Introduction
Rectal cancer is a type of malignant tumor of the
digestive tract. Although the overall incidence of rectal cancer is
slowly decreasing year by year, the incidence in adults <65
years old is still increasing (1).
Most patients with rectal cancer have low rectal cancer (tumor
distance from anus <5 cm) (2).
Abdominoperineal resection (Miles operation) has been used to treat
low rectal cancer; however, patients require permanent abdominal
wall fistulation following the operation. Although this operation
can achieve local radical resection of the tumor, a permanent stoma
in the abdominal wall can affect the postoperative life of
patients. Miles operation has a marked influence on some normal
functions of patients after operation, such as defecation, voiding
and sexual functions (3). With the
development of surgical instruments and the improvement of surgical
technology, clinicians have aimed to identify methods to cure
tumors and preserve the anus (4).
To protect the anus, total mesorectal excision (TME),
intersphincteric resection and anterior resection have been widely
used. With the progression of surgical approaches, it is now
possible to perform sphincter-preserving resection (SPR) on
patients with low rectal cancer. However, novel problems have also
emerged, with 60–90% of patients with rectal cancer experiencing
defecation disorders after SPR. These disorders are known as low
anterior resection syndrome (LARS) (5). LARS may reduce the quality of life of
patients after operation, and severe LARS will negatively affect
the daily life of patients (6).
Currently, there is no specific treatment for LARS, and most of the
treatments are symptomatic; therefore, the prevention of LARS is
necessary (7). Notably, accurate
prediction of the influencing factors of LARS is of great
significance in reducing the incidence of LARS. Numerous theories
on the pathogenesis and influencing factors of LARS have been put
forward; however, there are some problems, such as small sample
size, incomplete research projects, inconsistent research results,
lack of convincing research results and lack of in-depth research
(8). Therefore, the present study
performed a meta-analysis to systematically evaluate the studies on
the influencing factors of LARS in rectal cancer, with the aim of
identifying accurate and reliable influencing factors of LARS and
providing a theoretical basis and reference for clinical work, to
reduce the incidence of LARS.
Materials and methods
Literature source
Studies on the efficacy of the risk factors for
severe LARS in patients with rectal cancer undergoing
sphincter-preserving surgery were searched using PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase
(www.embase.com), Cochrane Library (https://www.cochranelibrary.com/), Scopus
(https://www.scopus.com/) and Web of Science
(https://www.webofscience.com/). The
studies published in these databases between January 1, 2000 and
December 31, 2022 on the influencing factors of LARS in patients
with rectal cancer undergoing sphincter-preserving surgery were
searched.
Search strategy
A systematic review and meta-analysis was performed
according to the Preferred Reporting Items for Systematic Reviews
and Meta-analysis guidelines (9).
The search strategy was carried out in the databases based on the
search string: [(Rectal Neoplasms (Medical Subject Headings, MeSH
Terms)) OR (Rectal Tumor (MeSH Terms)) OR ((MeSH Tumor, Rectal
Terms)) OR (Neoplasms (MeSH Terms)) OR (Cancer of Rectum (MeSH
Terms))] AND [(Anterior resection syndrome) OR (Fecal incontinence)
OR (Postoperative complication) OR (LARS) OR (Low Anterior
Resection Syndrome)] AND [Radiofrequency ablation] AND
[(Influencing factors) OR (Interfering factors)]. All detected
studies were assessed for eligibility.
Literature inclusion criteria
The inclusion criteria were as follows: i) Published
literature on the related influencing factors of severe LARS for
patients with rectal cancer; ii) all articles used the LARS score
(6) to evaluate severe LARS (LARS
score >29) and its risk factors; iii) all subjects were patients
with rectal cancer diagnosed by colonoscopy and pathology before
operation; iv) there was no defecation dysfunction or pelvic
surgery history before the operation; v) the research results
described odds ratio (OR) and 95% CI values, or OR and 95% CI could
be calculated using the literature data; and vi) Newcastle Ottawa
scale (NOS) (10) score ≥6
points.
Literature exclusion criteria
The exclusion criteria were as follows: i) Reviews,
animal studies, preliminary reports of research, case reports,
letters to editors, meeting minutes, commentaries and studies
published in languages other than English; ii) published in
repeated publications in different databases; iii) the purpose of
the study was not defined, and/or the data were not detailed or
inconsistent; and iv) the diagnosis of the patient was not clear,
and the LARS score was not used for LARS diagnosis in the
article.
Literature screening and data
extraction
According to the unified retrieval strategy, two
independent researchers retrieved and imported the studies into
EndNote 21 software (https://endnote.com/). Literature screening, data
extraction and risk of bias assessment were all carried out by two
reviewers independently. Conflicts were resolved by a third
independent reviewer. The EndNote software automatically deleted
reviews, animal experiments, repetitive literature, case reports
and literature published prior to 2000. All studies were read and
the literature was excluded if it contained inconsistent research
content, incorrect research methods (diagnosis of the case was not
clear and LARS was not diagnosed using the LARS score) or no
extractable data that met the inclusion criteria. The sample size,
the source of patients, the research methods, and the factors
affecting the incidence and occurrence of LARS were extracted from
the studies.
