Introduction
Laryngeal cancer, characterized by an increasing
annual incidence, is the second most common head and neck type of
cancer, accounting for ~20% of all head and neck cancer cases
(1,2). According to the 2018 Global Cancer
Statistics report, the incidence of laryngeal cancer was 2/100,000
individuals, with a mortality rate of 1/100,000 (3).
Laryngeal cancers are predominantly (95%) squamous
cell carcinomas (SCC), followed by neuroendocrine neoplasms of the
larynx, which are the most common non-squamous tumors of the
larynx, despite their rarity (4,5).
Comprehensive treatment approaches, such as surgery,
radiotherapy, chemotherapy and concurrent chemotherapy and
radiotherapy have provided a higher 5-year survival rate (50–80%)
in patients with laryngeal cancer (6). However, despite the current
therapeutic advances, the survival rate of patients with laryngeal
cancer remains poor due to the advanced stage of diagnosis, the
high tumor recurrence rate, and distant metastases (7).
Early diagnosis serves a crucial role in the early
detection of relapses and in reducing mortality via enhancing the
effectiveness of the currently available therapeutic approaches.
Therefore, identifying effective diagnostic and prognostic
biomarkers for laryngeal cancer is critical to guide disease
management and improve treatment outcomes.
The members of the chromogranin family, and more
particular chromogranin A (CGA) and its proteolytically derived
peptides, are widely used to identify particular types of tumors
with a neuroendocrine-like phenotype (8) and allow the assessment of the
malignancy grade and metastatic potential of tumors. Bartolomucci
et al (9) demonstrated that
CGA is expressed in several types of endocrine and neuroendocrine
tumors, such as prostate cancer (10), gastrointestinal neuroendocrine
tumors (11) and neuroendocrine
carcinomas of the head and neck, including laryngeal cancer
(11–13).
In addition to CGA, other secretogranins have been
also identified as potential endocrine tumor markers (14), including the VGF (non-acronymic)
polypeptide, identified from the ‘V’ clone of the PC12 cDNA library
(15).
In humans, VGF encodes a precursor protein
(pro-VGF), which produces several peptides involved in food intake,
energy balance and metabolism, water and electrolyte homeostasis,
reproduction, pain, learning and memory (16). In addition, it has been reported
that pro-VGF can promote neuronal growth and prevent apoptosis
(17,18), while it serves a significant role in
the pathogenesis of several types of neuroendocrine tumors
(19–21).
In addition to neuroendocrine tumors, previous
studies suggest that VGF could exhibit anticancer effects in
non-endocrine tumors, such as breast (22), testicular (23) and ovarian cancer (24), thus indicating that VGF could be a
potential biomarker in the above types of cancer. Therefore,
dysregulation of VGF expression and processing could be dependent
on tumor type.
As studies regarding the expression and role of VGF
in laryngeal cancer are lacking, the present study aimed to
investigate the expression profile of VGF in LSCC tumor tissues. In
addition, since a previous study indicated that CGA levels in the
blood of patients with endocrine tumors could act as a potential
biomarker (25), the measurement of
VGF in blood could provide an additional tool for the diagnosis and
monitoring of laryngeal tumors.
Materials and methods
Patients
A total of 15 patients with LSCC were included in
the present study. The protocol conformed to the Declaration of
Helsinki and its later amendments and was approved by the internal
Institutional Review Board (Ethical Committee of Sapienza
University and Policlinico Umberto I, Rome, Italy; approval number:
6129).
The sites of the tumors and staging and grading were
established according to the American Joint Committee on Cancer
(26). Archival formalin-fixed
paraffin-embedded (FFPE) tumor tissue from a laryngeal
neuroendocrine carcinoma was used as positive control in the
immunofluorescence experiments. Serum from five age-matched healthy
subjects was used to quantitate VGF by western blotting for
comparison with that of five of the patients with LSCC included in
the present study. A clinical synopsis of the patients with LSCC
included in the present study is in Table I.
 | Table I.Clinical synopsis of the patients
with laryngeal squamous cell carcinoma included in the present
study. |
Table I.
