Introduction
Breast cancer (BC) is the most commonly diagnosed
cancer and the second leading cause of cancer death among women
worldwide (1). Although adjuvant
chemotherapy confers a one-third reduction in the 10-year risk of
mortality from BC (2), a large
number of patients will experience recurrence and BC-associated
mortality (3). To improve the
prognosis and sensitivity to chemotherapy of patients with BC, a
new chemotherapy interval protocol has been proposed called
intensive dose-dense (DD) chemotherapy, which consists of using the
same chemotherapy agents and dosing but with shorter intervals
between treatment cycles (4).
Generally, the usual chemotherapy once every 3 weeks is shortened
to once every 2 weeks to shorten the treatment time, improve the
treatment effect, prolong the survival time of patients and improve
the quality of life.
To the best of our knowledge, no studies have
previously investigated which pathological type of patients is more
suitable for DD chemotherapy. Zhu et al (5) revealed that patients with hormone
receptor (HR)-positive (HR+) BC treated with DD
chemotherapy may benefit more from treatment (5). Lambertini et al showed that
patients with Her2+ BC receive less benefit under DD
chemotherapy (6). Furthermore, the
lack of an increased risk of serious adverse events with intensive
chemotherapy for DD suggests that shorter chemotherapy intervals
can be considered for follow-up treatments (7). Therefore, intensive DD chemotherapy
may be more suitable for some patients.
However, the most suitable pathological BC subtype,
including patients with HR− or HR+ BC, that
is most responsive to DD chemotherapy remains to be identified.
Currently, it is unknown whether intensive DD chemotherapy is
beneficial for patients with different pathological subtypes. To
the best of our knowledge, no previous systematic review (8–10) has
provided a comprehensive overview using pairwise and network
meta-analyses to evaluate which pathological type of BC is the most
responsive to DD chemotherapy regimens. The present study aimed to
determine the most suitable pathological subtype of BC to benefit
from intensive DD chemotherapy.
Materials and methods
Protocol registration
The present network meta-analysis was conducted and
reported following the Preferred Reporting Items for Systematic
Reviews and Meta-Analysis (PRISMA) (11) and the Cochrane Collaboration
guidelines (12). This systematic
review was registered with the PROSPERO online system as no.
CRD2022420351567 (13).
Search strategy and eligibility
criteria
The present study searched studies registered in
PubMed(ncbi.nlm.nih.gov/pubmed), Web of
Science(apps.webofknowledge.com), the Cochrane Library(www.cochranelibrary.com/) and China National
Knowledge Infrastructure(https://www.cnki.net/)_ from the date of database
inception to October 2022, and an upgraded search was conducted on
March 10, 2023. The keywords ‘breast cancer’, ‘intensive dose-dense
chemotherapy’ and their MeSH terms were used (see details in
Data S1). The Gene Expression
Omnibus and The Cancer Genome Atlas databases do not have relevant
data and were therefore not included. The studies were required to
have written the inclusion and exclusion criteria distinctly and
clearly based on PICOS as follows: P, patients with BC; I,
intensive DD chemotherapy; C, standard chemotherapy; O, survival
rate and adverse effects; and S, randomized controlled trials
(RCTs) and retrospective studies. Studies that met the
aforementioned PICOS criteria were included in the present
meta-analysis. After selection, the present study included RCTs and
retrospective studies comparing intensive DD chemotherapy with
standard chemotherapy in patients with BC, and patients with
available information on pathological subtype and clinical stage
were included. Studies with different durations of intensive DD
chemotherapy and no available survival data were excluded. For DD
chemotherapy, the experimental arm was designed to narrowly deliver
agents over a shorter interval in the same cycle and dosage as the
conventional schedule in the control arm. Survival data were
synthesized and merged for analysis. No language restrictions were
set, and reference lists from previous similar systematic reviews
were also identified for potentially eligible studies.
Data extraction and quality
assessment
A total of two reviewers (SD and ZTQ) independently
performed the selection of the title and abstract and the
evaluation of the full text of potentially eligible studies.
Discrepancies between the two authors were resolved through
discussion and by consultation with an experienced reviewer (ZYS).
Eligible studies were analyzed for first author, publication year,
study type, sample size (age range, before or after surgery,
pre/postmenopausal status), tumor size (≤2.0, 2.1–5.0, ≥5.1) or
tumor stage (T0-1/T2/T3-4), lymph node status (pN1/pN2-3) or
(cN0-1/cN2-3), tumor grade (G1/G2/G3), estrogen/progesterone
receptor (ER/PR) status, HER2 status and Ki-67 positivity
(20%/>20%); DD type, agents and treatment cycle were extracted
as baseline characteristics. For eligible RCTs, the Cochrane tool
for assessing risk of bias (RoB 2.0) (14) was used to rate RCTs as follows: All
low-risk domains were considered low-risk studies, one high-risk
domain was considered a high-risk study and all other studies were
considered unknown-risk studies. The quality of the eligible
retrospective study was assessed using the Newcastle-Ottawa Scale
score (15), with a score >4
considered acceptable.
To evaluate the effects of DD chemotherapy in
patients with BC, overall survival (OS), disease-free survival
(DFS), event-free survival (EFS), recurrence-free survival (RFS),
pathologic complete response (pCR) and objective remission rate
(ORR) were analyzed. BC subgroups of overall HR+,
HR−, Her2+, Her2 and triple-negative breast
cancer (TNBC) were also evaluated. Luminal A and B classification
could not be analyzed in subgroups due to limited data.
Furthermore, the safety results related to DD chemotherapy included
anemia, leukocytopenia, fatigue, diarrhea, nausea, vomiting and
febrile neutropenia. The present study also used the Grading of
Recommendations Assessment, Development, and Evaluation scales
(16) to evaluate the quality of
the outcomes from pairwise meta-analysis.
Statistical analysis
The present study performed pairwise meta-analyses
for all direct comparisons with at least two different pathological
types available and random effects network meta-analysis with a
frequented approach to simultaneously combine direct and indirect
evidence of all pathological types. For both pairwise meta-analyses
and network meta-analysis, a random effects model to prevent
inconsistencies was estimated. The present study assumed the
variance of the heterogeneity model, which reported P<0.05 or
I2>50%, indicating heterogeneity in the results
(17).
For all results, the odds ratios (OR) and the hazard
ratio (HR) with their corresponding 95% credible intervals (95% CI)
were used to confirm significance of the meta-analysis results.
Furthermore, a meta-analysis P<0.05 was used to determine
whether a specific factor was the source of heterogeneity (18). Furthermore, Begg's and Egger's tests
were performed to assess publication bias for available
comparisons, and P<0.05 indicated the existence of publication
bias.