Quality evaluation
The quality of the literature was evaluated
according to the NOS scale (10).
The case-control NOS scale was scored based on three aspects: Case
group and control group selection, comparability and exposure.
There were eight scoring conditions, with a total score of 9.
Literature with a score of ≥7 was considered high-quality, whereas
that with a score of ≤4 was considered low-quality literature.
Statistical analysis
Data for meta-analysis were entered into MS Excel
2019 (Microsoft Corporation). Meta-analysis was carried out using
RevMan 5.2 (The Cochrane Collaboration) and STATA (version 14.0;
StataCorp LLC) software. Forest plots were drawn using RevMan 5.2
software. P<0.05 was considered to indicate a statistically
significant difference. Q test and I2 test were used to
determine the heterogeneity of the results. The random-effects
models were used to pool the effect estimates in this
meta-analysis. The publication bias was assessed using a Begg's
funnel chart using STATA (11). The
source of heterogeneity was identified by sensitivity analysis or
subgroup analysis. The sensitivity analysis was carried out using a
random-effects model. Heterogeneity was evaluated by the Cochran's
Q test and I2 statistic. I2 ≥50% indicated
greater heterogeneity. Subgroup analysis was created to explore the
source of heterogeneity. Subgroup analysis was based on the study
population, measurement method, number of adjusted variables and
study quality.
Results
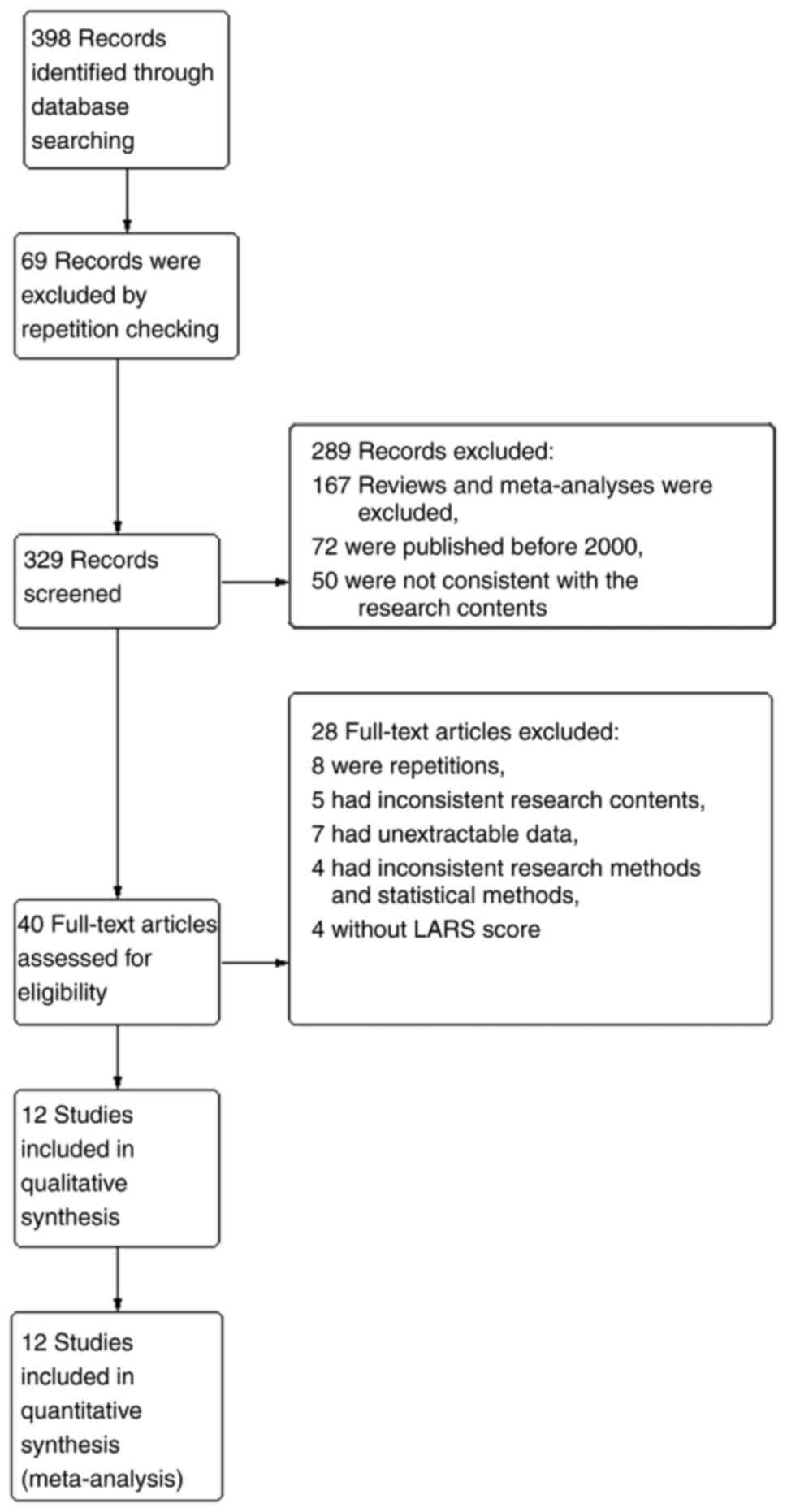
Literature retrieval results
A total of 398 original pieces of literature were
obtained, all of which were imported into the EndNote 21 software;
69 records were excluded by repetition checking, 167 studies were
excluded as they were reviews or meta-analyses, and 72 articles
published before 2000 were excluded. The abstracts of the remaining
literature were preliminarily screened, 50 articles that were not
consistent with the research contents were excluded, and the
remaining 40 articles were left after preliminary screening. After
reading the complete text, eight repetitive studies, five articles
with inconsistent research contents, seven articles with
unextractable data, four articles with inconsistent research
methods and statistical methods, and four articles without LARS
scores were excluded. A total of 12 articles were included for
quality evaluation (Fig. 1).
