Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common types of cancer in Southeast Asia and is particularly
observed in Southern China, including Guangxi Zhuang Autonomous
Region (1,2). NPC had an incidence of >20 cases
per 100,000 individuals and a mortality risk of 6 cases per 100,000
individuals in Southern China between 2004 and 2005 (2). At initial diagnosis, >80% of
patients present with locoregionally advanced NPC (LA-NPC)
(3). Despite use of
intensity-modulated radiation therapy (IMRT) and concurrent
chemotherapy (CCRT), treatment of LA-NPC is not satisfactory, with
a 5-year survival rate of 80% (4).
The latest phase 3 trial suggests that application of gemcitabine
combined with cisplatin induction therapy before chemoradiotherapy
significantly extends recurrence-free and overall survival of
patients with LA-NPC; however, 15.3% of patients develop local
recurrence and 9.5% experience distant metastasis, which are
primary reasons for treatment failure in these patients (5). The addition of targeted therapy to
CCRT increases local control rate and decreases occurrence of
distant metastasis (6,7).
Anlotinib is a small-molecule, multi-tyrosine kinase
inhibitor targeting tumor angiogenesis and proliferation (8); it may inhibit vascular endothelial
growth factor receptor (VEGFR), fibroblast and platelet-derived
growth factor receptor, epidermal growth factor receptor (EGFR) and
kinases (8,9). Anlotinib was approved by the National
Medical Products Administration as a third-line treatment for
advanced non-squamous non-small cell lung cancer without a driver
gene and small cell lung cancer and as a second-line treatment for
soft tissue sarcoma and locally advanced or metastatic medullary
thyroid carcinoma (8,10–12).
EGFR and VEGFR are highly expressed in 60–80% of
patients with LA-NPC and this is associated with poor prognosis
(13,14). This suggests potential effectiveness
of anlotinib against LA-NPC. An interim analysis of a phase 2 trial
shows promising efficacy of anlotinib as a third-line or further
treatment for advanced NPC (trial no. NCT03906058) (15). Nevertheless, the role of CCRT +
anlotinib for LA-NPC is still uncertain. Therefore, the present
study aimed to assess the effect of CCRT + anlotinib in patients
with LA-NPC.
Materials and methods
Study design
To assess the safety and effectiveness of CCRT +
anlotinib vs. CCRT alone in patients with LA-NPC (www.chictr.org.cn; Chinese Clinical Trial Registry
identifier: ChiCTR1900022969), patients were recruited from four
tertiary hospitals in Guangxi, China between July 1, 2019, and
February 20, 2021. The four hospitals were Guiping People's
Hospital, The First People's Hospital of Nanning, The People's
Hospital of Guangxi Zhuang Autonomous Region and The First
Affiliated Hospital of Guangxi Medical University. The present
study was approved by the Ethics Committees of these four
hospitals.
Participants
Eligible patients were aged 18–70 years with newly
treated stage III–IVA NPC, as diagnosed according to the 8th Union
for International Cancer Control and American Joint Committee on
Cancer (16), with histologically
confirmed non-keratinization. Other inclusion criteria included
Karnofsky performance status (KPS) score ≥70 and adequate bone
marrow (white blood cell count >4.0×109/l; absolute
neutrophil count >2.0×109/l; hemoglobin >90 g/l;
platelet count >100×109/l), renal and hepatic
function (creatinine clearance rate >60 ml/min; urinary protein
<2+; serum total bilirubin ≤1.5×ULN; aspartate aminotransferase
≤2.5×ULN; alanine aminotransferase ≤2.5×ULN; seralbumin >28
g/l). The exclusion criteria included previous malignancy, previous
antitumor treatment of NPC, contraindication to magnetic resonance
imaging (MRI), pregnancy or lactation, severe bleeding tendency or
any severe coexisting disease. Patients aged ≥70 years were not
included since elderly patients are generally less tolerant of
adverse events (17). In addition
to complete patient history and physical exam, routine pretreatment
assessments also included hematology (key items include white blood
cell count, absolute neutrophil count, hemoglobin and platelet
count) and biochemistry analyses (key items include creatinine
clearance rate, urinary protein, serum total bilirubin, aspartate
aminotransferase, alanine aminotransferase, seralbumin and fasting
blood glucose), as well as NP fiber-optic endoscopy,
histopathological diagnosis, chest and abdomen computed tomography
(CT) scan, nasopharynx and neck enhanced MRI and skeletal
scintigraphy. Written informed consent to participate was obtained
from all patients included in the study.
Randomization and masking
Randomization was performed (via sealed envelopes)
centrally at Guiping People's Hospital (Guiping, China). Patients
were randomized to the CCRT + anlotinib or the CCRT alone cohort.
The random allocation of the treatment was not concealed.