Clinical synopsis of the patients
with laryngeal squamous cell carcinoma included in the present
study.
| Patient number | Sex | Age | Tumor location | pTNM stage | American Joint
Committee on Cancer stage | Grade |
|---|
| 1 | Male | 79 | Glottis | pT4aN0M0 | IVa | G2 |
| 2 | Male | 78 | Supraglottis | pT3N3bM0 | IVb | G2 |
| 3 | Male | 77 | Supraglottis | pT4aN0M0 | IVa | G2 |
| 4 | Male | 61 | Supraglottis | pT4aN1M0 | IVa | G3 |
| 5 | Female | 74 | Glottis | pT4aN0M0 | IVa | G2 |
| 6 | Male | 63 | Glottis | pT4aN2aM0 | IVa | G2 |
| 7 | Male | 71 | Glottis | pT3N0M0 | III | G2 |
| 8 | Male | 56 | Supraglottis | pT4aN1M0 | IVa | G2 |
| 9 | Male | 58 | Glottis | pT3N3bM0 | IVb | G2 |
| 10 | Female | 75 | Supraglottis | pT3N0M0 | III | G2 |
| 11 | Male | 73 | Supraglottis | pT3N3bM0 | IVb | G2 |
| 12 | Female | 77 | Glottis | pT3N0M0 | III | G2 |
| 13 | Male | 63 | Glottis | pT4aN0M0 | IVa | G2 |
| 14 | Male | 78 | Supraglottis | pT3N3bM0 | IVb | G2 |
| 15 | Male | 78 | Glottis | pT3N0M0 | III | G2 |
Reagents
The following antibodies were used: Anti-VGF mouse
monoclonal antibody (cat. no. sc-515482; Santa Cruz Biotechnology,
Inc.); dilution for immunofluorescence, 1:100; dilution for western
blotting (WB), 1:500; anti-GAPDH mouse monoclonal antibody (cat.
no. sc-47724; Santa Cruz Biotechnology, Inc.; dilution for WB,
1:500); anti-Vimentin rabbit monoclonal antibody (cat. no. ab92547;
Abcam; dilution for immunofluorescence, 1:500); anti-CD3 monoclonal
antibody (cat. no. ab699; Abcam; dilution for immunofluorescence,
1:100). The secondary antibodies conjugated to horseradish
peroxidase were purchased from Jackson ImmunoResearch Laboratories
(cat. no. 111-035-003; cat. no. 115-035-003) and used at a dilution
of 1:5,000. The Alexa Fluor488- and Alexa Fluor594-conjugated
secondary antibodies were purchased from Thermo Fisher Scientific
Inc. (cat. nos. A-11029 and A-11012) and were used at a dilution of
1:250. TRIzol® was purchased from Thermo Fisher
Scientific Inc. Complete protease and phosphatase inhibitor
cocktail (cOmplete, EDTA-free Protease and PhosSTOP tablets) were
from Roche Diagnostics and the Chemiluminescence ECL kit was from
Cytiva. Ponceau S Staining Solution and ProLong with DAPI were from
Thermo Fisher Scientific Inc.
RNA extraction, retro-transcription
and reverse transcription-quantitative (RT-q) PCR
The total RNA from frozen tumor and adjacent
non-tumor tissue samples were extracted using TRIzol®
reagent according to the manufacturer's instructions and was then
reverse transcribed using a High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific). qPCR was performed
using an iCycler Detection System (Bio-Rad Laboratories, Inc.). The
cDNAs were amplified using iQ SYBR Green Supermix (Bioline;
Meridian Bioscience) and specific sense and antisense human primers
for the interest gene: VGF (Eurofins Genomics). Each reaction was
performed in triplicate under the same thermal cycling conditions
as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 30
sec, 60°C and 72°C for 30 sec, to obtain the cycle time (Ct) mean.
A reaction mixture without cDNA was used as control and
post-amplification dissociation curves were performed to verify the
presence of a single amplification product and the absence of
genomic DNA contamination. The Ct mean value of the target gene was
normalized to the Ct mean value of the house-keeping gene, 18S
rRNA, and the comparative method (2-∆∆Cq) (27) was obtained for each patient using
gene expression value of normal tissues as calibrator. Data were
reported as fold increase of the target gene mRNA compared to the
normal tissues. The human primer sequences used in the present
study were: VGF: F: 5′-AGCATAAAGAGCCGGTAGCC-3′, R:
5′-GGAAAAGCTCTCCCTCGTCC-3′; 18SrRNA F: 5′-ACCGGGTTGGTTTTGATCTG-3′,
R: 5′-ATCCTGCCAGTAGCATATGC-3′.
Protein extraction
Protein extraction was carried out as previously
described (28). Briefly, frozen
tumor and adjacent non-tumor tissue samples were processed in lysis
buffer (1% SDS; 1% NP-40, 5% glycerol and 5 mM EDTA) supplemented
with complete protease and phosphatase inhibitor cocktail using a
homogenizer (7 mm, OMNI International). Samples were boiled for 10
min and centrifuged for 20 min at 12,000 × g at 4°C. Supernatants
were collected and protein concentration was measured by a Qubit
fluorometer (Invitrogen; Thermo Fisher Scientific, Inc.), according
to the manufacturer's instructions. Protein extracts were stored at
−80°C until use.