The inconsistency between indirect sources of
evidence was statistically assessed using a global
(design-by-treatment inconsistency model) and a local method (back
calculation) (19,20). The mean rank and relative treatment
rankings were assessed for each intervention node according to the
surface under the cumulative ranking curve (SUCRA) values and
produced rankograms for the results of the OS and DFS analyses. The
SUCRA score ranged from 0–100%, and a higher SUCRA indicated that
patients with this pathological classification were more suitable
for DD chemotherapy. Comparison-adjusted funnel plots were produced
to explore the publication bias for network meta-analysis outcomes.
All analyses were performed using StataSE version 15.1 (College
Station, Texas 77845 USA).
Results
Study selection
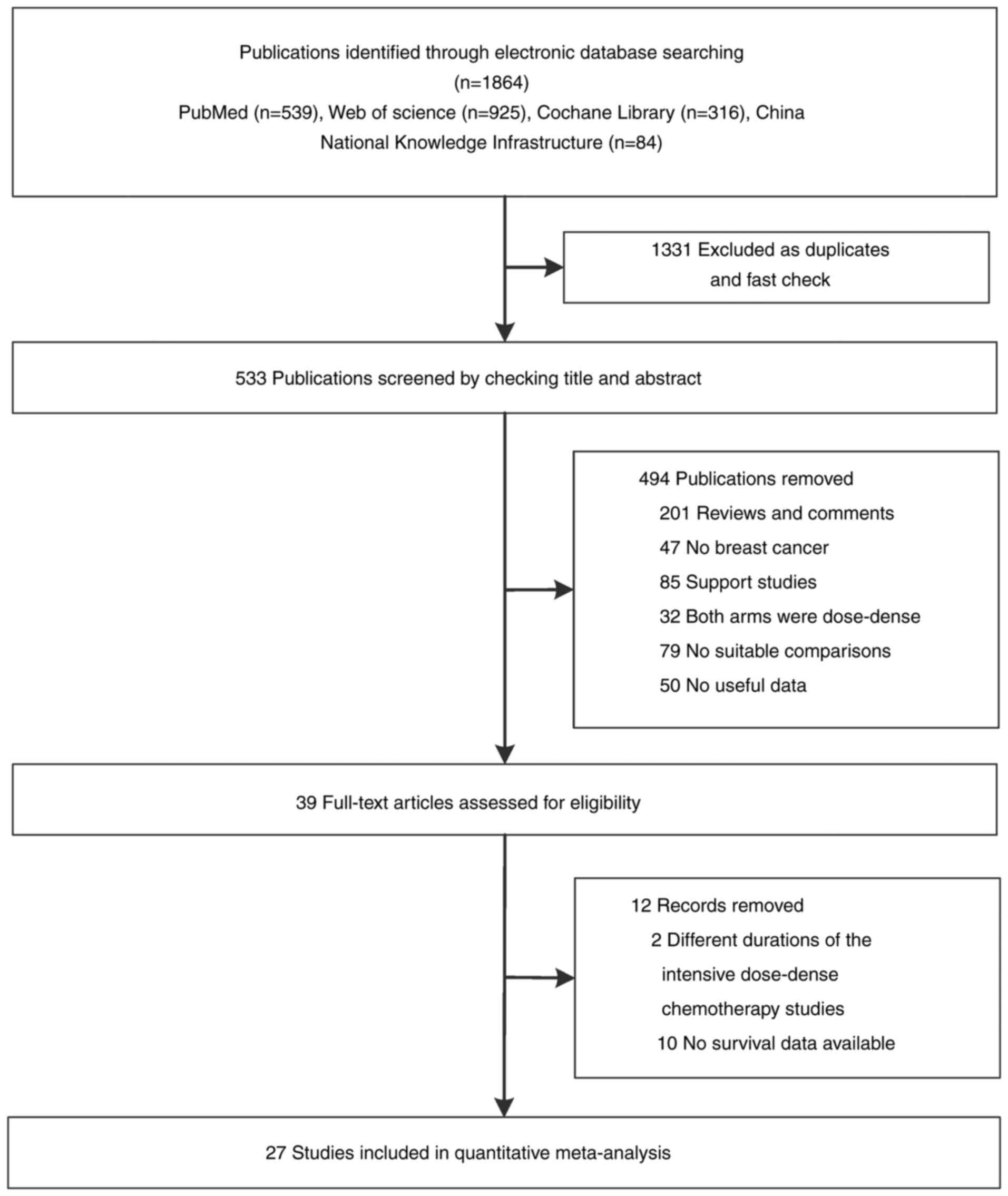
A total of 449 publications were evaluated for
eligibility after the removal of duplicates. After screening at the
title and abstract level, 33 articles were left for full-text
assessment. Furthermore, six records were removed based on a priori
study selection criteria. A total of 27 studies (21–47)
that included 27,580 patients were included in primary
meta-analyses (Fig. 1). Of these,
25 studies were RCTs, including 12 that were phase III trials, and
the remaining two were retrospective studies.
Study characteristics
The summarized characteristics of the studies are
reported in Table I by pathological
type and outcomes (see details in Table SI), with a sample size range of 43
to 3,264; of these, 17/43 included survival outcomes, such as the
pathological subtype, which could provide data for the network
meta-analysis (Table I). The
baseline was balanced in terms of indicators before or after
surgery, menopausal status, tumor size, lymph node status, ER/PR
status, HER2 status and Ki-67 positivity (Table II). The worst tumor grade was
determined in the DD chemotherapy group (Table II). Regarding quality assessment,
all included studies achieved acceptable quality (Fig. S1; Table SII).
 | Table I.Summarized characteristics of
included studies. |
Table I.
Summarized characteristics of
included studies.