Treatment procedure
The present study compared CCRT plus anlotinib with
CCRT alone. All patients received radical IMRT at a cumulative dose
of ≥70 Gy to the primary tumor at 2.12–2.27 Gy/fraction with five
daily fractions/week for 6–7 weeks, 66–70 Gy to associated areas of
the neck, 60 Gy to high-risk subclinical areas and 54 Gy to
low-risk subclinical areas. MRI fusion with treatment planning CT
images was proposed to determine total tumor volume, including the
primary tumor and metastatic lymph nodes. High-risk clinical target
tumor volume was defined as the total nasopharynx tumor volume with
a 5–10 mm margin (2–3 mm posteriorly if contiguous to the brainstem
or spinal cord) encompassing the entire nasopharynx and high-risk
areas of microscopic extension. Low-risk clinical target tumor
volume was defined as high-risk clinical target tumor volume with
5–10 mm margin (2–3 mm posteriorly if contiguous to the brainstem
or spinal cord) encompassing the low-risk areas of microscopic
extension, including retropharyngeal nodal regions, skull base,
clivus, sphenoid sinus, parapharyngeal space, pterygoid fossae,
posterior third of the nasal cavity or maxillary sinuses including
the pterygopalatine fossae and level II–V lymph node regions. The
level IB nodal region was radiated in the case of any nodal
involvement at this level. The ipsilateral level IB nodal region
should be covered if there is gross involvement of the ipsilateral
submandibular gland, anterior half of the nasal cavity, oral cavity
or ipsilateral level IIA lymph nodes with extra-capsular extension
or maximum nodal axial diameter >2 cm (18).
All patients received intravenous cisplatin (100
mg/m2) on day 1 of every cycle of 3 weeks, with three
cycles in total. Oral anlotinib (12 mg/day) was administrated to
patients in the CCRT + anlotinib cohort on days 1–14 of every cycle
of 3 weeks, with three cycles in total. Both cisplatin and
anlotinib were administered concurrently with radiotherapy.
Treatment response rates were evaluated using
Response Evaluation Criteria in Solid Tumors (version 1.1)
(19) through physical examination,
nasopharyngoscopy and head and neck MRI at 1 week and 3 and 6
months post-radiotherapy. Patients were evaluated at least once
every 3 months for 2 years post-radiotherapy and every 6 months
thereafter. The nasopharynx and neck MRI, chest CT scan and
abdominal sonography were conducted every 3 months for the first 2
years post-radiotherapy and every 6 months thereafter. Biopsy was
conducted when tumor relapse was suspected. Acute toxic effects
were assessed weekly during treatment and graded according to the