Essential characteristics of the
included literature
A total of 12 articles were included in the present
meta-analysis. The cases were from eight countries: China, Denmark,
South Korea, Thailand, Spain, UK, Netherlands and Germany (12–23). A
total of 3,877 patients were included in the study, of which 1,589
patients had severe LARS (Table I).
The factors that could affect the incidence of LARS were numbered
and outlined. The basic information of the included studies is
shown in Table I.
 | Table I.Basic information of the included
literature. |
Table I.
Basic information of the included
literature.
| First author/s,
year | Country | Study design | Sample size | Severe LARS
cases | NOS score | Influencing
factors | (Refs.) |
|---|
| Bondeven et
al, 2015 | Denmark | Retrospective | 125 | 47 | 8 | 1 | (12) |
| Bregendahl et
al, 2013 | Denmark | Retrospective | 938 | 383 | 8 | 1,2,3,4 | (13) |
| Cheong et
al, 2019 | South Korea | Prospective | 203 | 106 | 7 | 1,2 | (14) |
| Ekkarat et
al, 2016 | Thailand | Retrospective | 129 | 23 | 6 | 1 | (15) |
| Emmertsen et
al, 2013 | Denmark | Retrospective | 193 | 84 | 9 | 1,4 | (16) |
| Jimenez-Gomez et
al, 2017 | Spain | Cross
sectional | 184 | 104 | 9 | 1,4 | (17) |
| Lynes and Thaha,
2016 | UK | Retrospective | 1,093 | 447 | 8 | 1,2 | (18) |
| Qin et al,
2017 | China | Cross
sectional | 142 | 63 | 8 | 1,5 | (19) |
| Sun et al,
2019 | China | Retrospective | 129 | 60 | 8 | 2 | (20) |
| Hughes et
al, 2017 | UK | Retrospective | 68 | 38 | 6 | 1 | (21) |
| van Heinsbergen,
2018 | Netherlands | Retrospective | 412 | 141 | 7 | 2,5 | (22) |
| Kupsch et
al, 2018 | Germany | Retrospective | 261 | 93 | 6 | 5 | (23) |
Quality evaluation of the included
literature