WB analysis
WB analysis was carried out as previously described
(28). Briefly, protein extracts
(30 µg/lane) were electrophoresed through 10% SDS-PAGE and
transferred onto nitrocellulose membranes (Cytiva). The membrane
was stained with Ponceau Solution for 5 min at room temperature,
and then washed. After blocking the proteins with 4% non-fat died
milk (PanReac; AppliChem) for 2 h at room temperature, the primary
antibodies incubation was performed overnight at 4°C. Membranes
were then washed with PBS three times for 10 min and incubated with
the secondary HRP-conjugated antibodies for 40 min at room
temperature. After three washes in PBS, immunodetection of the
reactive bands was revealed by chemiluminescence (ECL kit; Cytiva)
and analyzed by iBright 1500 (Thermo Fisher Scientific Inc.).
ImageJ v1.53a (National Institutes of Health) was used for
densiometric analysis.
Immunofluorescence
Immunofluorescence analysis of FFPE samples was
performed as described previously (28). Briefly, paraffin-embedded sections
were dewaxed by two changes of xylene (5 min each) and hydrated in
graded ethanol solutions (100, 90, and 70%, ethanol, for 2 min
each). Sections were incubated in the antigen retrieval solution
(10 mM sodium citrate, 0.05% Tween 20, pH 6.1) for 3×2 min and 4×30
sec into a microwave oven at 750 W. After cooling to room
temperature slides were rinsed in PBS and blocked with 1% BSA in
PBS for 1 h at room temperature. Samples were incubated at 4°C
overnight using the appropriate primary antibodies; washed three
times in PBS/0.1% Tween 20; and incubated at room temperature with
the appropriate secondary antibodies for 1 h. Slides were mounted
with ProLong with DAPI (Thermo Fisher Scientific, Inc.) and
examined by an epifluorescence microscope (Olympus BX53; Olympus
Corporation) equipped with a SPOT RT3 camera. Images were merged
using the image analysis software IAS 2000 (Delta Sistemi).
Blood collection
Peripheral blood samples of 5 ml were available from
5 of 15 LSCC patients included in the present study, and 5
age-matched healthy subjects. The samples were collected in BD
Vacutainer Serum Separation Tubes (BD Biosciences) and centrifuged
at 1,000 × g for 15 min at 4°C to separate serum from plasma. Serum
was then stored at −80°C, until use.
Statistical analysis
All experiments were performed for at least three
independent replicates. Data are presented as mean ± standard
deviation Statistical analysis was performed using GraphPad Prism
9.4.1 software (GraphPad Software; Dotmatics). Data were analyzed
using both the unpaired and paired t-test. P<0.01 was considered
to indicate a statistically significant difference.
Results
Patients
A total of 15 patients with LSCC were enrolled in
the present study. Among them, 11 patients provided tissue samples
for WB and qPCR analysis, four patients for immunofluorescence
staining and five patients for serum analysis. The clinical
characteristics of patients with LSCC are listed in Table I.
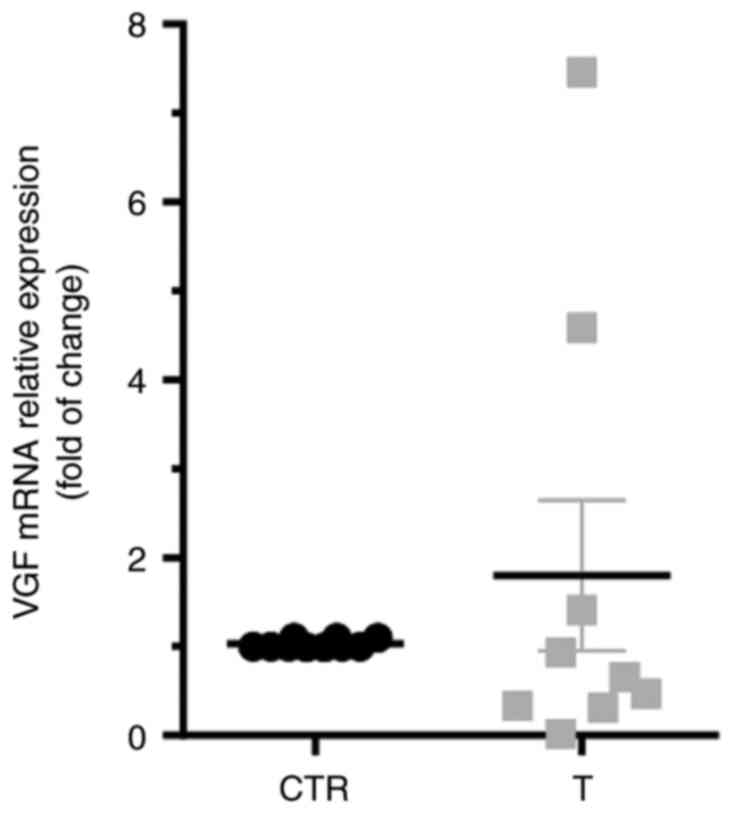
Expression of VGF in LSCC tissues
To evaluate the expression profile of VGF in LSCC
tissues, its mRNA and protein expression levels were detected by
qPCR and WB, respectively (n=11 subjects for each assay). For each
patient, the expression levels of VGF in LSCC tissues (T) were
compared with those in normal adjacent tissues (CTR). As shown in
Fig. 1, no significant differences
were observed in the amount of VGF mRNA in tumor samples compared
with CTR. In Fig. 2A,
representative immunoblots obtained from 11 patients with LSCC are
shown. WB revealed the presence of an immunoreactive band with a
molecular weight of ~70 kDa, corresponding to human pro-VGF
(19). Pro-VGF was mainly detected
in CTR. WB was carried out using samples derived from 11 patients
with LSCC and the expression levels of VGF were normalized to those
of GAPDH. The results showed that pro-VGF was downregulated in
tumor samples compared with CTR (Fig.