Common Terminology Criteria for Adverse Events (version 5.0)
(20).
Statistical analysis
Statistical analysis was performed using SPSS 26.0
software (IBM Corp.). All categorical variables are reported as
numbers with percentages. Continuous variables are expressed as
median and range. P-values were calculated using χ2 test
or Fisher's exact test for categorical variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
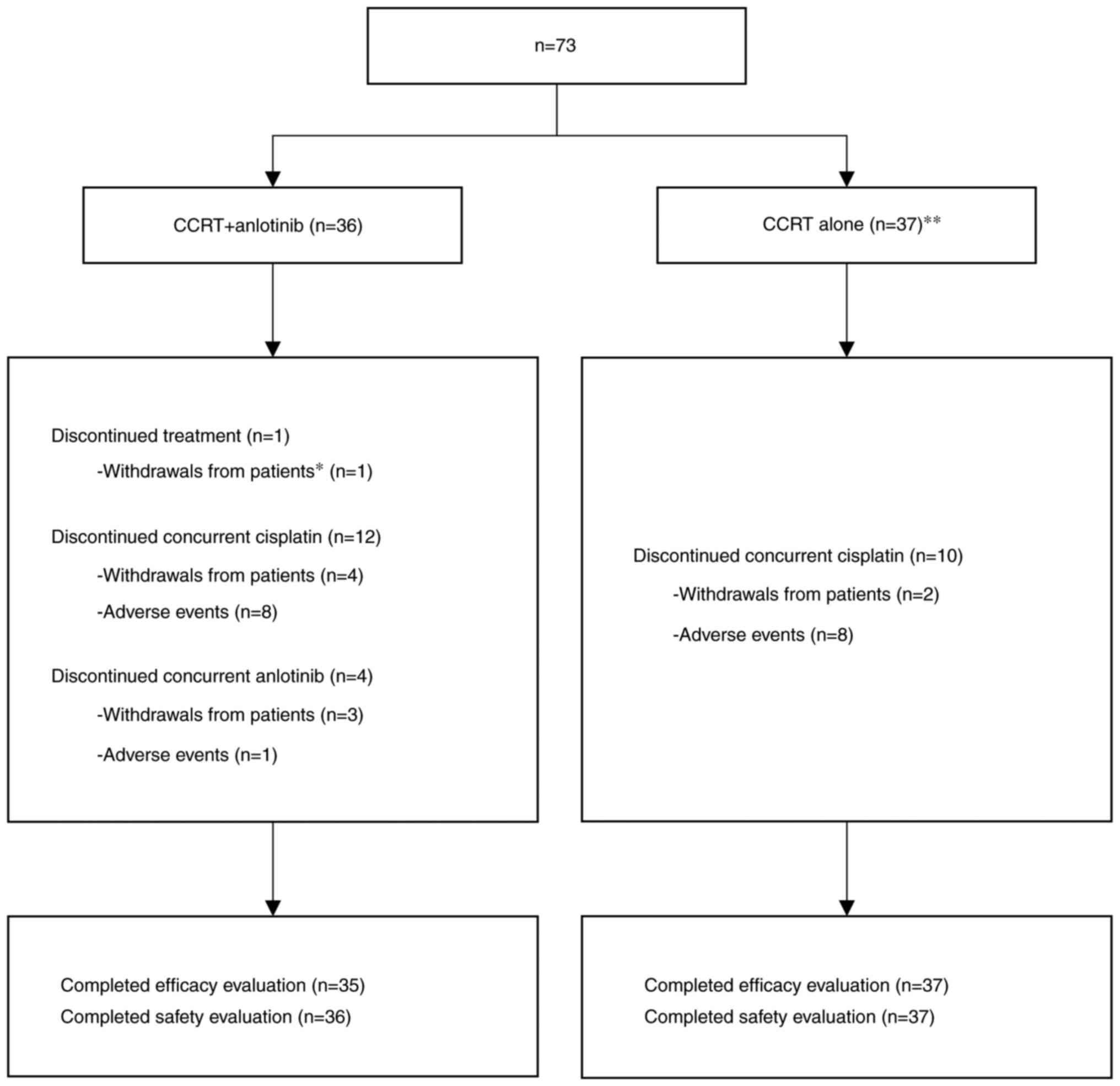
From July 2019 to February 2021, 73 patients with
LA-NPC randomly received CCRT + anlotinib (n=36) or CCRT alone
(n=37) (Fig. 1). The baseline
characteristics of patients were well balanced between the two
treatment cohorts (Table I).
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
| Characteristic | CCRT + anlotinib
(n=36) | CCRT alone
(n=37) |
|---|
| Sex, n (%) |
|
|
| Male | 26 (72.2) | 29 (78.4) |
|
Female | 10 (27.8) | 8 (21.6) |
| Median
age, years (range) | 47 (22–69) | 53 (28–70) |
| ECOG performance
status, n (%) |
|
|
| 0 | 27 (75.7) | 26 (70.3) |
| 1 | 9 (24.3) | 11 (29.7) |
| T stage, n (%) |
|
|
| T1 | 4 (11.8) | 2 (5.4) |
| T2 | 7 (20.6) | 9 (24.3) |
| T3 | 15 (44.1) | 14 (37.8) |
| T4 | 8 (23.5) | 13 (35.1) |
| N stage, n (%) |
|
|
| N0 | 1 (2.8) | 0 (0.0) |
| N1 | 2 (5.6) | 4 (10.8) |
| N2 | 24 (66.7 | 31 (83.8) |
| N3 | 9 (25.0) | 2 (5.4) |
| Clinical stage, n
(%) |
|
|
|
III | 19 (55.9) | 23 (62.2) |
|
IVA | 16 (44.1) | 14 (37.8) |
All patients in both treatment arms completed the
scheduled dose of radiotherapy. However, one patient from the CCRT
+ anlotinib arm withdrew from treatment after 3 weeks. In the CCRT
+ anlotinib cohort, 24 patients (66.7%) achieved three cisplatin
treatment cycles as planned, but 12 (33.3%) did not achieve this
therapy target. A total of eight patients and four patients reached
two and one cycle, respectively; four patients were unwilling to
continue chemotherapy and eight patients experienced adverse
events. A total of 32 patients (88.9%) achieved three concurrent
anlotinib treatment cycles; four patients (11.1%) did not achieve
three anlotinib treatment cycles (three and one patient reached two
cycles and one cycle, respectively; three patients were unwilling
to continue anlotinib treatment and one patient had experienced
adverse events). In the CCRT cohort, 27 patients (73.0%) achieved
three concurrent cisplatin treatment cycles; ten patients (27.0%)
did not reach the planned therapy target (nine and one patient
reached two cycles and one cycle, respectively; two patients were
unwilling to continue chemotherapy and eight experienced adverse
events). Treatment procedures and outcomes are shown in Fig. 1.