The quality of the 12 articles that met the criteria
for inclusion in the present meta-analysis was evaluated. After
quality evaluation, nine high-quality articles with a NOS score of
≥7 and three articles with a NOS score of 6 were obtained (Table I).
Effect of radiotherapy and
chemotherapy on severe LARS in rectal cancer
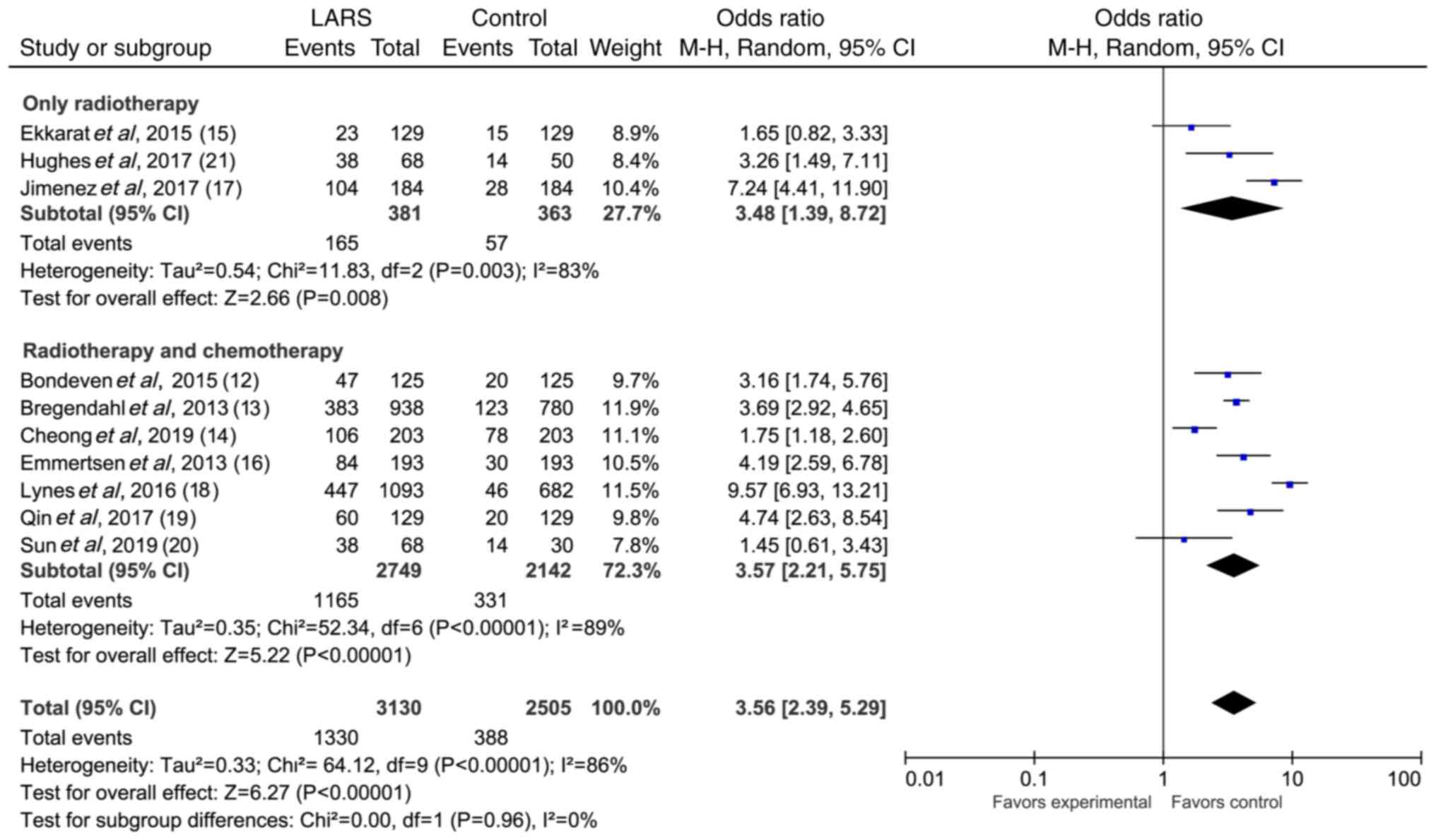
A total of 10 studies reported on the association
between radiotherapy and chemotherapy and severe LARS (Table II). The forest plot of the
random-effects meta-analysis of the effect of radiotherapy and
chemotherapy on severe LARS in rectal cancer is shown in Fig. 2 (OR, 3.45; 95% CI, 2.29–5.21; Z,
5.91; P<0.00001). Sensitivity analysis was carried out to assess
the stability of the results. No significant variations were
observed when eliminating any one article. According to whether
only radiotherapy was used as the standard, the eligible articles
were divided into only radiotherapy and non-only radiotherapy
subgroups. The results of the subgroup analysis are shown in
Fig. 3. The heterogeneity of only
radiotherapy articles was I2=83%, P=0.003. The
heterogeneity of non-only radiotherapy articles was
I2=89%, P<0.00001. These findings indicated that
radiotherapy and chemotherapy were risk factors for severe LARS,
and the radiotherapy alone group had a higher risk of LARS than the
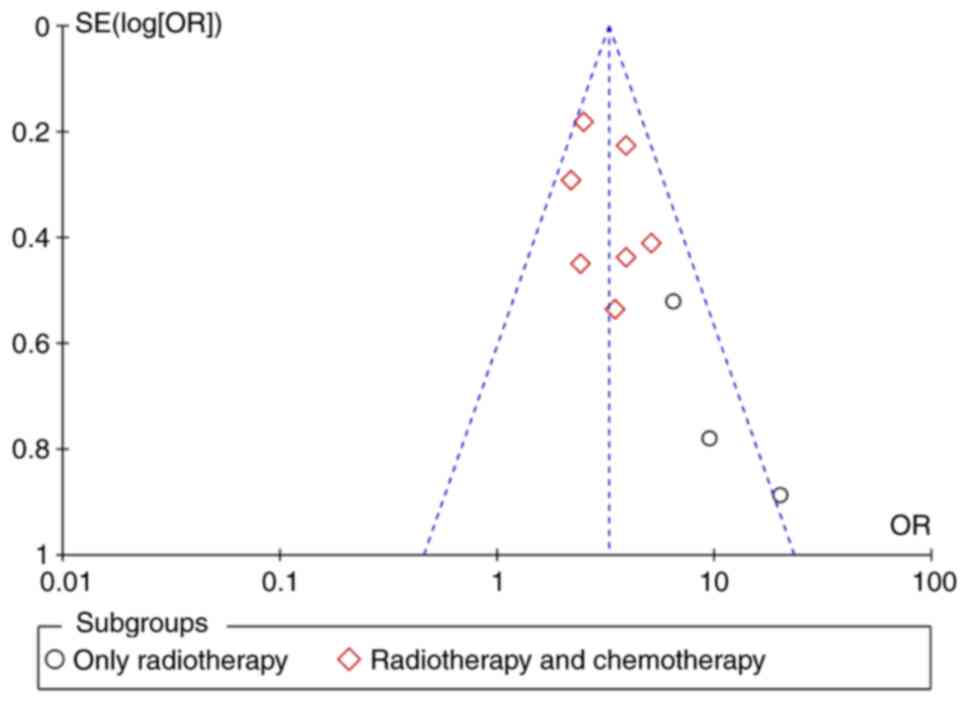
non-only radiotherapy group. A Begg's funnel map was used to
perform publication bias analysis for radiotherapy and chemotherapy
(Fig. 4). The results indicated
that there was a particular publication bias, but the bias was
slight.
 | Table II.Radiotherapy and chemotherapy as risk
factors for severe LARS. |
Table II.