| Author, year | Pathological
type | Outcome | Study type | Sample size
(DD/S) | BC type | (Refs.) |
|---|
| Schneeweiss,
2022 | HR+,
HR−, HER2−, HER2+, TNBC | OS, DFS | RCT, phase III | 470/475 | Early | (21) |
| Gogas, 2012 |
|
|
| 551/535 | All | (36) |
| Schneeweiss,
2019 | HR+,
HR−, HER2−, HER2+ | OS | RCT, phase III | 470/475 | Early | (25) |
| Foukakis, 2016 |
| OS, DFS, EFS,
RFS | RCT, phase III | 1001/1002 | Early | (31) |
| Venturini,
2005 |
| OS, EFS | RCT, phase III | 604/610 | Early | (44) |
| van Rossum,
2018 | HR+,
HER2−, TNBC | OS, RFS | RCT, phase III | 332/332 | All | (27) |
| Blondeaux,
2020 | Overall,
HR+, HR− | OS, DFS, EFS | RCT, phase III | 604/610 | Early | (23) |
| Del Mastro,
2015 |
| OS, DFS | RCT, phase III | 500/544;
502/545 | Early | (32) |
| Untch, 2011 |
|
|
| 363/370 | All | (39,40) |
| Untch, 2009 |
|
|
| 330/335 | All | (43) |
| Lambertini,
2017 |
| OS | RCT, phase III | 267/261 | All | (29) |
| Burnell, 2010 |
| RFS | RCT | 701/702 | All | (41) |
| Moebus, 2010 |
| OS, EFS | RCT, phase III | 643/612 | All | (42) |
| Liu, 2021 | TNBC | OS, RFS | RCT, phase II | 50/50 | All | (22) |
| Bao, 2016 |
| DFS | RCT | 23/20 | All | (30) |
| Jin, 2012 |
|
|
| 23/22 | All | (37) |
| Zhou, 2015 |
|
| Retrospective
study | 43/- | All | (33) |
| Möbus, 2018 |
| OS | RCT, phase III | 643/612 | All | (26) |
| Cameron, 2017 |
|
|
| 1086/1116;
1084/1105 | All | (28) |
| Therasse, 2003 |
|
|
| 224/224 | Advanced | (47) |
| Swain, 2013 |
| OS, DFS | RCT, phase III | 1634/1630 | Early | (34) |
| Zhu, 2013 |
| DFS | RCT | 24/27 | Advanced | (35) |
| Arun, 2011 |
|
| RCT, phase III | 99/100 | All | (38) |
| Baldini, 2003 |
|
|
| 73/77 | Advanced | (45) |
| Citron, 2003 |
|
| RCT | 493/484;
495/501 | All | (46) |
| He, 2020 |
| pCR | Retrospective
study | 111/761 | All | (24) |
 | Table II.Summary baseline indicators in the
network meta-analysis. |
Table II.
Summary baseline indicators in the
network meta-analysis.
| Baseline
indicator | OR (95% CI) | Heterogeneity (p,
I2) | Baseline
information balance |
|---|
| Before or after
surgery | 1.040 (0.984,
1.099) | 0.524, 0.0% | Yes |
| Menopausal status
(Pre/post) | 1.019 (0.952,
1.090) | 0.048, 36.6% | Yes |
| Tumor size (≤5.0,
≥5.1) or (T0-T2/T3-4) | 1.016 (0.935,
1.105) | 0.095, 27.0% | Yes |
| Lymph node status
(pN1/pN2-3) or (cN0-1/cN2-3) | 0.902 (0.774,
1.052) | 0.000,
88.0%b | Yes |
| Tumor grade
(G1-G2/G3) | 1.111 (1.017,
1.213)a | 0.018, 45.1% | No |
| ER/PR status
(positive/not) | 1.045
(0.958,1.140) | 0.000,
63.4%b | Yes |
| HER2 status
(positive/not) | 0.945 (0.820,
1.090) | 0.000,
68.0%b | Yes |
| Ki-67 positive
(≤20%/>20%) | 1.160 (0.936,
1.436) | 0.106, 47.5% | Yes |
Evaluation of the effectiveness of DD
chemotherapy from pairwise meta-analysis
All included studies included reporting of survival
data for DD chemotherapy compared with standard chemotherapy. For
OS, 13 studies provided HR data, and 30 of them provided OR data.
Significant differences were found in the general group with low
heterogeneity (HRa as in hazard ratios=0.82; 95% CI, 0.76 to 0.89;
P=0.205; I2=23.6%). Furthermore, the HR+ and
HR− subgroups also scored significant differences (0.75,
0.67 to 0.83; 0.77, 0.67 to 0.83). Furthermore, no significant
difference in the OR results was found, with no sources of
heterogeneity detected among meta-regressions that indicated low
publication bias, ranging from very low to low grade (Table III).
 | Table III.Subgroup meta-analysis of summary
both HRa and OR outcomes from different pathological types on the
efficacy of intensive DD chemotherapy for BC. |
Table III.
Subgroup meta-analysis of summary
both HRa and OR outcomes from different pathological types on the
efficacy of intensive DD chemotherapy for BC.
|
|
|
|
|
|
| Pairwise
meta-analysis outcomes from OR value |
|---|
|
|
|
|
|
|
|
|
|---|
|
|
| Pairwise
meta-analysis outcomes from HR value |
|
|
|
| Publication bias
(P-values from Begg's test) | Publication bias
(P-values from Egger's test) |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Outcome type | Pathological