2B; P<0.01). In the current study, the expression levels of
other pro-VGF-related peptides were not detected.
Localization of VGF in laryngeal tumor
tissues
Immunofluorescence staining of tissues from
primitive LSCC and LSCC with lymph node metastases was performed to
evaluate the localization of VGF in SCC. Sections from FFPE
laryngeal neuroendocrine carcinoma tissue samples served as a
positive control. The neoplastic cells, as expected, were negative
for vimentin. The cells immunoreactive for vimentin are ‘stromal
cells’ (i.e., they are distributed among the nests) (29). As expected, a strong VGF
immunoreactivity was observed in laryngeal neuroendocrine carcinoma
tissues (Fig. 3A). By contrast, no
immunoreactivity was obtained in LSCC tissues (Fig. 3B) and LSCC tissues with lymph node
metastasis (Fig. 3C). However, a
moderate immunoreactivity was detected around and within the SCC
nests, co-localizing with vimentin, possibly representing
tumor-(Fig. 3B) and nodal-related
(Fig. 3C) T-lymphocytes (30,31).
SCC tissues were also subjected to dual immunostaining for CD3
(T-lymphocytes marker) and vimentin (Fig. 3D). As expected, a CD3 immunostaining
(green) was observed around and within the tumoral nests,
co-localizing with vimentin (red; Fig.
3D), confirming the presence of T-lymphocytes. No
immunoreactivity for VGF was observed in the epithelial lining of
the larynx in sections from both LSCC and positive control tissues.
However, unavailability of images of adjacent non-tumor tissue
staining represents a limitation of the present study.
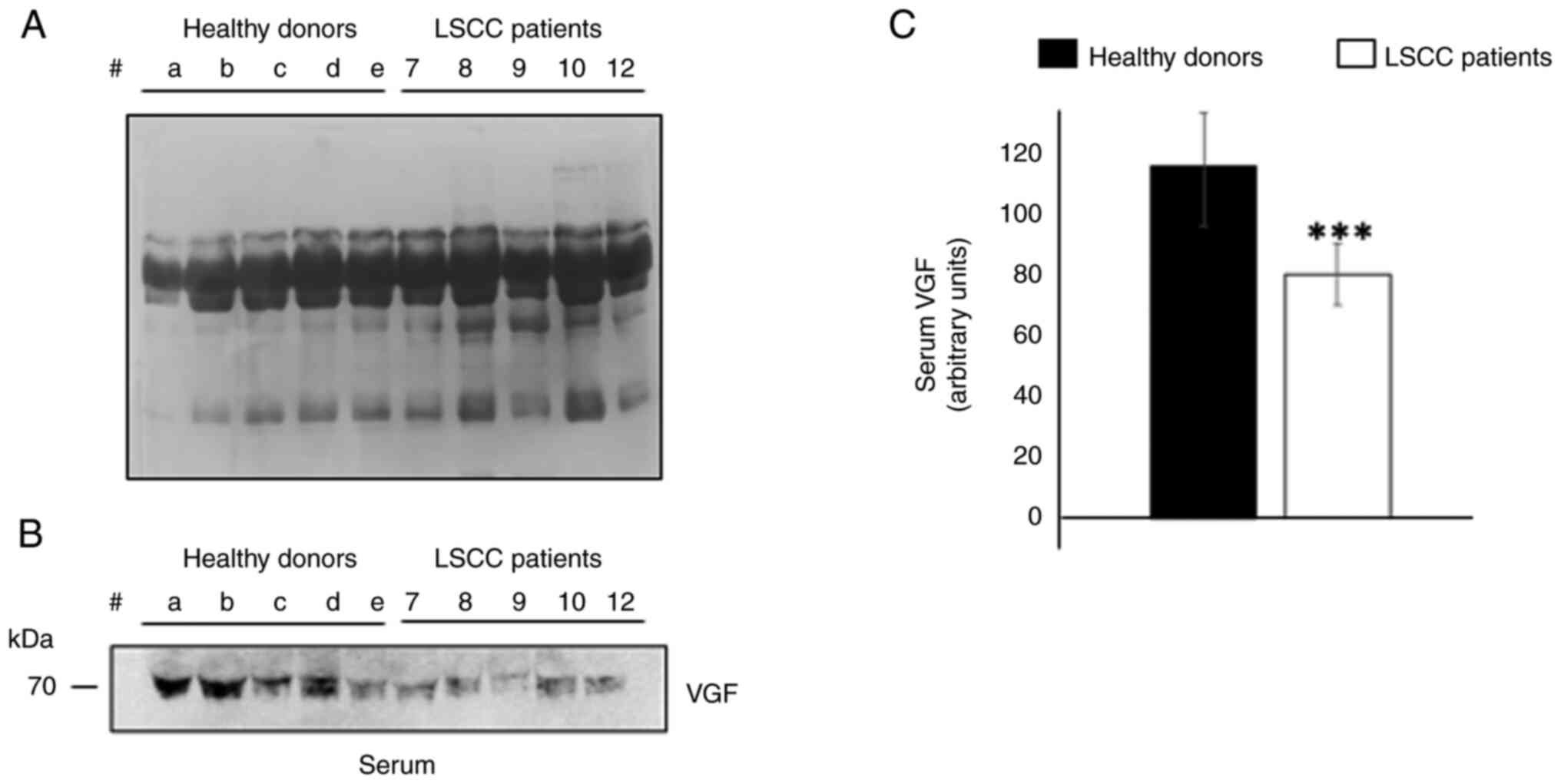
VGF levels in the serum of patients
with LSCC
Since the protein expression levels of pro-VGF were
decreased in tissues derived from patients with LSCC compared with
CTR, the VGF content in the serum derived from a subgroup of five
patients with LSCC were detected by WB. The serum levels of VGF in
patients with LSCC were compared with those in age-matched healthy
