Introduction
Targeted therapy and immunotherapy are emerging as
significant tools that can aid understanding of cancer development
and the treatment of cancer in addition to the conventional
treatments of surgery, chemotherapy and radiotherapy (1). New methods including genetic
techniques and whole genome sequencing are now used to treat
cancer. However, drug resistance is a clinical problem in
chemotherapy, which is primarily because of the failure to
eradicate cancer cells (2).
Chronic myeloid leukemia (CML) is a clonal disorder
characterized by the Philadelphia chromosome, which results from
aberrant BCR-ABL tyrosine kinase activity (3). BCR-ABL is the fusion oncogene of a
chromosome translocation between chromosome 9 and chromosome 22,
and promotes the over proliferation of leukemic cells (3). No apparent symptoms which mark the
initial stages of CML development have been reported. With the
gradual progression of the disease, the chronic phase progresses to
a blast crisis often after several years and even evolved into
acute leukemia (4). A clinical
trial reported that imatinib has strong efficacy in CML,
particularly in BCR-ABL-positive CML (5). Moreover, a study using transgenic
mice, which were obtained by cloning the promoter of the mouse
tec gene, demonstrated, through its deletion, that p53
serves a significant role in the development of CML and increases
BCL-ABL kinase expression in hematopoietic cells (6). These examples indicate the importance
of the research and development of anticancer drugs based on
molecular therapy. However, drug resistance and disease recurrence
remain as major clinical problems.
In recent years, certain novel methods have been
developed to address the early detection and treatment of cancer as
well as the development of anticancer drugs (7). For example, near-infrared (NIR)
fluorescence probes, fluorescent methods and multispectral imaging
technology are well developed (8).
A recent study using a NIR probe for the detection of cancer
indicated that the tumor could be observed through
protease-activated probes. In the NIR channel, the
target-to-background signal ratio of tumor and normal mucosa are
relatively high, which indicates the cancer selectivity displayed
by such fluorescence reagents (9).
Furthermore, a neutral pH NIR fluorescence probe was developed to
complete real-time imaging and in situ detection of the pH
value of cancer cells (10).
The present study, we developed one fluorescent
compound and explored its potential biological effects. CML cells
were used as the research model together with a zebrafish xenograft
organism.
Materials and methods
Reagent and cell culture assay
The tested compound
[2-[2-[3-[2-(5-carboxy-1-ethyl-3,3-dimethylindol-1-ium-2-yl)ethenyl]-2-chlorocyclohex-2-en-1-ylidene]ethylidene]-1-ethyl-3,3-dimethylindole-5-carboxylic
acid] was supplied by Tianjin Medical University and was
reconstituted in 10 mM stock in DMSO (cat. no. D8418;
Sigma-Aldrich) and stored at −20°C. We termed this chemical as
compound A02 in this present research. K562 cells were stably
transfected with the Kusabira Orange (K562-KOr) fluorescent
protein, which could be imaged using the Tritc filter. K562 cells
were transfected with 2.5 µg phKO1-MN1 vector (cat. no. AM-V0046M;
Amalgaam, Tokyo, Japan) with Kusabira-orange (KOr) fluorescent
protein expression using the reagent Lipofectamine 2000 (cat. no.
11668027; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. After transfection for 24 h at 37°C,
cells were selected using 800 µg geneticin/ml concentration for
seven days culture, the positive KOr-expressing cells were sorted
by FACSAria flow cytometry (BD Biosciences). The cells are cultured
in RPMI-1640 medium (cat. no. 11875119; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated FBS (cat.
no. 10099141C; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
G and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C in
a 5% CO2 atmosphere. The cell proliferation assay was
performed to test the relative cell viability using a CellTiter-Glo
luminescent cell viability assay kit (cat. no. G7517; Promega
Corporation) according to the manufacturer's instructions. For the
cell staining assays, the cells were centrifuge at 200 × g for 5
min for 3 times at room temperature. Zebrafish blood and K562
cancer cells, they were exposed to 20 µM of the compound A02 for 30
min in medium at 37°C in a 5% CO2 atmosphere,
respectively. ALDH(−) and ALDH(+) K562 cells, they were stained
using the compound A02 (2 µM) for 24 h in medium at 37°C in a 5%
CO2 atmosphere, respectively.