types | No. of studies | HRa (95% CI) | Heterogeneity (p,
I2) | Meta-regression
P-value | No. subjects | OR (95% CI) | Heterogeneity (p,
I2) | Meta-regression
P-value | Grade |
|---|
| Overall
survival | Overall | 13 | 0.82
(0.76,0.89)a | 0.205, 23.6% | 0.348 | 30 (22639) | 0.92
(0.82,1.04) | 0.000,
72.5%b | 0.283 | 0.530 | 0.238 | VERY LOW |
|
| HR+ | 13 | 0.75
(0.67,0.83)a | 0.442, 0.30% |
| 2 (959) | 0.62
(0.18,2.16) | 0.040,
76.2%b |
| 1.000 | - | LOW |
|
| HR− | 11 | 0.77
(0.67,0.87)a | 0.482, 0.0% |
| 3 (788) | 1.37
(0.93,2.02) | 0.881, 0.0% |
| 0.602 | 0.918 | LOW |
|
|
Her2+ | 3 | 0.82
(0.52,1.11) | 0.689, 0.0% | 0.678 | 3 (845) | 1.57
(0.96,2.56) | 0.333, 9.1% | 0.656 | 0.602 | 0.530 | LOW |
|
|
Her2− | 6 | 0.83
(0.55,1.11) | 0.073,
50.4%b |
| 5 (1,978) | 1.11
(0.78,1.07) | 0.157, 39.6% |
| 0.327 | 0.439 | VERY LOW |
| Disease-free
survival | Overall | 9 | 0.85
(0.79,0.91)a | 0.474 0.0% | 0.324 | 20 | 1.05 (0.90,
1.21) | 0.000,
74.5%b | 0.647 | 0.292 | 0.440 | LOW |
|
| HR+ | 6 | 0.62
(0.30,0.95)a | 0.003,
72.0%b |
| 2 | 0.67
(0.34,1.35) | 0.083,
66.8%b |
|
|
| VERY LOW |
|
| HR− | 5 | 1.07
(0.71,1.44) | 0.067,
54.5%b |
| 1 |
|
|
|
|
|
|
|
|
Her2+ | 2 | 0.62
(0.30,0.95)a | 0.573, 0.0% | 0.080c | 2 | 0.80
(0.44,1.47) | 0.137,
54.9%b | - |
|
|
|
|
|
Her2− | 2 | 0.45
(0.21,0.70)a | 0.903, 0.0% |
| 2 | 0.69
(0.32,1.50) | 0.059,
72.0%b |
|
|
|
|
|
| TNBC | 3 | 1.18
(0.83,1.52) | 0.412, 0.0% |
| 4 | 1.58 (1.03,
2.43)a | 0.600, 0.0% |
| 0.117 | 0.176 | VERY LOW |
| Event-free
survival | Overall | 4 | 0.77
(0.70,0.84)a | 0.517, 0.0% | 0.739 | 6 | 0.96
(0.72,1.27) | 0.000,
83.4%b | 0.771 | 1.000 | 0.962 | LOW |
|
| HR+ | 4 | 1.01
(0.86,1.17) | 0.905, 0.0% |
|
|
|
|
|
|
|
|
|
| HR− | 4 | 0.76
(0.65,0.88)a | 0.909, 0.0% |
|
|
|
|
|
|
|
|
| Recurrence-free
survival | Overall | 4 | 0.76
(0.61,0.91)a | 0.193, 36.5% |
| 3 | 0.99
(0.65,1.51) | 0.004,
81.6%b |
| 1.000 | 0.937 | VERY LOW |
| Pathologic complete
response |
|
|
|
|
| 8 (4112) | 1.19 (0.93,
1.52) | 0.164, 33.1% |
| 0.536 | 0.691 | LOW |
| Objective remission
rate |
|
|
|
|
| 6 (2575) | 1.48 (1.08,
2.04)a | 0.126, 41.9% |
| 0.707 | 0.789 | LOW |
For DFS, significant differences could also be found
in the overall sample [0.85 (95% CI, 0.79 to 0.91)] and in the
HR+ [0.62 (95% CI, 0.30 to 0.95)], Her2+
[0.62 (95% CI, 0.30 to 0.95)] and Her2− [0.45 (95% CI,
0.21, 0.70)] subgroups when considering outcomes and in the OR
results of the TNBC subgroup [1.58 (95% CI, 1.03 to 2.43)], which
had low heterogeneity in most subgroups and a small publication
bias. Furthermore, meta-regression revealed that Her2+
or HER1− status may have had a significant effect on the
overall results (P=0.080). Significant differences in EFS could
also be found in the overall sample [0.77 (95% CI, 0.70 to 0.84)]
and for the HR− status group [0.76 (95% CI, 0.65, 0.88)]
with no heterogeneity; likewise, there were significant differences
in the overall RFS [0.76 (95% CI, 0.61 to 0.91)] for HRa outcomes
and the ORR [1.48 (95% CI, 1.08 to 2.04)] with OR outcome (Table III).
Altogether, the present study revealed that DD
chemotherapy successfully improved patient survival, especially
with regard to OS and DFS. Subsequently, network meta-analysis was
used to determine the most suitable pathological type of patients
for DD chemotherapy. Furthermore, compared with OR, the HRa not
only reflected the existence of events at the endpoint but also the
time taken to reach the endpoint and the censored data. Therefore,
the present study used HRa data for subsequent network
meta-analysis.
Pathological subtypes of patients with
BC suitable for DD chemotherapy derived from the network
meta-analysis
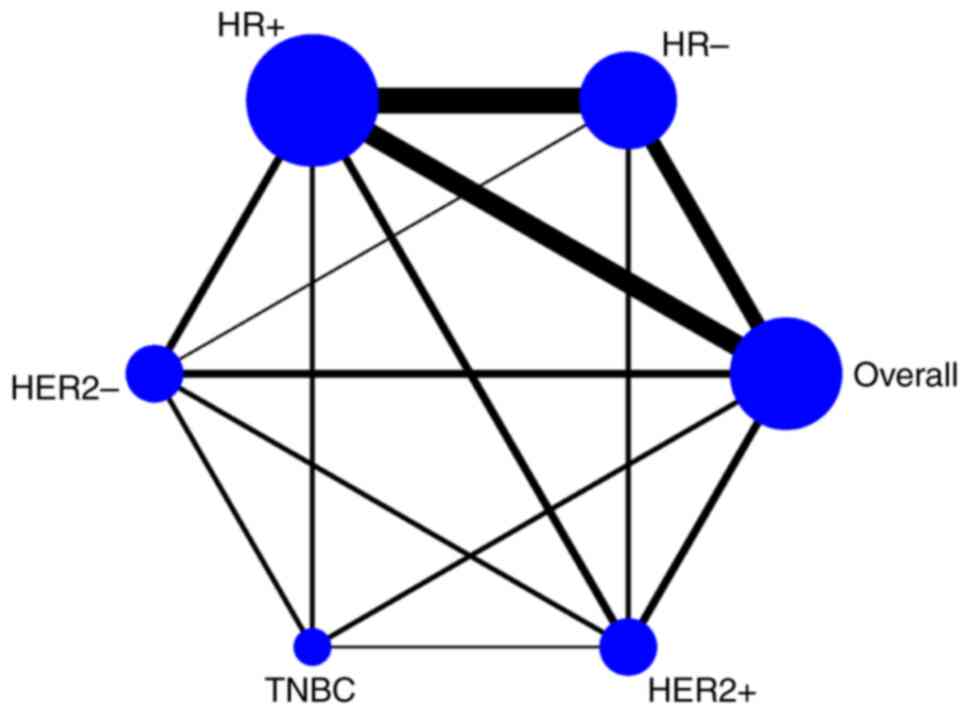
The network graphs of direct comparisons between the
13 studies included in the network meta-analysis that provided OS
data are shown in Fig. 2. Regarding
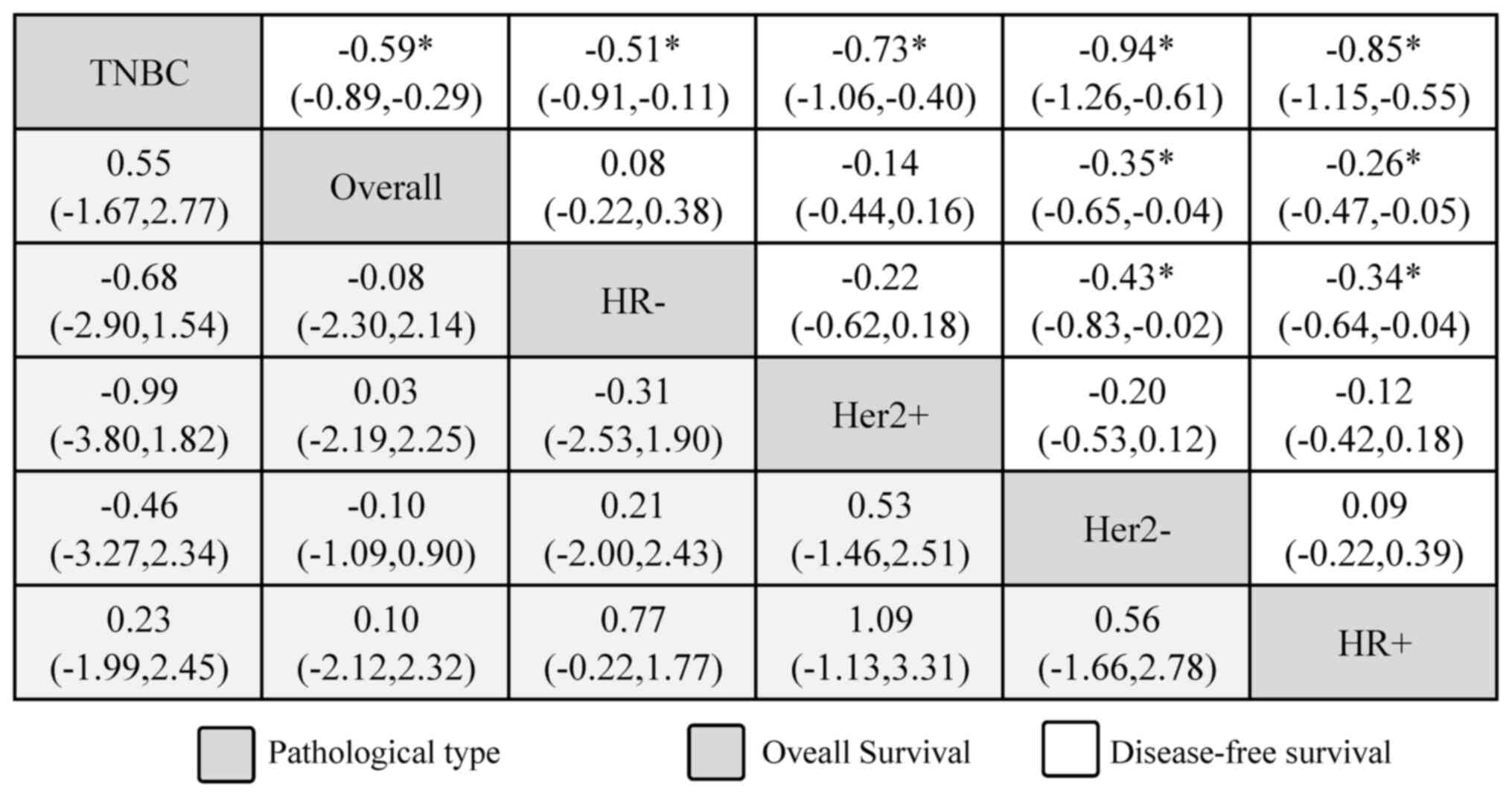
the HRa outcome for OS, TNBC ranked the lowest with the lowest
SUCRA score, and the second to last group was the overall patient