Therapeutic effects
Treatment responses were evaluated for eligible
patients (Table II). The complete
response (CR) rate in the CCRT + anlotinib group 1 week
post-radiotherapy was 81.1% compared 60.0% in the CCRT group.
However, this difference was not significant (P=0.10). At 3 months
post-radiotherapy, the CR rates in the CCRT + anlotinib cohort and
in the CCRT cohort were 91.4 and 81.1%, respectively, without
significant difference (P=0.35). CR rates increased to 97.1 and
91.9%, respectively, at 6-month follow-up; however, the difference
was not significant (P=0.65). Furthermore, no significant
differences were found between groups at 3 months post-radiotherapy
for nasopharyngeal (P=0.50) and neck lymph node lesions
(P=0.77).
 | Table II.Response to treatment. |
Table II.
Response to treatment.
| A, All sites |
|---|
|
|---|
|
| CCRT +
anlotiniba (n=35) | CCRT alone
(n=37) | P-value |
|---|
|
|
|
|
|
|---|
| Best response | 1 week, n (%) | 3 months, n
(%) | 6 months, n
(%) | 1 week, n (%) | 3 months, n
(%) | 6 months, n
(%) | 1 week | 3 months | 6 months |
|---|
| CR | 21 (60.0) | 32 (91.4) | 34
(97.1)b | 15 (40.5) | 30 (81.1) | 34
(91.9)c | 0.10d | 0.35d | 0.65e |
| PR | 14 (40.0) | 3 (8.6) | 0 (0.0) | 16 (43.2) | 6 (16.2) | 2 (5.4) | - | - | - |
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (16.2) | 1 (2.7) | 0 (0.0) | - | - | - |
| PD | 0 (0.0) | 0 (0.0) | 1
(2.9)f | 0 (0.0) | 0 (0.0) | 1
(2.7)g | - | - | - |
|
| B,
Nasopharyngeal lesions |
|
|
| CCRT +
anlotiniba
(n=35) | CCRT alone
(n=37) | P-value |
|
|
|
|
|
| Best
response | 1 week, n
(%) | 3 months, n
(%) | 6 months, n
(%) | 1 week, n
(%) | 3 months, n
(%) | 6 months, n
(%) | 1 week | 3
months | 6
months |
|
| CR | 22 (62.9) | 32 (94.3) | 34 (97.1) | 20 (54.1) | 32 (86.5) | 36 (97.3) | 0.64d | 0.50e | 1.00e |
| PR | 10 (28.6) | 3 (8.6) | 1 (2.9) | 8 (21.6) | 4 (10.8) | 0 (0.0) | - | - | - |
| SD | 3 (8.6) | 0 (0.0) | 0 (0.0) | 9 (24.3) | 1 (2.7) | 1 (2.7) | - | - | - |
| PD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - | - |
|
| C, Lymph node
lesions |
|
|
| CCRT +
anlotiniba
(n=35) | CCRT alone
(n=37) | P-value |
|
|
|
|
|
| Best
response | 1 week, n
(%) | 3 months, n
(%) | 6 months, n
(%) | 1 week, n
(%) | 3 months, n
(%) | 6 months, n
(%) | 1 week | 3
months | 6
months |
|
| CR | 23 (65.7) | 32 (94.3) | 35 (100.0) | 20 (54.1) | 32 (86.5) | 36 (97.3) | 0.31d | 0.77e | 1.00e |
| PR | 11 (31.4) | 2 (5.7) | 0 (0.0) | 12 (32.4) | 5 (13.5) | 1 (2.7) | - | - | - |
| SD | 1 (2.9) | 0 (0.0) | 0 (0.0) | 5 (13.5) | 0 (0.0) | 0 (0.0) | - | - | - |
| PD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - | - |
With a median follow-up of 13.6 months (range
6.1–26.2 months), 2 (5.5%) and 4 (10.8%) of patients in the CCRT +
anlotinib cohort and CCRT alone cohort, respectively, experienced
tumor progression. In the CCRT + anlotinib cohort, one patient
developed bone metastasis at 7.6 months and another developed liver
metastasis 6 months after randomization. In the CCRT cohort, one
patient experienced lung metastasis at 5.1 months, while two
patients experienced liver metastasis and another patient developed
bone metastasis at 10.7, 11.0 and 12.3 months, respectively, after
randomization.