Aldehyde dehydrogenase (ALDH)-positive
putative CSC preparation
ALDH positive (putative CSCs) and negative cells
were sorted using an ALDEFLUOR™ assay kit (cat. no. 01700; Stemcell
Technologies, Inc.) followed by FACSAria flow cytometry (BD
Biosciences) according to the manufacturer's instructions.
Mitochondrial staining
Mitochondrial staining in viable cells was performed
using the Rhodamine 123 dye (cat. no. R302; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
K562 cells were stained using 2 µM Rhodamine 123 and 20 µM tested
compound A02 for 30 min at 37°C in a 5% CO2 atmosphere.
The cells were centrifuged at 200 × g for 5 min and washed out for
3 times. Then the cells were observed and imaged in the bright
field and fluorescence filters (green and red) using the LSM-510
confocal microscope (Zeiss, Thornwood, USA).
Cancer mass in vivo imaging
ALDH-positive and negative K562 cell populations
were injected into the yolk sac (~100 cancer cells per fish) of the
transparent 48 h postfertilization transgenic zebrafish line
(fli-EGFP; China Zebrafish Resource Center). The xenografted
zebrafish were subsequently maintained at 32°C and the successful
cancer xenograft models with visible cancer mass were collected the
next day [1 day postinjection (dpi)] and obtained the images.
Subsequently, the fluorescent compound A02 was subsequently added
into the E3 egg water (0.17 mM KCl, 5 mM NaCl, 0.4 mM CaCl2, and
0.16 mM MgSO4 in dd water) and the images were obtained 2 days
later, at 3 dpi after washing out using fresh E3 egg water for 3
times. The images for each zebrafish were obtained using an
‘angiogenesis based target area best focusimaging acquisition
program’ (version 4.0, Molecular Devices, LLC) under FITC filter
(zebrafish fli-EGFP signal detection), Tritc filter
(Kusabira-orange, KOr fluorescent protein signal detection) and Cy5
filter (tested compound A02 signal detection) in an
ImageXpressMICRO Device (Molecular Devices, LLC) automatically. The
experiments involving zebrafish were performed according to
international guidelines and approved by the Laboratory Animal
Welfare Ethics Committee of Yunnan University (approval no.
YNU20210100). Following the experiments, zebrafish used were
sacrificed by an overdose of anesthesia using the final 0.1%
tricaine concentration and subsequently stored at −20°C for further
professional waste disposal.
Statistical analysis
Data are presented as mean ± SEM. Comparisons
between multiple groups and a single control group were calculated
using Dunnett's test following one way ANOVA and comparisons
between the two groups were performed using unpaired Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Compound A02 displays slight toxic
effects on biological organisms
To evaluate the novel application of biological
chemicals, compound A02 displaying interesting biological
characteristics was identified following screening of a series of
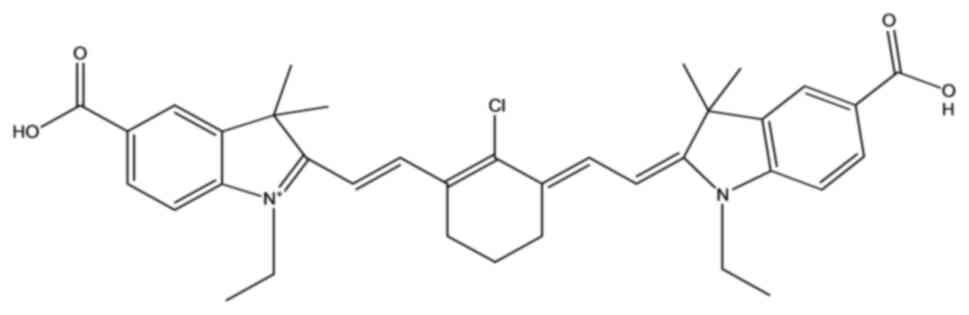
compounds. The chemical structure is presented in Fig. 1. Treatment of 3 days
postfertilization (dpf) zebrafish embryos with the compound A02 (20
µM) for 48 h had little effect on zebrafish survival and
morphology; however, a very weak red fluorescence was detected in
the yolk sac using the DsRed microscopy filter (Fig. 2). Overall, these data suggested that
compound A02 was relatively safe with no apparent toxic effects on
the model organism used.