Radiotherapy and chemotherapy as risk
factors for severe LARS.
| First author/s,
year | Risk factors | OR | OR_LL | OR_UL | (Refs.) |
|---|
| Bondeven et
al, 2015 | Neoadjuvant therapy
(yes/no) | 3.50 | 1.15 | 9.40 | (12) |
| Bregendahl et
al, 2013 | Neoadjuvant therapy
(yes/no) | 2.48 | 1.73 | 3.55 | (13) |
| Cheong et
al, 2019 | Chemoradiation
(yes/no) | 3.89 | 2.98 | 16.60 | (14) |
| Ekkarat et
al, 2015 | Radiation therapy
(yes/no) | 6.50 | 2.37 | 3.55 | (15) |
| Emmertsen et
al, 2013 | Neoadjuvant therapy
(yes/no) | 2.41 | 1.00 | 5.83 | (16) |
| Hughes et
al, 2017 | Neoadjuvant
treatment (radiotherapy) (yes/no) | 19.90 | 3.50 | 113.10 | (21) |
| Jimenez-Gomez et
al, 2017 | Postoperative
radiotherapy (yes/no) | 9.52 | 1.74 | 3.00 | (17) |
| Lynes and Thaha,
2016 | Neoadjuvant
chemoradiotherapy (yes/no) | 3.89 | 2.49 | 6.07 | (18) |
| Qin et al,
2017 | Neoadjuvant therapy
(NCRT/NCT) | 5.13 | 2.29 | 11.49 | (19) |
| Sun et al,
2019 | Neoadjuvant therapy
(NCRT/NCT) | 2.20 | 1.24 | 3.91 | (20) |
Effect of sex on severe LARS in rectal
cancer
A total of four studies reported on the association
between sex and the incidence of severe LARS, and the
random-effects model was used to examine the effects of sex on
severe LARS. As shown in Fig. 5,
the heterogeneity was apparent: I2=94%, P<0.00001.
Through sensitivity analysis, it was revealed that Cheong et
al (14) was the source of
heterogeneity, and the forest plot of the random-effects
meta-analysis after elimination is shown in Fig. 6. The results revealed that the risk
of severe LARS in female patients was slightly higher (OR, 6.54;
95% CI, 3.63–11.76; Z, 6.27; P<0.00001).
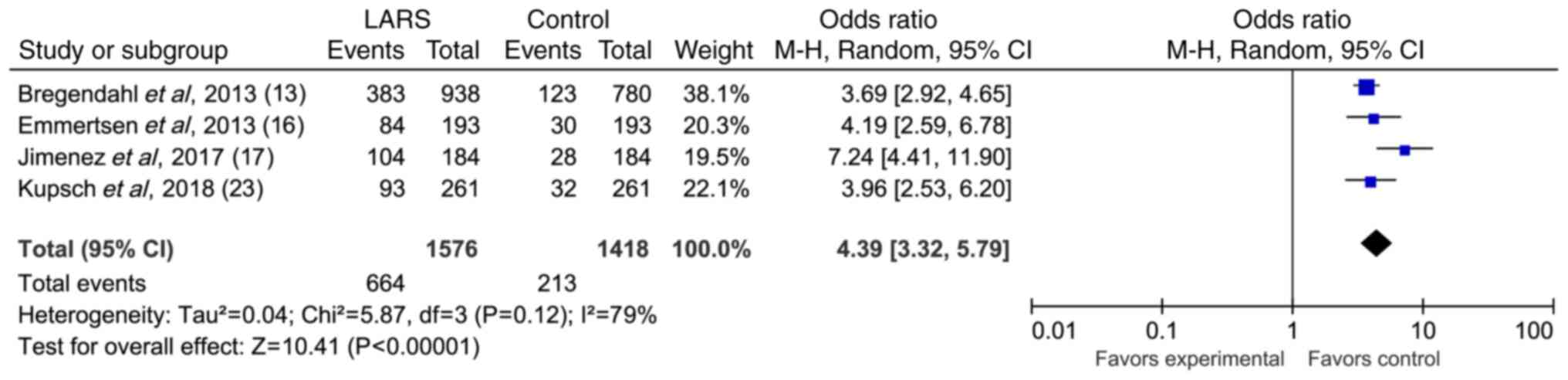
Effect of TME on severe LARS in rectal
cancer
A total of four articles reported the relationship
between TME and severe LARS. The forest plot of the random-effects
meta-analysis showed a significant association between TME and the
incidence of severe LARS (OR, 4.39; 95% CI, 3.32–5.79; Z, 10.41;
P<0.00001; Fig. 7). These
findings indicated that TME was an influencing factor of severe
LARS in rectal cancer.