subjects. As shown in Fig. 4B, the
levels of VGF-related peptide (~70 kDa) were significantly reduced
in the serum from patients with LSCC compared with those in the
serum of healthy donors (Fig. 4C;
P<0.01).
Discussion
The present pilot study aimed to investigate the
expression and the putative role, if any, of VGF in LSCC.
Therefore, the expression profile of VGF-derived peptides in tumor
tissues and serum of patients with LSCC was determined.
As expected, the results indicated that, at least in
the larynx, SCC cells did not express VGF. This finding was
consistent with that obtained in a previous study showing that only
a very small fraction of head and neck SCCs could express
neuroendocrine markers (12).
VGF is an active neuroendocrine regulatory
polypeptide, mainly expressed in the human hypothalamus, in the
medial and lateral frontal gyrus and in several neuroendocrine
tissues, including the pituitary gland and various gastrointestinal
and pancreatic neuroendocrine cells (19). In addition to nerve growth factor,
several stimuli can induce VGF expression, such as cell
depolarization, growth factors, IL-6, insulin and cyclic adenosine
monophosphate (19).
The VGF gene encodes a precursor protein, namely
pro-VGF, with a molecular weight of ~70 kDa, which in humans
consists of 615 amino acids (19).
Pro-VGF is then processed by pro-protein convertases (PC1/3 or
PC2), resulting in a series of VGF-related peptide fragments, which
are stored in dense core granules and secreted via regulated
pathways (32). It has been
reported that several low molecular weight VGF-encoded peptides,
covering ~20% of the pro-VGF sequence, including TLQP-21, TLQP-62
and AQEE-30, with total lengths of 21, 62 and 30 amino acids,
respectively, exhibit several biological functions (15,33,34).
Processing at different sites or under diverse conditions can
result in different acting end products. However, the significance
of VGF remains currently poorly understood (19).