Anticancer effects and staining of
cancer cells, particularly CSCs in vitro
The data indicated that the compound A02 could
significantly inhibit the proliferation of BCR-ABL+ CML
K562 cells at 10 and 20 µM following 24 h exposure compared with
the control (Fig. 3A). Exposure of
cells to 10 µM compound killed >50% of the cancer cells.
 | Figure 3.Effects on cancer cell proliferation
and cell imaging. (A) Cell proliferative status of
BCR-ABL+ CML K562 cells, which were treated with the
compound for 24 h at different concentrations, n=3. (B) Zebrafish
blood cells and K562 cancer cells were treated with 20 µM
fluorescent compound A02 for 30 min. The fluorescent signal was
detected in K562 cancer cells using the Cy5 filter following
washing out of the dyes. DAPI was used to stain the cell nuclei.
Scale bar, 100 µm. (C) Zebrafish blood cells and K562-KOr cancer
cells were mixed together and subsequently exposed to 20 µM
fluorescent dye for 30 min. DAPI was used to stain the cell nuclei.
The compound fluorescent signals were detected using the Cy5 filter
in these K562-KOr cancer cells and the KOr signal was detected
using the Tritc filter, following washing out of the dyes. Scale
bar, 100 µm. (D) The percentage of positive K562-KOr cells was
quantified. (E) The ALDH(−) and ALDH(+) K562 cells were stained
with 2 µM fluorescent dye for 24 h and imaged using the Cy5 filter.
DAPI was used to stain the cell nuclei. Scale bar, 100 µm. (F) The
fluorescence intensity of ALDH(−) and ALDH(+) K562 cells was
quantified using the Cy5 filter. *P<0.05 and **P<0.01. CML,
chronic myeloid leukemia; n.s., not significant; Cy5, Cyanine-5;
K562-KOr, Kusabira Orange; ALDH, aldehyde dehydrogenase; CML,
chronic myeloid leukemia. |
Zebrafish blood and K562 cancer cells were exposed
to 20 µM compound A02 for 30 min. The fluorescent signal of
compound A02 was clearly detected in K562 cancer cells; however, it
was not present in the normal zebrafish blood cells when imaged
using the Cyanine-5 (Cy5) filter following washing out of the dyes
(Fig. 3B). Subsequently, the
zebrafish blood and K562-KOr, expressing Kusabira Orange protein
fluorescence, (Fig. S1), cancer
cells were mixed and exposed to the compound (20 µM) for 30 min.
K562-KOr cancer cells were clearly visible using the fluorescent
signal of the compound following washing out (Fig. 3C). No significant differences were
noted between the percentage of positively stained cells detected
using the Tritc (KOr) and Cy5 (compound A02 signal) filters
(Fig. 3D). Moreover, the ALDH(−)
and ALDH(+) K562 cells were sorted (Fig. S2) and stained using compound A02 (2
µM) for 24 h; subsequently, they were imaged using the Cy5 filter
(Fig. 3E). The fluorescent
intensity of ALDH(−) and ALDH(+) K562 cells was quantified and the
results demonstrated that ALDH(+) cells displayed significantly
stronger fluorescence compared with the ALDH(−) cells. Taken
together, these data suggested that the compound could be used to
selectively image cancer cells, notably the ALDH (+) putative
CSCs.
Effects of staining on cancer cells in
vivo
As the novel fluorescent dye displayed cancer cell
selectivity in vitro as indicated by the aforementioned
experiments, in vivo effects were subsequently examined.
K562-KOr cells were injected into zebrafish embryos. The
successfully established xenografts (1 dpi) were imaged.
Subsequently, these embryos were seeded in egg water either with or
without the novel fluorescent compound A02 (2.5 µM) for 48 h and
imaged again on 3 dpi. The representative images of the K562-KOr
zebrafish xenograft on 1 and 3 dpi were shown (Fig. 4). The results suggested that this
compound could be used to preferentially stain cancer cells in
vivo.
Accumulation of mitochondria in cancer
cells
The initial experiments indicated that K562 cells
were selectively stained by the novel compound A02; therefore the
subcellular localization of the compound was assessed. To label the
organelles, the Rhodamine 123 fluorescent dye was used to stain the
mitochondria and microscopic imaging indicated that the staining of
the fluorescent compound A02 signal (red) colocalized with the
Rhodamine 123 labelled mitochondria green signal (Fig. 5). These data demonstrated that the
main intracellular dye accumulation site of this novel compound A02
was the cancer cell mitochondria.
Discussion
CSCs refer to cancer cells that can self-renew and
eventually form heterogeneous malignant tumors (11). CSCs are associated with tumor
recurrence, metastasis and chemoresistance, and are associated with
certain abnormal signaling pathways, such as the Hedgehog, WNT and
Notch signaling pathways (12).