Effect of distance between tumor and
anal margin on severe LARS in rectal cancer
Two articles reported on the relationship between
the distance between the tumor and anal margin and severe LARS. The
forest plot of the random-effects meta-analysis showed that there
was a significant association between the distance between the
tumor and anal margin and severe LARS (OR, 2.74; 95% CI, 0.86–8.72;
Z, 1.70; P<0.00001; Fig. 8).
These findings suggested that the distance between the tumor and
anal margin was an influencing factor of severe LARS in rectal
cancer.
Discussion
Numerous studies have reported on the influencing
factors of postoperative LARS in rectal cancer; however, the
results vary (24,25). The present study collected
literature on the influencing factors of severe LARS in rectal
cancer between January 1, 2000 and December 31, 2020, and
systematically analyzed the association between the influencing
factors and the incidence of LARS. The meta-analysis results
demonstrated that female sex, radiotherapy and chemotherapy,
distance between the tumor and anal margin, and TME were the
influencing factors of severe LARS in rectal cancer.
Tanaka et al (26) conducted a 5-year follow-up study on
506 patients with rectal cancer following anus-preserving surgery.
The results revealed that female patients had a higher risk of
developing LARS than male patients. The present results showed that
the risk of LARS in women was higher than that in men, which was
consistent with the results of this previous study. This may be
because the anal sphincter of women is congenitally thinner than
that in men, and most patients with rectal cancer are elderly
patients. Most female elderly patients have a reproductive history;
natural delivery through the birth canal can relax the pelvic floor
muscles, which may be why the risk of LARS in female patients is
higher than that in men (27).
Furthermore, there are intrauterine organs in the female pelvis,
which reduces the functional space during the operation to a
certain extent (28). If the
surgical technique is unsuccessful, it can increase the injury of
the pelvic autonomic nerve and anal sphincter (29).
With the development of radiotherapy and
chemotherapy, patients with colorectal cancer have a higher
resection rate, sphincter preservation success rate, survival rate
and clinical cure rate (30).
However, the present meta-analysis found that radiotherapy and
chemotherapy could increase the incidence of severe LARS. After
subgroup analysis, it was observed that the radiotherapy alone
group (OR=3.48) had a higher risk of LARS than the radiotherapy and
chemotherapy group (OR=3.57). Emerging evidence has suggested that
the incidence of severe LARS after neoadjuvant radiotherapy and
chemotherapy is higher than that of severe LARS after neoadjuvant
chemotherapy (30,31). In addition, a recent study has
demonstrated that compared with those receiving relatively simple
chemotherapy, patients receiving radiotherapy and chemotherapy have
a higher incidence of postoperative complications (32,33). A
recent study concluded that even patients who received radiotherapy
without surgery developed severe LARS, possibly because
radiotherapy was more toxic than sphincter-preserving resection
(31). However, radiotherapy and
chemotherapy can reduce the intestinal function of patients with
colorectal cancer, which may be caused by damage to the anal
sphincter, nerves in the pelvic cavity and intestinal microecology
(34).
The present study demonstrated that the distance
between the tumor and the anal margin, and TME were risk factors
for severe LARS. A previous study reported that when the distance
from the tumor to the anal margin decreases by 1 cm, the incidence
of severe LARS increases by 1.29, and patients with relatively high
positions of ultra-low rectal cancer have worse intestinal function
(35). It has also been reported
that a residual rectal length of ≤4 cm can lead to severe
intestinal dysfunction (36). The
reason may be that the lower tumor leads to a shorter residual
rectal length, and the shorter rectal residue affects the rectal
compliance of the patient, thus increasing the sense of urgency of
defecation. Compared with traditional surgery, TME surgery reduces
the local recurrence rate and the incidence of postoperative
complications (37). However, TME
surgery for the inferior mesenteric artery and its branches can
lead to changes in residual intestinal blood supply, which may lead
to intestinal dysfunction (38).
Based on the existing research, the results of the
present meta-analysis were reliable but still had some limitations.
First, the studies were conducted in various Asian and European
countries, on patients with different ethnicities who used
different languages, which may lead to differences in measurement
tools, treatment options and some definitions. Second, the included
literature did not mention specific radiotherapy and chemotherapy
regimens, and surgical anastomoses were not introduced. There was a
slight heterogeneity among the included studies, which may affect
the results. Finally, more literature on other factors, such as age
and anastomotic leakage, should be included; therefore, larger
samples and multicenter studies are needed to clarify these
factors.
In conclusion, radiotherapy and chemotherapy, TME,
the distance between the tumor and anal margin, and female sex were
revealed to be risk factors for severe LARS, which can seriously
affect the intestinal function of patients with rectal cancer
post-operation. Notably, clinicians should pay more attention to
the differences in female pelvic organs during surgery and
strengthen multidisciplinary cooperation to formulate more
personalized radiotherapy and chemotherapy programs and surgical
methods so that patients with rectal cancer have an improved
intestinal function and a higher survival rate.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangsu Postgraduate
Practice and Innovation Plan (grant no. SJCX22_0742) and the Hubei
Chen Xiaoping Science and Technology Development Fund ‘Huai'er
Special Fund for Cancer Prevention and Treatment Research’ in 2020
(grant no. CXPJJH12000002-202035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LLX and TCC were involved in study methodology,
investigation and data curation, and wrote the original draft. PC
was responsible for research design and conducted the experiments.
