and more:
FDA-approved novel therapeutic drugs for solid tumors from 1991 to
2021. J Hematol Oncol. 15:143–205. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology Breast
Cancer. Version 4. NCCN; Plymouth Meeting, PN: 2023, View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu D, Ma C, Lu P, Gong J, Ye D, Wang S,
Peng P, Bai Y, Song Y, Chen J, et al: Dose escalation and expansion
(phase Ia/Ib) study of GLS-010, a recombinant fully human
antiprogrammed death-1 monoclonal antibody for advanced solid
tumors or lymphoma. Eur J Cancer. 148:1–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin N, Zhang M, Bai H, Liu H, Cui J, Ke X,
Zhang H, Liu L, Yan D, Jiang Y, et al: Efficacy and safety of
GLS-010 (zimberelimab) in patients with relapsed or refractory
classical Hodgkin lymphoma: A multicenter, single-arm, phase II
study. Eur J Cancer. 164:117–126. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Markham A: Zimberelimab: First approval.
Drugs. 81:2063–2068. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X, Xia L, Zhou Q, Zhu J, Wang K, Chen
J, Huang Y, Kurb G, Chang B, Zhao W, et al: GLS-010 (Zimberelimab),
a novel fully human anti-PD-1 mAb in Chinese patients with
recurrent metastatic cervical cancer results from a multicenter,
open-label, single-arm phase II trial. Int J Gynecol Cancer.
30:A1472020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung HC, Ros W, Delord JP, Perets P,
Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L,
Zeigenfuss S, et al: Efficacy and safety of pembrolizumab in
previously treated advanced cervical cancer results from the phase
II KEYNOTE-158 study. J Clin Oncol. 37:1470–1478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Health Commission Of The People's
Republic Of China, . Chinese guidelines for diagnosis and treatment
of breast cancer 2018 (English version). Chin J Cancer Res.
31:259–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: ASCO/CAP guideline update. J
Clin Oncol. 38:1346–1366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, Jessup JM, Brirley JD, Gaspar LE, Schilsky RL, Balch
CM, et al: AJCC Cancer Staging Manual. 8th edition. Springer; New
York, NY: pp. 589–636. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ignatov T, Gorbunow F, Eggemann H, Ortmann
O and Ignatov A: Loss of HER2 after HER2-targeted treatment. Breast
Cancer Res Treat. 175:401–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Branco FP, Machado D, Silva FF, André S,
Catarino A, Madureira R, Pinto JM, Godinho JP, Simões PD, Brito M,
Casa-Nova M, Moreira AR and Passos-Coelho JL: Loss of HER2
amplification and disease prognosis after neoadjuvant treatment of
HER2 amplified breast cancer. Eur J Cancer. 92:S942018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niikura N, Tomotaki A, Miyata H, Iwamoto
T, Kawai M, Anan K, Hayashi N, Aogi K, Ishida T, Masuoka H, et al:
Changes in tumor expression of HER2 and hormone receptors status
after neoadjuvant chemotherapy in 21,755 patients from the Japanese
breast cancer registry. Ann Oncol. 27:480–487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niikura N, Liu J, Hayashi N, Mittendorf
EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN
and Ueno NT: Loss of human epidermal growth factor receptor 2
(HER2) expression in metastatic sites of HER2-overexpressing
primary breast tumors. J Clin Oncol. 30:593–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mittendorf EA, Wu Y, Scaltriti M,
Meric-Bernstam F, Hunt KK, Dawood S, Esteva FJ, Buzdar AU, Chen H,
Eksambi S, et al: Loss of HER2 amplification following
trastuzumab-based neoadjuvant systemic therapy and survival
outcomes. Clin Cancer Res. 15:7381–7388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Modi S, Park H, Murthy RK, Iwata H, Tamura
K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, et
al: Antitumor activity and safety of trastuzumab deruxtecan in
patients with HER2-low-expressing advanced breast cancer: Results
from a phase Ib Study. J Clin Oncol. 38:1887–1896. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Modi S, Jacot W, Yamashita T, Sohn J,
Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al:
Trastuzumab deruxtecan in previously treated HER2-low advanced
breast cancer. N Engl J Med. 387:9–20. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Z, Li J, Chen J, Liu Y, Wang K, Nie
J, Wang X, Hao C, Yin Y, Wang S, et al: Chinese society of clinical
oncology (CSCO) breast cancer guidelines 2022. Transl Breast Cancer
Res. 3:13–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology Breast
Cancer. Version 8. NCCN; Plymouth Meeting, PN: 2021, View Article : Google Scholar : PubMed/NCBI
|
|
26
|
von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab Emtansine for residual invasive
HER2-positive breast cancer. N Engl J Med. 380:617–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Xu MZ, Wang L, Jiang J, Dong LH,
Chen F, Dong K and Song HF: Conjugating MMAE to a novel anti-HER2
antibody for selective targeted delivery. Eur Rev Med Pharmacol
Sci. 24:12929–12937. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rinnerthaler G, Gampenrieder SP and Greil
R: HER2 directed antibody-drug-conjugates beyond T-DM1 in breast
cancer. Int J Mol Sci. 20:11152019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li L, Zhang D, Liu B, Lv D, Zhai J, Guan
X, Yi Z and Ma F: Antibody-drug conjugates in HER2-positive breast
cancer. Chin Med J (Engl). 135:261–267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muller P, Kreuzaler M, Khan T, Thommen DS,
Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, et al:
Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly
susceptible to CTLA-4/PD-1 blockade. Sci Transl Med.
7:315ra1882015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwata TN, Ishii C, Ishida S, Ogitani Y,
Wada T and Agatsuma T: A HER2-targeting antibody-drug conjugate,
trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a
mouse model. Mol Cancer Ther. 17:1494–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang L, Wang R, Xie K, Zhang J, Tao F, Pi
C, Feng Y, Gu H and Fang J: A HER2 target antibody drug conjugate
combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in
hPD-1 transgenic mouse model and contributes immune memory
formation. Breast Cancer Res Treat. 191:51–61. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang T, Kang L, Li D and Song Y:
Immunotherapy for HER-2 positive breast cancer. Front Oncol.
13:10979832023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Emens LA, Esteva FJ, Beresford M, Saura C,
De Laurentiis M, Kim SB, Im SA, Wang Y, Salgado R, Mani A, et al:
Trastuzumab emtansine plus atezolizumab versus trastuzumab
emtansine plus placebo in previously treated, HER2-positive
advanced breast cancer (KATE2): A phase 2, multicentre, randomised,
double-blind trial. Lancet Oncol. 21:1283–1295. 2020. View Article : Google Scholar : PubMed/NCBI
|