Cluster of differentiation (CD) 44, CD24, ALDH1A1, CD133 and CD13
have been reported to be cell surface markers of CSCs (13–15).
Leukemia stem cells (LSCs) are the root cause of the occurrence,
development and recurrence of leukemia (16). A previous study using a mouse model
reported that CD27, a TNF receptor family member, promoted the
development of CML in LSCs (17).
Compounds, such as WNT signaling inhibitors, may serve as a
potential therapeutic agent for CML (18). Therefore, targeted therapy is a
method to eliminate LSCs and prevent their self-renewal, which
could be used in the clinical therapy in future.
Mitochondria are an important target for cancer
therapy due to their role in the occurrence, metastasis and
recurrence of tumors (19).
Mitochondria are subcellular organelles serve key roles in
biological process. For example, mitochondria serve an active role
in the efficacy of anticancer drugs and regulate cellular processes
by releasing cytochrome C (20).
Cancer treatment may interrupt the mitochondrial respiratory chain,
reduce ATP production and disrupt redox homeostasis. Reactive
oxygen species (ROS), a highly reactive chemical species with
oxygen free radicals, are thought to be involved in the
pathogenesis of different cancers. Apoptosis is induced by
increasing ROS levels and destroying mitochondrial DNA. Hypoxia
leads to increased ROS levels, which regulates the TGF-β signaling
pathway to promote self-renewal of CSCs and protects them from cell
death caused by exposure to drugs or radiation (21). Therefore, mitochondria have become
an important target in cancer research and target drug
development.
In the present study, the potential toxicity or side
effects of compound A02 were assessed. The data indicated that
exposure of zebrafish to 20 µM of A02 demonstrated no apparent
effects on their embryonic development; which suggested that the
compound was relatively safe within a possible therapeutic window,
given that this concentration had already demonstrated
antiproliferative ability against cancer cells under certain
conditions. The data further demonstrated cancer cell mitochondrial
accumulation. The applied concentrations of the dye in the present
study were similar with those used for certain other dyes (such as
IR-780 iodide) reported in published studies, which were relatively
safe for in vivo applications (22). For example, a previously reported
near-infrared fluorescent heptamethine indocyanine dye indicated
accumulation by in vivo imaging with no apparent side
effects at a dose as high as 0.2 mg/kg in a mouse model and
displayed cancer cell preference at a dose of 10 µM in in
vitro studies (22). Additional
tests should be performed in future studies to assess the in
vivo profile of this compound by using other animal models.
Furthermore, the optimization of the compound structure could
enable the identification of additional candidates for future
medical or diagnostic applications. Possible models for the
application of the fluorescent compound A02 in cancer are presented
(Fig. 6). The compound A02
permeated into cancer cells and targeted the mitochondria, which
may induce mitochondrial damage and subsequent cell death by
regulating the associated transcription factors. Although the
underlying mechanism requires further investigation, the present
study suggests that this compound will be further explored due to
its low toxicity to the host and its selective cancer cell imaging
and killing abilities. In conclusion, the present study
demonstrated a novel fluorescent dye that could selectively image
and kill cancer cells, including CSCs, by invading the
mitochondria. The future development of this reagent may provide an
alternative strategy in cancer therapy, including CML and other
cancer types.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Project of the Department
of Science and Technology of Guangxi Zhuang Autonomous Region,
China (grant no. Guike AB19110052), Yunnan Fundamental Research
Project (grant no. 202201AT070198) and the National Natural Science
Foundation of China (grant no. 82000167).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TR, YJH, NX and BBZ contributed to the study
conception and experimental design. TR, MZY, LJQ, SCZ, NNC and JS
performed measurements and drafted the manuscript. TR, WMZ, YJH,
HBS, NX and BBZ aided in the composition of the manuscript,
performed the experiment analysis, interpretation of data. NX, HBS
and BBZ confirm the authenticity of the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Experiments involving zebrafish were approved by the
Laboratory Animal Welfare Ethics Committee of Yunnan University
(approval no. YNU20210100).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saeed A, Park R and Sun W: The integration
of immune checkpoint inhibitors with VEGF targeted agents in
advanced gastric and gastroesophageal adenocarcinoma: A review on
the rationale and results of early phase trials. J Hematol Oncol.