NJX and ZWJ were responsible for data analysis, and reviewed and
edited the manuscript. XXL was involved in the concepts and
supervision, and reviewed and edited the manuscript. LLX and TCC
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiao Y, Qiu M, Huang W, Hu S, Tan C, Nan
F, Jiang X, Wu D, Li M, Li Q and Qin C: Global status of research
on radiotherapy for rectal cancer: A bibliometric and visual
analysis. Front Public Health. 10:9622562022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keller DS, Berho M, Perez RO, Wexner SD
and Chand M: The multidisciplinary management of rectal cancer. Nat
Rev Gastroenterol Hepatol. 17:414–429. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toiyama Y and Kusunoki M: Changes in
surgical therapies for rectal cancer over the past 100 years: A
review. Ann Gastroenterol Surg. 4:331–342. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Serra-Aracil X, Mora-Lopez L,
Alcantara-Moral M, Caro-Tarrago A, Gomez-Diaz CJ and Navarro-Soto
S: Transanal endoscopic surgery in rectal cancer. World J
Gastroenterol. 20:11538–11545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Annicchiarico A, Martellucci J, Solari S,
Scheiterle M, Bergamini C and Prosperi P: Low anterior resection
syndrome: Can it be prevented? Int J Colorectal Dis. 36:2535–2552.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keane C, Fearnhead NS, Bordeianou LG,
Christensen P, Basany EE, Laurberg S, Mellgren A, Messick C,
Orangio GR, Verjee A, et al: International consensus definition of
low anterior resection syndrome. Dis Colon Rectum. 63:274–284.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Varghese C, Wells CI, Bissett IP, O'Grady
G and Keane C: The role of colonic motility in low anterior
resection syndrome. Front Oncol. 12:9753862022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garfinkle R and Boutros M: Low anterior
resection syndrome: Predisposing factors and treatment. Surg Oncol.
43:1016912022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo CK, Mertz D and Loeb M:
Newcastle-Ottawa Scale: Comparing reviewers' to authors'
assessments. BMC Med Res Methodol. 14:452014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin T, Chen ZH, Liang PP, Li ZD, He FJ,
Chen ZW, Hu JK and Yang K: A Gastrectomy for early-stage gastric
cancer patients with or without preserving celiac branches of vagus
nerves: A meta-analysis. Surgery. 173:375–382. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bondeven P, Emmertsen KJ, Laurberg S and
Pedersen BG: Neoadjuvant therapy abolishes the functional benefits
of a larger rectal remnant, as measured by magnetic resonance
imaging after restorative rectal cancer surgery. Eur J Surg Oncol.
41:1493–1499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bregendahl S, Emmertsen KJ, Lous J and
Laurberg S: Bowel dysfunction after low anterior resection with and
without neoadjuvant therapy for rectal cancer: A population-based
cross-sectional study. Colorectal Dis. 15:1130–1139. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheong C, Oh SY, Choi SJ and Suh KW:
Ultralow anterior resection and coloanal anastomosis for low-lying
rectal cancer: An appraisal based on bowel function. Dig Surg.
36:409–417. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ekkarat P, Boonpipattanapong T,
Tantiphlachiva K and Sangkhathat S: Factors determining low
anterior resection syndrome after rectal cancer resection: A study
in Thai patients. Asian J Surg. 39:225–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Emmertsen KJ and Laurberg S; Rectal Cancer
Function Study Group, : Impact of bowel dysfunction on quality of
life after sphincter-preserving resection for rectal cancer. Br J
Surg. 100:1377–1387. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jimenez-Gomez LM, Espin-Basany E, Trenti
L, Martí-Gallostra M, Sánchez-García JL, Vallribera-Valls F,
Kreisler E, Biondo S and Armengol-Carrasco M: Factors associated
with low anterior resection syndrome after surgical treatment of
rectal cancer. Colorectal Dis. Sep 29–2017.(Epub ahead of print).
PubMed/NCBI
|
|
18
|
Rosen H, Sebesta CG and Sebesta C:
Management of Low Anterior Resection Syndrome (LARS) Following
Resection for Rectal Cancer. Cancers (Basel). 15:7782023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin Q, Huang B, Cao W, Zhou J, Ma T, Zhou
Z, Wang J and Wang L: Bowel dysfunction after low anterior
resection with neoadjuvant chemoradiotherapy or chemotherapy alone
for rectal cancer: A cross-sectional study from China. Dis Colon
Rectum. 60:697–705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun W, Dou R, Chen J, Lai S, Zhang C, Ruan
L, Kang L, Deng Y, Lan P, Wang L and Wang J: Impact of long-course
neoadjuvant radiation on postoperative low anterior resection
syndrome and quality of life in rectal cancer: Post hoc analysis of
a randomized controlled trial. Ann Surg Oncol. 26:746–755. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hughes DL, Cornish J and Morris C; LARRIS
Trial Management Group, : Functional outcome following rectal
surgery-predisposing factors for low anterior resection syndrome.