Rindi et al (35) demonstrated that the expression of
VGF-related peptides, such as that of pro-VGF, in human
neuroendocrine cells could promote endocrine hyperplasia and
neoplasia, depending on the cell type-specific processing of
pro-VGF. This finding was further supported by the finding that
88/102 endocrine tumors tested were positive for the expression of
VGF peptides, thus indicating that VGF could mark an
active/proliferative state in response to specific stimuli
(35). The increased expression and
release of VGF-related fragment peptides have been also verified in
large-cell neuroendocrine carcinoma of the lungs (21,36)
and in breast cancer with neuroendocrine features (20). The present study also demonstrated
that, in addition to CGA, VGF was also upregulated in
neuroendocrine carcinomas of the larynx, thus suggesting that VGF
could be considered as a potential novel biomarker for
neuroendocrine tumors.
However, emerging evidence has also suggested that
VGF exhibits different roles, as it possesses a protective effect
on non-endocrine tumors, such as breast (22), testicular (23) and ovarian cancer (24). Therefore, it was hypothesized that
the abnormal expression and processing of VGF could depend on tumor
type.
RT-qPCR analysis revealed that the mRNA expression
levels of VGF were comparable in LSCC tissues compared with the
adjacent non-tumor tissues. By contrast, WB showed that the protein
expression levels of pro-VGF were significantly reduced in LSCC
tissues compared with CTR tissues. No other lower molecular weight
bands were present in the membrane, at least not under the
experimental conditions of the present study.
In addition, immunofluorescence assays verified the
weak VGF immunoreactivity in primary LSCC and LSCC with lymph node
metastasis. Notably, VGF immunoreactivity was observed in
vimentin-positive cells within the stromal tissue in tumor samples,
possibly corresponding to T-lymphocytes (30,31).
Pro-VGF levels were also notably reduced in the serum of patients
with LSCC compared with healthy donors, thus indicating that VGF
could be downregulated both locally and systemically through
post-transcriptional mechanisms.
The present results, obtained in the serum of a
limited number of LSCC patients using WB, should be confirmed by
quantifying the level of VGF in a larger number of patients,
possibly using a more selective analysis, such as an ELISA
test.
The current study also aimed to uncover the meaning
of VGF downregulation in tissues and serum derived from patients
with LSCC, as the significance of VGF precursor in the diagnosis or
prognosis of patients is worth further exploration. Indeed,
additional in vivo and in vitro experiments could
confirm these preliminary findings and support evidence of abnormal
expression and processing of VGF in this type of tumor. By using
cellular models of human laryngeal carcinoma, it is hoped to
provide further evidence of the VGF implication in cancer-relevant
behaviors.
Given the role of VGF in regulating energy
homeostasis and metabolism, it was hypothesized that VGF depletion
in various types of tumor, such as LSCC, could promote the
proliferation and spread of neoplastic cells. Indeed, previous
studies show that VGF knockout mice are hyperactive and
hypermetabolic (37).
Hypermetabolism is a well-known feature of cancer, which allows
tumor cells to undergo uncontrolled cell division and proliferation
(38).
Growing evidence has also supported the significance
of enhanced metabolism and thus energy production in the tumor
microenvironment. The above effect negatively affects the
availability of nutrients to immune cells, and more particular in
tumor-invasive T cells, which also require a high metabolic energy
status to function efficiently. Therefore, immune cells should
compete with cancer cells for the available energy resources
(39). However, whether VGF
downregulation in SCC also serves a significant role in other sites
either within or outside the head and neck region should be further
investigated.
Acknowledgements
Not applicable.
Funding
The present study was supported by Medio Progetto di Ateneo 2019
(grant no. RM11916B7E5A0D4) to Massimo Ralli.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MRa contributed to conception and design,
responsible for supervision, funding acquisition and writing the
original draft of the manuscript. CS was responsible for
supervision and writing the original draft of the manuscript and
performed the formal analysis. MV was responsible for supervision
and performign formal analysis. AnC, AlC, MC, RL, RP and AG were
responsible for investigation, writing, reviewing and editing; ER,
MRi and EP were responsible for formal analysis and data curation.
FG and DM were responsible for formal analysis, writing, reviewing
and editing. MRa and CS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and approved by the Institutional
Review Board (or Ethics Committee) of Sapienza University and
Policlinico Umberto I, Rome, Italy; approval number: 6129. Informed
consent was obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNC
|
head and neck cancer
|
|
SCC
|
squamous cell carcinoma
|
|
LSCC
|
laryngeal squamous cell carcinoma
|
|
CGA
|
chromogranin A
|
|
NGF
|
nerve growth factor
|
|
proVGF
|
VGF precursor
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
qPCR
|
quantitative real-time PCR
|
|
WB
|
western blotting
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
CD3
|
cluster of differentiation 3
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Prim. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlito A, Silver CE, Bradford CR and
Rinaldo A: Neuroendocrine neoplasms of the larynx: An overview.