sample. In terms of reduced survival risk, HR+ was
classified first, followed by Her2−, Her2+
and HR− BC subtypes, with no significant differences.
Furthermore, DFS was classified as OS, and significance was found
in subgroups of HR+ [-0.85 (95% CI, −1.15 to −0.55)],
Her2− [-0.94 (95% CI, −1.26 to −0.61)], Her2+
[-0.73 (95% CI, −1.06 to −0.40)], HR− [-0.51 (95% CI,
−0.91 to −0.11)] and the overall sample [-0.59 (95% CI, −0.89 to
−0.29)] compared with TNBC. Significant differences were also
identified when comparing the overall group in terms of
HR+ [-0.26 (95% CI, −0.47 to −0.05)] and
Her2− [-0.35 (95% CI, −0.65 to −0.04)] status subgroups
and when comparing the HR+ and Her vs. HR−
status subgroups (Fig. 3). In
summary, DD chemotherapy may be more effective in patients with
HR+ and Her2− pathological subtypes.
Evaluation of the effectiveness of DD
chemotherapy from pairwise meta-analysis
Grade 3 to 4 toxicity of DD chemotherapy compared
with standard chemotherapy was reported in only 17 studies. DD
chemotherapy did not increase the risk of serious leukocytopenia,
fatigue, diarrhea, vomiting or febrile neutropenia. However, DD
chemotherapy resulted in significant increases in the serious risk
of anemia [3.024 (95% CI, 2.173 to 4.208)] and nausea [1.482 (1.096
to 2.004)], with low to substantial heterogeneity, low publication
bias and low to moderate grade (Table
IV).
 | Table IV.Most frequent toxicities (grade III
or higher) for patients treated with DD vs. standard treatment. |
Table IV.
Most frequent toxicities (grade III
or higher) for patients treated with DD vs. standard treatment.
| Outcome | No. of studies | OR (95% CI) | Heterogeneity (p,
I2) | Publication
bias(P-values from Begg's test) | Publication bias
(P-values from Egger's test) | Grade |
|---|
| Anemia | 12 | 3.024 (2.173,
4.208)a | 0.402, 4.3% | 1.000 | 0.932 | MODERATE |
| Leukocytopenia | 10 | 1.360 (0.342,
5.407) | 0.000,
98.8%b | 0.592 | 0.785 | LOW |
| Fatigue | 8 | 1.223 (0.838,
1.786) |
0.000,91.4%b | 0.174 | 0.279 | LOW |
| Diarrhea | 15 | 1.227 (0.779,
1.934) | 0.000,
78.9%b | 0.621 | 0.993 | LOW |
| Nausea | 14 | 1.482 (1.096,
2.004)a | 0.000,
77.6%b | 0.228 | 0.951 | LOW |
| Vomiting | 17 | 1.273 (0.940,
1.722) | 0.000,
74.5%b | 0.773 | 0.573 | LOW |
| Febrile
neutropenia | 11 | 0.815 (0.430,
1.544) | 0.000,
96.2%b | 0.876 | 0.639 | LOW |
Discussion
The present network meta-analysis systematically
reviewed the efficacy and safety of DD chemotherapy in patients
with BC and determined the most suitable pathological subtype that
would benefit from DD chemotherapy. A total of 27 studies involving
27,580 patients were included and the baseline indicators were
balanced, except for tumor grade. In addition, from the pairwise
meta-analysis of efficacy outcomes of DD chemotherapy, significant
differences were frequently found in the general, HR+
and Her2− status subgroups in terms of HRa outcome.
Moreover, the meta-regression revealed stratified patients with
Her2 status and the heterogeneity source. Furthermore, the network
meta-analysis indicated that patients with the HR+,
Her2− pathological BC subtype may be more responsive to
DD chemotherapy. In addition, chemotherapy for DD can cause serious
anemia and nausea. For patients with BC with HR+ and
Her2− pathology, chemotherapy for DD was
recommended.
This network meta-analysis adhered to the PRISMA
guidelines and was registered with the PROSPERO website, which
means that the review was systematic and robust. The present study
concluded that patients with BC with HR+ and
Her2− states were the pathological subtypes most
suitable for intense DD chemotherapy. Similar conclusions were
obtained by Puglisi et al, whereby DD adjuvant chemotherapy
showed a consistent benefit in patients with early BC with
HR+/HER2− disease, although its effects
varied according to the composite measure of the risk of recurrence
(48). Lambertini et al
concluded that DD chemotherapy is associated with a significant
improvement in survival in patients with high-risk breast cancer.