Int J Colorectal Dis. 32:691–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Heinsbergen M, Janssen-Heijnen ML,
Leijtens JW, Slooter GD and Konsten JL: Bowel dysfunction after
sigmoid resection underestimated: Multicentre study on quality of
life after surgery for carcinoma of the rectum and sigmoid. Eur J
Surg Oncol. 44:1261–1267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kupsch J, Jackisch T, Matzel KE, Zimmer J,
Schreiber A, Sims A, Witzigmann H and Stelzner S: Outcome of bowel
function following anterior resection for rectal cancer-an analysis
using the low anterior resection syndrome (LARS) score. Int J
Colorectal Dis. 33:787–798. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asnong A, Tack J, Devoogdt N, De Groef A,
Geraerts I and D'Hoore A: Exploring the pathophysiology of LARS
after low anterior resection for rectal cancer with high-resolution
colon manometry. Neurogastroenterol Motil. 34:e144322022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eid Y, Bouvier V, Dejardin O, Menahem B,
Chaillot F, Chene Y, Dutheil JJ, Juul T, Morello R and Alves A:
‘French LARS score’: Validation of the French version of the low
anterior resection syndrome (LARS) score for measuring bowel
dysfunction after sphincter-preserving surgery among rectal cancer
patients: A study protocol. BMJ Open. 10:e0342512020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka N, Mizuno R, Ito K, Shirotake S,
Yasumizu Y, Masunaga A, Ito Y, Miyazaki Y, Hagiwara M, Kanao K, et
al: External validation of the MSKCC and IMDC risk models in
patients treated with targeted therapy as a first-line and
subsequent second-line treatment: A Japanese multi-institutional
study. Eur Urol Focus. 2:303–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu C, Liang H, Wang B, Liang F, Liu E and
Xiang N: The association between reproductive history and the
multidimensional health of older adults in rural China and its
gender differences: Evidence from the Chinese longitudinal healthy
longevity survey. Front Public Health. 10:9526712022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shabanian S, Khazaie M, Ferns GA and
Arjmand MH: Local renin-angiotensin system molecular mechanisms in
intrauterine adhesions formation following gynecological
operations, new strategy for novel treatment. J Obstet Gynaecol.
42:1613–1618. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chi P and Wang XJ: Significance of the
intact of the fascia propria in protection of pelvic plexus during
total mesorectal excision. Zhonghua Wei Chang Wai Ke Za Zhi.
24:297–300. 2021.(In Chinese). PubMed/NCBI
|
|
30
|
Xu J, Tang B, Li T, Jia B, Yao H, Zhao R,
Yuan W, Zhong M, Chi P, Zhou Y, et al: Robotic colorectal cancer
surgery in China: A nationwide retrospective observational study.
Surg Endosc. 35:6591–6603. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He S, Zhang J, Wang R, Li L, Shi L, Ren D,
Wang J, Deng Y and Dou R: Impact of long-course neoadjuvant
radiation on postoperative low anterior resection syndrome and
stoma status in rectal cancer: Long-term functional follow-up of a
randomized clinical trial. BJS Open. 6:zrac1272022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, An L, Yu R, Peng J, Yu K, Huang
M, Li L and Wang X: The impact of neoadjuvant chemotherapy on low
anterior resection syndrome after rectal cancer resection: A 6
Months longitudinal follow-up. Asian J Surg. 44:1260–1265. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YH, Qiu XY, Lin GL, Zhou JL, Xiao Y and
Qiu HZ: Prognosis and complications of local excision for rectal
cancer after neoadjuvant chemoradiotherapy. Zhonghua Wei Chang Wai
Ke Za Zhi. 24:344–351. 2021.(In Chinese). PubMed/NCBI
|
|
34
|
Shen D, Luo J, Chen L, Ma W, Mao X, Zhang
Y, Zheng J, Wang Y, Wan J, Wang S, et al: PARPi treatment enhances
radiotherapy-induced ferroptosis and antitumor immune responses via
the cGAS signaling pathway in colorectal cancer. Cancer Lett.
550:2159192022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L and Ma T: Cross suture closure
technique of the perineal wound following abdominoperineal
resection. Zhonghua Wei Chang Wai Ke Za Zhi. 21:936–939.
2018.PubMed/NCBI
|
|
36
|
Cianci R, Cristel G, Agostini A, Ambrosini
R, Calistri L, Petralia G and Colagrande S: MRI for rectal cancer
primary staging and restaging after neoadjuvant chemoradiation
therapy: How to do it during daily clinical practice. Eur J Radiol.
131:1092382020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knol J and Keller DS: Total mesorectal
excision technique-past, present, and future. Clin Colon Rectal
Surg. 33:134–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pandit N, Deo KB, Gautam S, Yadav TN,
Kafle A, Singh SK and Awale L: Extended total mesorectal excision
(e-TME) for locally advanced rectal cancer. J Gastrointest Cancer.
53:253–258. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pieniowski EHA, Nordenvall C, Palmer G,
Johar A, Tumlin Ekelund S, Lagergren P and Abraham-Nordling M:
Prevalence of low anterior resection syndrome and impact on quality
of life after rectal cancer surgery: Population-based study. BJS
Open. 4:935–942. 2020. View Article : Google Scholar : PubMed/NCBI
|