Head Neck. 31:1634–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunt JL, Barnes L, Triantafyllou A, Gnepp
DR, Devaney KO, Stenman G, Halmos GB, Bishop JA, Skálová A, Willems
SM, et al: Well-differentiated neuroendocrine carcinoma of the
larynx: Confusion of terminology and uncertainty of early studies.
Adv Anat Pathol. 26:246–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Machiels JP, René Leemans C, Golusinski W,
Grau C, Licitra L and Gregoire V; EHNS Executive Board, ESMO
Guidelines Committee and ESTRO Executive Board, : Reprint of
‘Squamous cell carcinoma of the oral cavity, larynx, oropharynx and
hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for
diagnosis, treatment and follow-up’. Oral Oncol. 113:1050422021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forastiere AA, Ismaila N, Lewin JS, Nathan
CA, Adelstein DJ, Eisbruch A, Fass G, Fisher SG, Laurie SA, Le QT,
et al: Use of larynx-preservation strategies in the treatment of
laryngeal cancer: American society of clinical oncology clinical
practice guideline update. J Clin Oncol. 36:1143–1169. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Portela-Gomes GM, Grimelius L, Wilander E
and Stridsberg M: Granins and granin-related peptides in
neuroendocrine tumours. Regul Pept. 165:12–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartolomucci A, Possenti R, Mahata SK,
Fischer-Colbrie R, Loh YP and Salton SRJ: The extended granin
family: Structure, function, and biomedical implications. Endocr
Rev. 32:755–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komiya A, Suzuki H, Imamoto T, Kamiya N,
Nihei N, Naya Y, Ichikawa T and Fuse H: Neuroendocrine
differentiation in the progression of prostate cancer. Int J Urol.
16:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massironi S, Conte D, Sciola V, Spampatti
MP, Ciafardini C, Valenti L, Rossi RE and Peracchi M: Plasma
chromogranin A response to octreotide test: Prognostic value for
clinical outcome in endocrine digestive tumors. Am J Gastroenterol.
105:2072–2078. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kusafuka K, Abe M, Iida Y, Onitsuka T,
Fuke T, Asano R, Kamijo T and Nakajima T: Mucosal large cell
neuroendocrine carcinoma of the head and neck regions in Japanese
patients: A distinct clinicopathological entity. J Clin Pathol.
65:704–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lewis JS Jr, Chernock RD and Bishop JA:
Squamous and neuroendocrine specific immunohistochemical markers in
head and neck squamous cell carcinoma: A tissue microarray study.
Head Neck Pathol. 12:62–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang KR, Jia YJ, Zhou SH, Wang QY, Bao YY,
Feng ZY, Yao HT and Fan J: Cutaneous and subcutaneous metastases
from atypical laryngeal carcinoids: Case report and review of the
literature. Medicine (Baltimore). 95:e27962016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salton SR, Ferri GL, Hahm S, Snyder SE,
Wilson AJ, Possenti R and Levi A: VGF: A novel role for this
neuronal and neuroendocrine polypeptide in the regulation of energy
balance. Front Neuroendocrinol. 21:199–219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartolomucci A, La Corte G, Possenti R,
Locatelli V, Rigamonti AE, Torsello A, Bresciani E, Bulgarelli I,
Rizzi R, Pavone F, et al: TLQP-21, a VGF-derived peptide, increases
energy expenditure and prevents the early phase of diet-induced
obesity. Proc Natl Acad Sci USA. 103:14584–14589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Severini C, Ciotti MT, Biondini L,
Quaresima S, Rinaldi AM, Levi A, Frank C and Possenti R: TLQP-21, a
neuroendocrine VGF-derived peptide, prevents cerebellar granule
cells death induced by serum and potassium deprivation. J
Neurochem. 104:534–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimazawa M, Tanaka H, Ito Y, Morimoto N,
Tsuruma K, Kadokura M, Tamura S, Inoue T, Yamada M, Takahashi H, et
al: An inducer of VGF protects cells against ER stress-induced cell
death and prolongs survival in the mutant SOD1 animal models of
familial ALS. PLoS One. 5:e153072010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Qin X, Han Y and Li B: VGF: A
prospective biomarker and therapeutic target for neuroendocrine and
nervous system disorders. Biomed Pharmacother. 151:1130992022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Annaratone L, Medico E, Rangel N,
Castellano I, Marchiò C, Sapino A and Bussolati G: Search for
neuro-endocrine markers (chromogranin A, synaptophysin and VGF) in
breast cancers. An integrated approach using immunohistochemistry
and gene expression profiling. Endocr Pathol. 25:219–228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumoto T, Kawashima Y, Nagashio R,
Kageyama T, Kodera Y, Jiang SX, Okayasu I, Kameya T and Sato Y: A
new possible lung cancer marker: VGF detection from the conditioned
medium of pulmonary large cell neuroendocrine carcinoma-derived
cells using secretome analysis. Int J Biol Markers. 24:282–285.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ostrow KL, Park HL, Hoque MO, Kim MS, Liu
J, Argani P, Westra W, Van Criekinge W and Sidransky D:
Pharmacologic unmasking of epigenetically silenced genes in breast
cancer. Clin Cancer Res. 15:1184–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brait M, Maldonado L, Begum S, Loyo M,
Wehle D, Tavora FF, Looijenga LHJ, Kowalski J, Zhang Z, Rosenbaum
E, et al: DNA methylation profiles delineate epigenetic
heterogeneity in seminoma and non-seminoma. Br J Cancer.