Adverse reactions
The acute toxicity during treatment is shown in
Table III. Grade 3/4 mucositis
and myelosuppression, particularly leucopenia, were the most common
severe toxicities. In the CCRT + anlotinib cohort, the incidence of
grade 3/4 mucositis and leucopenia were 36.1 and 27.7%,
respectively, compared with incidence rates of 29.7 and 21.6% in
the CCRT arm (P=0.56 and P=0.54, respectively). A total of five
patients displayed grade 3 hypertension during treatment, one
developed grade 3 hepatotoxicity due to a hepatitis B flare up, one
reported grade 4 nephrotoxicity after three cycles of concurrent
cisplatin and required short-term dialysis therapy and two reported
hemorrhage (grade 1 or 2). No patient presented with grade 3/4
hemorrhage or adverse event-associated death.
 | Table III.Acute adverse events. |
Table III.
Acute adverse events.
|
| CCRT + anlotinib
(n=36) | CCRT alone
(n=37) |
|
|---|
|
|
|
|
|
|---|
| Adverse event | Grade 1/2, n
(%) | Grade 3/4, n
(%) | Grade 1/2, n
(%) | Grade 3/4, n
(%) | P-value (grade
3/4) |
|---|
| Any | 12 (33.3) | 24 (66.7) | 16 (43.2) | 21 (56.8) | 0.38a |
| Hematological |
|
|
|
|
|
|
Leucopenia | 23 (63.9) | 10 (27.7) | 25 (67.6) | 8 (21.6) | 0.54a |
|
Neutropenia | 20 (55.6) | 6 (16.7) | 25 (67.6) | 5 (13.5) | 0.70a |
|
Neutropenic infection | 0 (0.0) | 1 (2.8) | 0 (0.0) | 1 (2.7) | 1.00b |
|
Anemia | 23 (63.9) | 3 (8.3) | 26 (70.3) | 5 (13.5) | 0.74b |
|
Thrombocytopenia | 7 (19.4) | 4 (11.1) | 6 (16.2) | 2 (5.4) | 0.65b |
|
Non-hematological |
|
|
|
|
|
|
Hypertension | 6 (16.7) | 5 (13.9) | 3 (8.1) | 0 (0.0) | 0.06b |
|
Hemorrhage | 2 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
|
Dermatitis | 29 (80.5) | 3 (8.3) | 25 (67.6) | 3 (8.1) | 1.00b |
|
Mucositis | 21 (58.3) | 13 (36.1) | 24 (64.9) | 11 (29.7) | 0.56a |
|
Vomiting | 17 (47.2) | 4 (11.1) | 19 (51.3) | 5 (13.5) | 1.00b |
|
Diarrhea | 2 (5.6) | 1 (2.8) | 1 (2.7) | 2 (5.4) | 1.00b |
|
Hepatotoxicity | 2 (5.6) | 1 (2.8) | 0 (0.0) | 0 (0.0) | 0.49b |
|
Nephrotoxicity | 2 (5.6) | 1 (2.8) | 2 (5.4) | 0 (0.0) | 0.49b |
Discussion
The present interim analysis reports on an ongoing
multicenter randomized controlled trial comparing CCRT + anlotinib
with CCRT alone in patients with LA-NPC. The primary objective was
to define the toxicity and compliance of chemoradiotherapy +
anlotinib in LA-NPC. The results revealed that concurrent
administration of chemoradiation + anlotinib had acceptable
toxicity profiles, good compliance and encouraging efficacy.
EGFR and VEGFR are highly expressed and associated
with increased therapeutic tolerance and poor prognosis in patients
with nasopharyngeal carcinoma (21–24).
EGFR- and VEGFR-blocking drugs may offer a potential treatment for
LA-NPC.
Studies have confirmed that anti-EGFR antibodies,
such as cetuximab, nimotuzumab and endostar, plus CCRT for patients
with LA-NPC is well tolerated and safe (25–27).
To the best of our knowledge, however, most studies have shown no
survival benefit (26,28,29).
Xu et al (30) performed a
contrasting study of induction chemotherapy followed by concomitant
cisplatin-chemoradiotherapy (CRT) or cetuximab-radiotherapy (ERT).
ERT was not superior to CRT. The three-year disease-free survival
rates of CRT and ERT were 78.3 and 85.7%, respectively (P=0.547),
and ERT was more likely to cause acute adverse events. In a phase 2
trial by Huang et al (7),
chemoradiation + nimotuzumab was well-tolerated, grade 3/4 serious
toxicity was observed in 14 of 23 (60.9%) patients, while grade 3/4
oral mucositis was recorded in 8 (34.8%) patients; CR rates of
nasopharynx and regional nodal involvement were 91.3 and 95.5%,
respectively; 2-year progression-free and overall survival reached
83.5 and 95.0%, respectively. However, the aforementioned study did
not establish a control cohort. By contrast, a study by Kang et
al (31) demonstrated that the
efficacy of radiotherapy + endostar is comparable to radiotherapy +
chemotherapy in LA-NPC but acute adverse reactions are milder.