Its benefit is smaller in patients with HER2+ disease
who received adjuvant trastuzumab (6). In terms of safety outcomes,
information regarding treatment with pertuzumab, trastuzumab and
common anthracycline-containing regimens for the neoadjuvant
treatment of early breast cancer resulted in cardiac and general
safety profiles and pCR rates that were consistent with those from
previous studies of pertuzumab (49). These studies support the present
results and suggest that the safety of DD chemotherapy is
acceptable.
Currently, standard chemotherapy based on
anthracycline and taxane is still recommended for patients with
HER2−/HR+ BC with a 21-day cycle. The present
study recommended a 14-day cycle of chemotherapy without changing
chemotherapy agents to achieve an improved patient benefit and
acceptable safety. The mechanism of DD chemotherapy is more
effective, as the DD regimen was less toxic to the immune system,
presented reduced immunosuppression by the tumor microenvironment,
and triggered macrophage recruitment and tumor-specific
CD8+ T-cell responses to tumors as determined by IL-2
and IFN-γ secretion (50).
Furthermore, other mechanisms of action may also be involved, such
as induction of apoptosis or inhibition of angiogenesis (51). Furthermore, DD chemotherapy may
contribute to overcoming drug resistance, giving a high response
rate, although durable remissions were achieved in few patients
(52). These studies indicate that
it is important to investigate non-traditional chemotherapy
interval options for the treatment of BC.
There are also several limitations among the studies
included in the meta-analysis. First, the baseline was not balanced
as an indicator of tumor grade, which may have influenced the
overall outcome. Second, the present study found that significant
differences in OS and DFS data often appeared in HRa but not in OR
data because HRa data were more accurate. Third, the present study
did not perform a subgroup analysis based on the classification of
chemotherapeutic agents or on the clinical stage because the
included studies did not provide enough HRa data. Fourth, the
sample size of the network meta-analysis may not have been large
enough to show the final conclusion. In the future, high-quality
studies with larger sample sizes should be conducted in an effort
to clearly report agents and clinical stages, thereby allowing
network meta-analysis determination of the most suitable
chemotherapeutic agents and clinical stage of patients to benefit
from intensive DD chemotherapy. Finally, the present study found
that repeated data were reported; for example, some data were
included in both the HR+ and Her2− groups,
which may have also influenced the overall results. In conclusion,
patients with HR+ and Her2− BC are the most
suitable pathological subtype to benefit from intense DD
chemotherapy with an acceptable safety profile.
The findings of the present network meta-analysis
represent studies with the best evidence base currently available
and provide a guide on the choice of intense DD chemotherapy or
standard chemotherapy regimens for patients. From a clinical
standpoint, it is important to also consider the most suitable
chemotherapy interval for different pathological subtypes in
patients with BC, and it is hoped that these results will improve
informed and shared decision-making processes for patients and
clinicians. The present study hypothesized that intensive DD
chemotherapy was most appropriate for patients with the luminal A
type. Future studies should focus on the specific characteristics
of patients to provide a personalized prediction of comparative
effectiveness and safety with respect to the applicability of DD
chemotherapy.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by National College Students
Innovation and Entrepreneurship Training Program of Shenyang
Pharmaceutical University (grant no. 202210163017), Science
Foundation of Shenyang Pharmaceutical University (grant no.
GGJJ2021107) and Scientific Research Fund of Liaoning Provincial
Education Department (grant no. LJKQZ20222349).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DS, TQZ, and YSZ contributed to the study conception
and design. Data collection and analysis were performed by DS, TQZ,
HMH, XYS, YL and XYW. The first draft of the manuscript was written
by DS and all authors commented on previous versions of the
manuscript. All authors read and approved the final manuscript. DS
and YSZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schopper D and de Wolf C: How effective
are breast cancer screening programmes by mammography? Review of
the current evidence. Eur J Cancer. 45:1916–1923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian Z, Tang J, Liao X, Yang Q, Wu Y and
Wu G: An immune-related prognostic signature for predicting breast
cancer recurrence. Cancer Med. 9:7672–7685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li BX, Chen XJ, Ding TJ, Liu YH, Ma TT,
Zhang GL and Wang XM: Potentially overestimated efficacy of
nanoparticle albumin-bound paclitaxel compared with solvent-based
paclitaxel in breast cancer: A systemic review and meta-analysis. J
Cancer. 12:5164–5172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu T, Xu F, Zhang L, Zhang Y, Yang C,
Cheng M, Chen F and Wang K: Measurement of molecular biomarkers
that predict the tumor response in estrogen receptor-positive
breast cancers after dose-dense (biweekly) paclitaxel/carboplatin
neoadjuvant chemotherapy. Oncotarget. 8:101087–101094. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lambertini M, Poggio F, Bruzzone M, Conte
B, Bighin C, de Azambuja E, Giuliano M, De Laurentiis M, Cognetti
F, Fabi A, et al: Dose-dense adjuvant chemotherapy in HER2-positive
early breast cancer patients before and after the introduction of
trastuzumab: Exploratory analysis of the GIM2 trial. Int J Cancer.
147:160–169. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomasello G, Valeri N, Ghidini M, Smyth
EC, Liguigli W, Toppo L, Mattioli R, Curti A, Hahne JC, Negri FM,
et al: First-line dose-dense chemotherapy with docetaxel,
cisplatin, folinic acid and 5-fluorouracil (DCF) plus panitumumab
in patients with locally advanced or metastatic cancer of the
stomach or gastroesophageal junction: Final results and biomarker
analysis from an Italian oncology group for clinical research
(GOIRC) phase II study. Oncotarget. 8:111795–111806. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou W, Chen S, Xu F and Zeng X: Survival
benefit of pure dose-dense chemotherapy in breast cancer: A
meta-analysis of randomized controlled trials. World J Surg Oncol.
16:1442018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldvaser H, Majeed H, Ribnikar D, Šeruga
B, Ocaña A, Cescon DW and Amir E: Influence of control group
therapy on the benefit from dose-dense chemotherapy in early breast
cancer: a systemic review and meta-analysis. Breast Cancer Res
Treat. 169:413–425. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petrelli F, Coinu A, Lonati V, Cabiddu M,
Ghilardi M, Borgonovo K and Barni S: Neoadjuvant dose-dense
chemotherapy for locally advanced breast cancer: A meta-analysis of
published studies. Anticancer Drugs. 27:702–708. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Tulder M, Furlan A, Bombardier C and
Bouter L; Editorial Board of the Cochrane Collaboration Back Review
Group, : Updated method guidelines for systematic reviews in the
cochrane collaboration back review group. Spine (Phila Pa 1976).