106:414–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brait M, Maldonado L, Noordhuis M, Begum
S, Loyo M, Poeta ML, Barbosa A, Fazio VM, Angioli R, Rabitti C, et
al: Association of promoter methylation of VGF and PGP9.5 with
ovarian cancer progression. PLoS One. 8:e708782013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nehar D, Lombard-Bohas C, Olivieri S,
Claustrat B, Chayvialle JA, Penes MC, Sassolas G and Borson-Chazot
F: Interest of chromogranin A for diagnosis and follow-up of
endocrine tumours. Clin Endocrinol (Oxf). 60:644–652. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gabanella F, Colizza A, Mottola MC,
Francati S, Blaconà G, Petrella C, Barbato C, Greco A, Ralli M,
Fiore M, et al: The RNA-binding protein SMN as a novel player in
laryngeal squamous cell carcinoma. Int J Mol Sci. 24:17942023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou J, Tao D, Xu Q, Gao Z and Tang D:
Expression of E-cadherin and vimentin in oral squamous cell
carcinoma. Int J Clin Exp Pathol. 8:3150–3154. 2015.PubMed/NCBI
|
|
30
|
Busse S, Steiner J, Micheel J, Dobrowolny
H, Mawrin C, Krause TJ, Adamaszek M, Bogerts B, Bommhardt U, Hartig
R and Busse M: Age-related increase of VGF-expression in T
lymphocytes. Aging (Albany NY). 6:440–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Busse S, Steiner J, Glorius S, Dobrowolny
H, Greiner-Bohl S, Mawrin C, Bommhardt U, Hartig R, Bogerts B and
Busse M: VGF expression by T lymphocytes in patients with
Alzheimer's disease. Oncotarget. 6:14843–14851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trani E, Giorgi A, Canu N, Amadoro G,
Rinaldi AM, Halban PA, Ferri GL, Possenti R, Schininà ME and Levi
A: Isolation and characterization of VGF peptides in rat brain.
Role of PC1/3 and PC2 in the maturation of VGF precursor. J
Neurochem. 81:565–574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferri GL, Noli B, Brancia C, D'Amato F and
Cocco C: VGF: An inducible gene product, precursor of a diverse
array of neuro-endocrine peptides and tissue-specific disease
biomarkers. J Chem Neuroanat. 42:249–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bartolomucci A, Possenti R, Levi A, Pavone
F and Moles A: The role of the vgf gene and VGF-derived peptides in
nutrition and metabolism. Genes Nutr. 2:169–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rindi G, Licini L, Necchi V, Bottarelli L,
Campanini N, Azzoni C, Favret M, Giordano G, D'Amato F, Brancia C,
et al: Peptide products of the neurotrophin-inducible gene vgf are
produced in human neuroendocrine cells from early development and
increase in hyperplasia and neoplasia. J Clin Endocrinol Metab.
92:2811–2815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang W, Chiu YF, Kuo MH, Lee KL, Lee AC,
Yu CC, Chang JL, Huang WC, Hsiao SH, Lin SE and Chou YT: Expression
of neuroendocrine factor VGF in lung cancer cells confers
resistance to EGFR kinase inhibitors and triggers
epithelial-to-mesenchymal transition. Cancer Res. 77:3013–3026.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest
CA, Kozak CA, Boozer CN, Peng B, McEvoy RC, Good P, et al: Targeted
deletion of the Vgf gene indicates that the encoded secretory
peptide precursor plays a novel role in the regulation of energy
balance. Neuron. 23:537–548. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu A and Curran MA: Tumor hypermetabolism
confers resistance to immunotherapy. Semin Cancer Biol. 65:155–163.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang CH, Qiu J, O'Sullivan D, Buck MD,
Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ,
et al: Metabolic competition in the tumor microenvironment is a
driver of cancer progression. Cell. 162:1229–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|