Several retrospective studies have explored the
effect of cetuximab or nimotuzumab in LA-NPC: Li et al
(28) reported that patients with
stage II–IV NPC receive no additional benefit from cetuximab +
CCRT, with similar 3-year progression-free, local relapse-free,
distant metastasis-free survival (DMFS) and overall survival but
resulted in more severe acute mucositis and acneiform rash. Similar
results were achieved in a retrospective study by You et al
(32), where cetuximab/nimotuzumab
+ IMRT produced similar disease-free, locoregional recurrence-free
and overall survival and DMFS compared with CCRT. Skin reaction and
mucositis appeared more frequently in the cetuximab/nimotuzumab
cohort.
The additional benefit of combined EGFR-blocking
drugs + CCRT for LA-NPC treatment remains controversial. The
aforementioned studies did not report positive results, which may
be due to small sample size with low statistical power or
EGFR-blocking drugs combined with radiotherapy instead of CCRT. For
studies where EGFR-blocking drugs were combined with CCRT, the
inclusion of low-risk stage II patients may be a possible cause of
failure.
Cai et al (15) reported that the disease control rate
of anlotinib as a third-line or further treatment for advanced NPC
is 76.5% (trial no. NCT03906058). In the present study, anlotinib
was combined with CCRT for stage III–IVA NPC. Only one patient
discontinued CCRT + anlotinib after one treatment cycle. A total of
66.7% of patients in the CCRT + anlotinib cohort achieved the
planned three cycles of cisplatin treatment, compared with 73.0% of
the CCRT cohort. The total dose of cisplatin in the CCRT alone
cohort was similar to those in previous studies (28,30–32).
The most common causes of interruption of concurrent cisplatin were
grade 3/4 treatment toxicity and patient refusal. Anlotinib was
also well-tolerated, with 91.7% of patients completing three cycles
of concurrent anlotinib therapy. The present data revealed a slight
increase in CR rate after anlotinib therapy, but this increase was
not significant. These preliminary results suggested
non-inferiority of CCRT + anlotinib compared with CCRT alone in
terms of CR rates. Nevertheless, long-term follow-up is required to
determine the long-term effects of anlotinib on survival. The
common acute adverse events during treatment were leucopenia,
neutropenia, anemia, thrombocytopenia, dermatitis, mucositis and
vomiting. The major grade 3/4 hematological events in the CCRT +
anlotinib group were mucositis (36.1%), leucopenia (27.7%) and
neutropenia (16.7%), which were manageable by using drugs for
symptomatic treatment. Grade 3/4 hypertension was observed in 5
patients in the CCRT + anlotinib group, whereas no grade 3/4
hypertension occurred in the CCRT group. The incidence of grade 3/4
hypertension (13.9%) was consistent with that reported by ALTER
0303 Trial (13.6%), a phase 3 trial confirming the efficacy of
anlotinib treatment for advanced non-small cell lung cancer
(11). No patient presented with
grade 3/4 hemorrhage or adverse event-related death. The incidences
of grade 3/4 neutropenia, anemia and thrombocytopenia were higher
than those in similar treatment cohorts reported in a previous
study by Zhang et al (5)
(13.5 vs. 10.5, 16.7 vs. 0.8, 5.4 vs. 1.3%, respectively), which
may be due to a higher number of enrolled patients aged >45
years in the present study.
The present study has limitations. Firstly, the
small sample size may lead to underpowered statistics and potential
selection bias. Secondly, the present study only collected
short-term follow-up data to assess therapy efficacy and acute
toxicity. Hence, a follow-up period of ≥5 years is required to
observe other treatment-associated events and evaluate the
therapeutic impact and late toxic effects of CCRT + anlotinib in
LA-NPC. Thirdly, some patients ceased treatment or were lost to
follow-up. Furthermore, the latest phase 3 trial (5) suggests that induction chemotherapy
with gemcitabine + cisplatin added to CCRT significantly improves
recurrence-free and overall survival of patients with high-risk
LA-NPC, which made induction chemotherapy with gemcitabine +
cisplatin followed by CCRT a standard treatment regimen for LA-NPC.
The present study was initiated earlier than the publication of the
phase 3 trial above, so induction chemotherapy with gemcitabine +
cisplatin was not performed in this study. However, to the best of
our knowledge, the present study is the first to assess the
effectiveness and toxicity of CCRT + anlotinib in LA-NPC.
In conclusion, concurrent anlotinib has acceptable
toxicity profiles, good compliance and encouraging efficacy with a
favorable CR rate in patients with LA-NPC. However, further studies
with larger cohorts, long-term follow-up and new induction regimens
are required to confirm the clinical value of CCRT + anlotinib.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Department of Health of
Guangxi Zhuang Autonomous Region Self-Raised Funds Project (grant
no. Z20190097), Scientific Research Projects funded by Guangxi
Health Department (grant no. Z20200024), and Nanning Qingxiu
District Science and Technology Plan Project (Key R&D Project
grant no. 2019042).