28:1290–1299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jinatongthai P, Kongwatcharapong J, Foo
CY, Phrommintikul A, Nathisuwan S, Thakkinstian A, Reid CM and
Chaiyakunapruk N: Comparative efficacy and safety of reperfusion
therapy with fibrinolytic agents in patients with ST-segment
elevation myocardial infarction: A systematic review and network
meta-analysis. Lancet. 390:747–759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366:l48982019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wells G: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of non-randomised studies in
meta-analyses. Symposium on Systematic Reviews: Beyond the Basics.
2014.
|
|
16
|
Guyatt GH, Oxman AD, Vist GE, Kunz R,
Falck-Ytter Y, Alonso-Coello P and Schünemann HJ; GRADE Working
Group, : GRADE: An emerging consensus on rating quality of evidence
and strength of recommendations. BMJ. 336:924–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang C, Xu C, Li X and Zhang Y, Zhang S,
Zhang T and Zhang Y: Could camrelizumab plus chemotherapy improve
clinical outcomes in advanced malignancy? A systematic review and
network meta-analysis. Front Oncol. 11:7001652021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng F, Jiang Q, Jia H, Sun H, Chai Y, Li
X, Rong G, Zhang Y and Li Z: Which is the best combination of TACE
and Sorafenib for advanced hepatocellular carcinoma treatment? A
systematic review and network meta-analysis. Pharmacol Res.
135:89–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins JPT, Jackson D, Barrett JK, Lu G,
Ades AE and White IR: Consistency and inconsistency in network
meta-analysis: Concepts and models for multi-arm studies. Res Synth
Methods. 3:98–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
König J, Krahn U and Binder H: Visualizing
the flow of evidence in network meta-analysis and characterizing
mixed treatment comparisons. Stat Med. 32:5414–5429. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schneeweiss A, Michel LL, Möbus V, Tesch
H, Klare P, Hahnen E, Denkert C, Kast K, Pohl-Rescigno E, Hanusch
C, et al: Survival analysis of the randomised phase III GeparOcto
trial comparing neoadjuvant chemotherapy of intense dose-dense
epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel,
liposomal doxorubicin (plus carboplatin in triple-negative breast
cancer) for patients with high-risk early breast cancer. Eur J
Cancer. 160:100–111. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y: Efficacy and long-term survival
outcomes of dose-dense carboplatin plus paclitaxel as neoadjuvant
chemotherapy for triple-negative breast cancer. PhD Thesis. Chin
Acad Med Sci. 2021.(In Chinese).
|
|
23
|
Blondeaux E, Lambertini M, Michelotti A,
Conte B, Benasso M, Dellepiane C, Bighin C, Pastorino S, Levaggi A,
Alonzo A, et al: Dose-dense adjuvant chemotherapy in early breast
cancer patients: 15-Year results of the phase 3 Mammella
InterGruppo (MIG)-1 study. Br J Cancer. 122:1611–1617. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He J: Application of Dose-dense
Chemotherapy in Neoadjuvant Therapy for Breast Cancer. PhD Thesis.
Hebei Med Univ. 2020.(In Chinese).
|
|
25
|
Schneeweiss A, Möbus V, Tesch H, Hanusch
C, Denkert C, Lübbe K, Huober J, Klare P, Kümmel S, Untch M, et al:
Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus
weekly paclitaxel, liposomal doxorubicin (plus carboplatin in
triple-negative breast cancer) for neoadjuvant treatment of
high-risk early breast cancer (GeparOcto-GBG 84): A randomised
phase III trial. Eur J Cancer. 106:181–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Möbus V, Jackisch C, Lück HJ, du Bois A,
Thomssen C, Kuhn W, Nitz U, Schneeweiss A, Huober J, Harbeck N, et
al: Ten-year results of intense dose-dense chemotherapy show
superior survival compared with a conventional schedule in
high-risk primary breast cancer: Final results of AGO phase III
iddEPC trial. Ann Oncol. 29:178–185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Rossum AGJ, Kok M, van Werkhoven E,
Opdam M, Mandjes IAM, van Leeuwen-Stok AE, van Tinteren H, Imholz
ALT, Portielje JEA, Bos MMEM, et al: Adjuvant dose-dense
doxorubicin-cyclophosphamide versus
docetaxel-doxorubicin-cyclophosphamide for high-risk breast cancer:
First results of the randomised MATADOR trial (BOOG 2004–04). Eur J
Cancer. 102:40–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cameron D, Morden JP, Canney P, Velikova
G, Coleman R, Bartlett J, Agrawal R, Banerji J, Bertelli G,
Bloomfield D, et al: Accelerated versus standard epirubicin
followed by cyclophosphamide, methotrexate, and fluorouracil or
capecitabine as adjuvant therapy for breast cancer in the
randomised UK TACT2 trial (CRUK/05/19): A multicentre, phase 3,
open-label, randomised, controlled trial. Lancet Oncol. 18:929–945.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lambertini M, Ceppi M, Cognetti F,
Cavazzini G, De Laurentiis M, De Placido S, Michelotti A, Bisagni
G, Durando A, Valle E, et al: Dose-dense adjuvant chemotherapy in
premenopausal breast cancer patients: A pooled analysis of the MIG1
and GIM2 phase III studies. Eur J Cancer. 71:34–42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bao Z, Chen Y, Ren H, Jiang Y, Yang J and
Li S: Clinical observation of dose-dense chemotherapy in the
postoperative treatment of triple-negative breast cancer. J Mod
Oncol. 24:2221–2224. 2016.(In Chinese).
|
|
31
|
Foukakis T, von Minckwitz G, Bengtsson NO,
Brandberg Y, Wallberg B, Fornander T, Mlineritsch B, Schmatloch S,
Singer CF, Steger G, et al: Effect of tailored dose-dense
chemotherapy vs standard 3-weekly adjuvant chemotherapy on
recurrence-free survival among women with high-risk early breast
cancer: A randomized clinical trial. JAMA. 316:1888–1896. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Del Mastro L, De Placido S, Bruzzi P, De
Laurentiis M, Boni C, Cavazzini G, Durando A, Turletti A, Nisticò
C, Valle E, et al: Fluorouracil and dose-dense chemotherapy in
adjuvant treatment of patients with early-stage breast cancer: An
open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet.
385:1863–1872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou Y: Clinical study of postoperative
intensive chemotherapy with pirarubicin for triple negative breast
cancer. J Clin Med. 2:6059–6062. 2015.(In Chinese).
|
|
34
|
Swain SM, Tang G, Geyer CE Jr, Rastogi P,
Atkins JN, Donnellan PP, Fehrenbacher L, Azar CA, Robidoux A,
Polikoff JA, et al: Definitive results of a phase III adjuvant
trial comparing three chemotherapy regimens in women with operable,
node-positive breast cancer: The NSABP B-38 trial. J Clin Oncol.