14:132021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murphy M and Stordal B: Erlotinib or
gefitinib for the treatment of relapsed platinum pretreated
non-small cell lung cancer and ovarian cancer: A systematic review.
Drug Resist Updat. 14:177–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah NP, Nicoll JM, Nagar B, Gorre ME,
Paquette RL, Kuriyan J and Sawyers CL: Multiple BCR-ABL kinase
domain mutations confer polyclonal resistance to the tyrosine
kinase inhibitor imatinib (STI571) in chronic phase and blast
crisis chronic myeloid leukemia. Cancer Cell. 2:117–125. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yotaro O: Genetic landscape of chronic
myeloid leukemia. Int J Hematol. 117:30–36. 2023. View Article : Google Scholar
|
|
5
|
Druker BJ, Talpaz M, Resta DJ, Peng B,
Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R,
Ohno-Jones S and Sawyers CL: Efficacy and safety of a specific
inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid
leukemia. N Engl J Med. 344:1031–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honda H, Ushijima T, Wakazono K, Oda H,
Tanaka Y, Aizawa Si, Ishikawa T, Yazaki Y and Hirai H: Acquired
loss of p53 induces blastic transformation in
p210(bcr/abl)-expressing hematopoietic cells: a transgenic study
for blast crisis of human CML. Blood. 95:1144–1150. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Deiry WS, Sigman CC and Kelloff GJ:
Imaging and oncologic drug development. J Clin Oncol. 24:3261–3273.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang BB, Liu JG, Bai XY, Huang YJ, Xu N
and Ren T: A novel fluorescent dye invades mitochondria to
selectively kill cancer stem cells via increased ROS production.
Bioinorg Chem Appl. 2021:47639442021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alencar H, Funovics MA, Figueiredo J,
Sawaya H, Weissleder R and Mahmood U: Colonic adenocarcinomas:
Near-infrared microcatheter imaging of smart probes for early
detection-study in mice. Radiology. 244:232–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang B, Yu F, Li P, Tong L, Duan X, Xie T
and Wang X: A near-infrared neutral pH fluorescent probe for
monitoring minor pH changes: Imaging in living HepG2 and HL-7702
cells. J Am Chem Soc. 131:3016–3023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borah A, Raveendran S, Rochani A, Maekawa
T and Kumar DS: Targeting self-renewal pathways in cancer stem
cells: Clinical implications for cancer therapy. Oncogenesis.
4:e1772015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henderson T, Chen M, Darrow MA, Li CS,
Chiu CL, Monjazeb AM, Murphy WJ and Canter RJ: Alterations in
cancer stem-cell marker CD44 expression predict oncologic outcome
in soft-tissue sarcomas. J Surg Res. 223:207–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Ma H, Zhang J, Zhu L, Wang C and
Yang Y: Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem
cell markers in tumorigenesis and metastasis. Sci Rep. 7:138562017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sudhalkar N, Rathod NP, Mathews A, Chopra
S, Sriram H, Shrivastava SK and Goda JS: Potential role of cancer
stem cells as biomarkers and therapeutic targets in cervical
cancer. Cancer Rep (Hoboken). 2:e11442019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krause DS and Van Etten RA: Right on
target: Eradicating leukemic stem cells. Trends Mol Med.
13:470–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schürch C, Riether C, Matter MS, Tzankov A
and Ochsenbein AF: CD27 signaling on chronic myelogenous leukemia
stem cells activates Wnt target genes and promotes disease
progression. J Clin Invest. 122:624–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jamieson CHM, Ailles LE, Dylla SJ,
Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating
A, et al: Granulocyte-macrophage progenitors as candidate leukemic
stem cells in blast-crisis CML. N Engl J Med. 351:657–667. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikawa K, Takenaga K, Akimoto M,
Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y and Hayashi
J: ROS-generating mitochondrial DNA mutations can regulate tumor
cell metastasis. Science. 320:661–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scheel C, Eaton EN, Li SH, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL and
Weinberg RA: Paracrine and autocrine signals induce and maintain
mesenchymal and stem cell states in the breast. Cell. 145:926–940.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang C, Liu T, Su Y, Luo S, Zhu Y, Tan X,
Fan S, Zhang L, Zhou Y, Cheng T and Shi C: A near-infrared
fluorescent heptamethine indocyanine dye with preferential tumor
accumulation for in vivo imaging. Biomaterials. 31:6612–6617. 2010.
View Article : Google Scholar : PubMed/NCBI
|