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
KC, GX, YL, PL, WG, ZL, WL, ZT, JC, XH and YX
contributed to the study conception and design. KC, GX, YL, PL and
WG analyzed data. KC and GX wrote the manuscript. All authors have
read and approved the final manuscript. KC and YX confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed in line with the
principles of the Declaration of Helsinki. Approval (no. 2019-001)
was granted by the Ethics Committee of Guiping People's Hospital
(Guiping, China), The First People's Hospital of Nanning (Nanning,
China), The People's Hospital of Guangxi Zhuang Autonomous Region
(Nanning, China) and The First Affiliated Hospital of Guangxi
Medical University (Nanning, China). Written informed consent to
participate was obtained from all participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
globocan estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Yu XQ, Zheng R, Zhang S, Zeng H,
Wang J, Liao Y, Zou X, Zuo T, Yang Z and Chen W: Spatial and
temporal patterns of nasopharyngeal carcinoma mortality in China,
1973–2005. Cancer Lett. 401:33–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB,
Sun Y, Li WX, Chen YY, Xie FY, Liang SB, et al: Adjuvant
chemotherapy in patients with locoregionally advanced
nasopharyngeal carcinoma: Long-term results of a phase 3
multicentre randomised controlled trial. Eur J Cancer. 75:150–158.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD,
Yang KY, Jin F, Shi M, Chen YP, Hu WH, et al: Gemcitabine and
cisplatin induction chemotherapy in nasopharyngeal carcinoma. N
Engl J Med. 381:1124–1135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Sullivan B: Nasopharynx cancer:
Therapeutic value of chemoradiotherapy. Int J Radiat Oncol Biol
Phys. 69 (Suppl 2):S118–S121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang JF, Zhang FZ, Zou QZ, Zhou LY, Yang
B, Chu JJ, Yu JH, Zhang HW, Yuan XP, Tai GM, et al: Induction
chemotherapy followed by concurrent chemoradiation and nimotuzumab
for locoregionally advanced nasopharyngeal carcinoma: Preliminary
results from a phase II clinical trial. Oncotarget. 8:2457–2465.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li
L, Wang F, Hao Y, Li C and Chi Y: Safety, pharmacokinetics, and
antitumor properties of anlotinib, an oral multi-target tyrosine
kinase inhibitor, in patients with advanced refractory solid
tumors. J Hematol Oncol. 9:1052016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taurin S, Yang CH, Reyes M, Cho S, Coombs
DM, Jarboe EA, Werner TL, Peterson CM and Janát-Amsbury MM:
Endometrial cancers harboring mutated fibroblast growth factor
receptor 2 protein are successfully treated with a new small
tyrosine kinase inhibitor in an orthotopic mouse model. Int J
Gynecol Cancer. 28:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Chi Y, Chen X, Ge M, Zhang Y, Guo Z,
Wang J, Chen J, Zhang J, Cheng Y, et al: Anlotinib in locally
advanced or metastatic medullary thyroid carcinoma: A randomized,
double-blind phase IIB trial. Clin Cancer Res. 27:3567–3575. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 Phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L,
Han B, Chen G, He J, Wang J, et al: Anlotinib vs placebo as third-
or further-line treatment for patients with small cell lung cancer:
A randomised, double-blind, placebo-controlled Phase 2 study. Br J
Cancer. 125:366–371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma BB, Poon TC, To KF, Zee B, Mo FK, Chan
CM, Ho S, Teo PM, Johnson PJ and Chan AT: Prognostic significance
of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth
factor receptor and HER2 receptor protein expression in
undifferentiated nasopharyngeal carcinoma-a prospective study. Head
Neck. 25:864–872. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Y, Chen D, Liang J, Gao J, Luo Z, Wang
R, Liu W, Huang C, Ning X, Liu M and Huang H: Administration of
nimotuzumab combined with cisplatin plus 5-fluorouracil as
induction therapy improves treatment response and tolerance in
patients with locally advanced nasopharyngeal carcinoma receiving
concurrent radiochemotherapy: A multicenter randomized controlled
study. BMC Cancer. 19:12622019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Q, Su N, Fang Y, Zou Q, Xia Y, Ma S,
Cai J, Liu P, Wang J and Zhang Y: Anlotinib for patients with
recurrent or metastatic nasopharyngeal carcinoma: A phase II study.