31:3197–3204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu X, Qin Q, Wei C, Zhu F, Mo G and Lian
B: Clinical effect analysis of intensive chemotherapy and
conventional adjuvant chemotherapy in patients with advanced breast
cancer. Pract Geriatr. 27:157–173. 2013.(In Chinese).
|
|
36
|
Gogas H, Dafni U, Karina M, Papadimitriou
C, Batistatou A, Bobos M, Kalofonos HP, Eleftheraki AG, Timotheadou
E, Bafaloukos D, et al: Postoperative dose-dense sequential versus
concomitant administration of epirubicin and paclitaxel in patients
with node-positive breast cancer: 5-Year results of the Hellenic
cooperative oncology group HE 10/00 phase III trial. Breast Cancer
Res Treat. 132:609–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin C, Zhang Y, Ma L, Zhou Y, Wei Y and Li
H and Li H: Clinical analysis of intensive chemotherapy with
perarubicin after high risk breast cancer surgery. Guide China Med.
10:558–559. 2012.(In Chinese).
|
|
38
|
Arun BK, Dhinghra K, Valero V, Kau SW,
Broglio K, Booser D, Guerra L, Yin G, Walters R, Sahin A, et al:
Phase III randomized trial of dose intensive neoadjuvant
chemotherapy with or without G-CSF in locally advanced breast
cancer: Long-term results. Oncologist. 16:1527–1534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Untch M, Fasching PA, Konecny GE, von Koch
F, Conrad U, Fett W, Kurzeder C, Lück HJ, Stickeler E, Urbaczyk H,
et al: PREPARE trial: A randomized phase III trial comparing
preoperative, dose-dense, dose-intensified chemotherapy with
epirubicin, paclitaxel and CMF versus a standard-dosed
epirubicin/cyclophosphamide followed by paclitaxel ± darbepoetin
alfa in primary breast cancer-results at the time of surgery. Ann
Oncol. 22:1988–1998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Untch M, von Minckwitz G, Konecny GE,
Conrad U, Fett W, Kurzeder C, Lück HJ, Stickeler E, Urbaczyk H,
Liedtke B, et al: PREPARE trial: A randomized phase III trial
comparing preoperative, dose-dense, dose-intensified chemotherapy
with epirubicin, paclitaxel, and CMF versus a standard-dosed
epirubicin-cyclophosphamide followed by paclitaxel with or without
darbepoetin alfa in primary breast cancer-outcome on prognosis. Ann
Oncol. 22:1999–2006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Burnell M, Levine MN, Chapman JA, Bramwell
V, Gelmon K, Walley B, Vandenberg T, Chalchal H, Albain KS, Perez
EA, et al: Cyclophosphamide, epirubicin, and fluorouracil versus
dose-dense epirubicin and cyclophosphamide followed by paclitaxel
versus doxorubicin and cyclophosphamide followed by paclitaxel in
node-positive or high-risk node-negative breast cancer. J Clin
Oncol. 28:77–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moebus V, Jackisch C, Lueck HJ, du Bois A,
Thomssen C, Kurbacher C, Kuhn W, Nitz U, Schneeweiss A, Huober J,
et al: Intense dose-dense sequential chemotherapy with epirubicin,
paclitaxel, and cyclophosphamide compared with conventionally
scheduled chemotherapy in high-risk primary breast cancer: Mature
results of an AGO phase III study. J Clin Oncol. 28:2874–2880.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Untch M, Möbus V, Kuhn W, Muck BR,
Thomssen C, Bauerfeind I, Harbeck N, Werner C, Lebeau A,
Schneeweiss A, et al: Intensive dose-dense compared with
conventionally scheduled preoperative chemotherapy for high-risk
primary breast cancer. J Clin Oncol. 27:2938–2945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Venturini M, Del Mastro L, Aitini E,
Baldini E, Caroti C, Contu A, Testore F, Brema F, Pronzato P,
Cavazzini G, et al: Dose-dense adjuvant chemotherapy in early
breast cancer patients: Results from a randomized trial. J Natl
Cancer Inst. 97:1724–1733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baldini E, Gardin G, Giannessi PG,
Evangelista G, Roncella M, Prochilo T, Collecchi P, Rosso R,
Lionetto R, Bruzzi P, et al: Accelerated versus standard
cyclophosphamide, epirubicin and 5-fluorouracil or
cyclophosphamide, methotrexate and 5-fluorouracil: A randomized
phase III trial in locally advanced breast cancer. Ann Oncol.
14:227–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Citron ML, Berry DA, Cirrincione C, Hudis
C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R,
Ingle JN, et al: Randomized trial of dose-dense versus
conventionally scheduled and sequential versus concurrent
combination chemotherapy as postoperative adjuvant treatment of
node-positive primary breast cancer: First report of intergroup
trial C9741/cancer and leukemia group B trial 9741. J Clin Oncol.
21:1431–1439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Therasse P, Mauriac L, Welnicka-Jaskiewicz
M, Bruning P, Cufer T, Bonnefoi H, Tomiak E, Pritchard KI, Hamilton
A and Piccart MJ; EORTC: Final results of a randomized phase III
trial comparing cyclophosphamide, epirubicin, and fluorouracil with
a dose-intensified epirubicin and cyclophosphamide + filgrastim as
neoadjuvant treatment in locally advanced breast cancer: An
EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 21:843–850. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Puglisi F, Gerratana L, Lambertini M,
Ceppi M, Boni L, Montemurro F, Russo S, Bighin C, De Laurentiis M,
Giuliano M, et al: Composite risk and benefit from adjuvant
dose-dense chemotherapy in hormone receptor-positive breast cancer.
NPJ Breast Cancer. 7:822021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Swain SM, Ewer MS, Viale G, Delaloge S,
Ferrero JM, Verrill M, Colomer R, Vieira C, Werner TL, Douthwaite
H, et al: Pertuzumab, trastuzumab, and standard anthracycline- and
taxane-based chemotherapy for the neoadjuvant treatment of patients
with HER2-positive localized breast cancer (BERENICE): A phase II,
open-label, multicenter, multinational cardiac safety study. Ann
Oncol. 29:646–653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chang CL, Hsu YT, Wu CC, Lai YZ, Wang C,
Yang YC, Wu TC and Hung CF: Dose-dense chemotherapy improves
mechanisms of antitumor immune response. Cancer Res. 73:119–127.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kumar A, Hoskins PJ and Tinker AV:
Dose-dense paclitaxel in advanced ovarian cancer. Clin Oncol (R
Coll Radiol). 27:40–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Crout CA, Koh LP, Gockerman JP, Moore JO,
Decastro C, Long GD, Diehl L, Gasparetto C, Niedzwiecki D, Edwards
J, et al: Overcoming drug resistance in mantle cell lymphoma using
a combination of dose-dense and intense therapy. Cancer Invest.
28:654–660. 2010. View Article : Google Scholar : PubMed/NCBI
|