J Clin Oncol. 40 (Suppl 16):e180202022. View Article : Google Scholar
|
|
16
|
Rice TW, Patil DT and Blackstone EH: 8th
edition AJCC/UICC staging of cancers of the esophagus and
esophagogastric junction: Application to clinical practice. Ann
Cardiothorac Surg. 6:119–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu TT, Hu CS and LiI BS: Clinical
consensus on the treatment of locally advanced squamous cell
carcinoma of the head and neck with anti-EGFR monoclonal antibody
(2023 edition). China Oncology. 33:81–94. 2023.
|
|
18
|
Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC,
Alhussain H, Corry J, Grau C, Grégoire V, Harrington KJ, et al:
International guideline for the delineation of the clinical target
volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol.
126:25–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
US Department of Health and Human
Services, . National Institutes of Health, National Cancer
Institute: Common Terminology Criteria for Adverse Events (CTCAE)
Version 5. Published:. November 27–2017.
|
|
21
|
Li HP, Huang CY, Lui KW, Chao YK, Yeh CN,
Lee LY, Huang Y, Lin TL, Kuo YC, Huang MY, et al: Combination of
epithelial growth factor receptor blockers and CDK4/6 inhibitor for
nasopharyngeal carcinoma treatment. Cancers (Basel). 13:29542021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo Y, Wang J, Wang F, Liu X, Lu J, Yu X,
Ma X, Peng X and Li X: Foxq1 promotes metastasis of nasopharyngeal
carcinoma by inducing vasculogenic mimicry via the EGFR signaling
pathway. Cell Death Dis. 12:4112021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wakisaka N, Hirota K, Kondo S,
Sawada-Kitamura S, Endo K, Murono S and Yoshizaki T: Induction of
lymphangiogenesis through vascular endothelial growth
factor-C/vascular endothelial growth factor receptor 3 axis and its
correlation with lymph node metastasis in nasopharyngeal carcinoma.
Oral Oncol. 48:703–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng QX, Han YW, Zhang YL, Hu J, Fan J, Fu
SZ, Xu S and Wan Q: Apatinib inhibits VEGFR-2 and angiogenesis in
an in vivo murine model of nasopharyngeal carcinoma. Oncotarget.
8:52813–52822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You R, Hua YJ, Liu YP, Yang Q, Zhang YN,
Li JB, Li CF, Zou X, Yu T, Cao JY, et al: Concurrent
chemoradiotherapy with or without Anti-EGFR-Targeted treatment for
Stage II–IVb nasopharyngeal carcinoma: Retrospective analysis with
a large cohort and long follow-up. Theranostics. 7:2314–2324. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin M, You R, Liu YP, Zhang YN, Zhang HJ,
Zou X, Yang Q, Li CF, Hua YJ, Yu T, et al: Beneficial effects of
anti-EGFR agents, Cetuximab Or Nimotuzumab, in combination with
concurrent chemoradiotherapy in advanced nasopharyngeal carcinoma.
Oral Oncol. 80:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin Y, Zhou Z, Li Z, Shen M, Qin Y, Yang
C, Wang R and Kang M: Efficacy of concurrent chemoradiotherapy plus
Endostar compared with concurrent chemoradiotherapy in the
treatment of locally advanced nasopharyngeal carcinoma: A
retrospective study. Radiat Oncol. 17:1352022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Chen QY, Tang LQ, Liu LT, Guo SS,
Guo L, Mo HY, Chen MY, Guo X, Cao KJ, et al: Concurrent
chemoradiotherapy with or without cetuximab for stage II to IVb
nasopharyngeal carcinoma: A case-control study. BMC Cancer.
17:5672017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang N, Wang K, Song F and Liu Y:
Cetuximab in combination with chemoradiotherapy for nasopharyngeal
carcinoma: A meta-analysis. Indian J Cancer. 55:196–200. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu T, Liu Y, Dou S, Li F, Guan X and Zhu
G: Weekly cetuximab concurrent with IMRT aggravated
radiation-induced oral mucositis in locally advanced nasopharyngeal
carcinoma: Results of a randomized phase II study. Oral Oncol.
51:875–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang M, Wang F, Liao X, Zhou P and Wang R:
Intensity-modulated radiotherapy combined with endostar has similar
efficacy but weaker acute adverse reactions than IMRT combined with
chemotherapy in the treatment of locally advanced nasopharyngeal
carcinoma. Medicine (Baltimore). 97:e111182018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
You R, Sun R, Hua YJ, Li CF, Li JB, Zou X,
Yang Q, Liu YP, Zhang YN, Yu T, et al: Cetuximab or nimotuzumab
plus intensity-modulated radiotherapy versus cisplatin plus
intensity-modulated radiotherapy for stage II–IVb nasopharyngeal
carcinoma. Int J Cancer. 141:1265–1276. 2017. View Article : Google Scholar : PubMed/NCBI
|