Introduction
Horseshoe kidney (HSK) is the most common renal
fusion anomaly, with a prevalence of 0.25% among the general
population and is more common in males (1,2). It
frequently consists of kidney fusion in the lower pole across the
midline and occurs during embryogenesis (2). Furthermore, this rare entity can be
detected as an incidentaloma during a diagnostic assessment for
other reasons or otherwise due to urogenital disorders. The
incidence of malignancies in HSKs can be 3–4 times higher than that
in normal kidneys (1–3). Renal cell carcinoma (RCC) is the most
frequent malignancy in patients with HSK (4–6). B
cell lymphoma in a HSK has not yet been reported in the literature,
according to a search performed in both the PubMed (7) and Google Scholar databases (8), using ‘horseshoe kidney’ and ‘B cell
lymphoma’ as key words. Therefore, to the best of our knowledge,
the present report describes the first case of B cell lymphoma in a
HSK to raise awareness among physicians regarding the importance of
a correct clinical evaluation and diagnostic workup to avoid
surgery, which is not easy and without complications (9), in patients with this kidney
anomaly.
Case report
A 69-year-old man was referred to the General
Surgery Division of University of Campania Luigi Vanvitelli
(Naples, Italy) in January 2023 due to a voluminous right kidney
neoplasm, previously detected on abdominal ultrasound. Ultrasound
revealed a voluminous neoplasm measuring 8×6 cm in the right
kidney, which was markedly vascularized on Doppler analysis. A HSK
with inferior pole fusion was also reported. The neoplasm occupied
the renal pelvis and caused calico-pielic dilation. The patient had
no urinary symptoms and was unaware of their kidney anomaly.
Routine laboratory tests were normal, except RBC, HCT, PLT, PCT,
phosphorous, creatine phosphokinase and transferrin that were lower
than the normal range, and MCH, bilirubin total, direct and
indirect, and ferritin that were higher than the normal range;
renal function also showed no signs of impairment (Table I). On clinical examination, a mass
in the right flank was palpated, occupying the entire right
hemi-abdomen. No abnormalities were found in the chest except for
the presence of a single, painless, mobile, enlarged (diameter, 3
cm) lymph node in the right axilla. There were no other enlarged
superficial lymph nodes. As part of the radiological work-up, a
total body CT scan was performed, which revealed a single right
axillary lymphadenopathy (37×28 mm; Fig. 1) and renal inferior pole fusion
consistent with a HSK, with a hypodense right renal neoplasm
(109×108×92 mm; Fig. 2) within its
context. This formation involved a large part of the right
hemi-abdomen resulting in anterior duodenum displacement and
posterior dislocation with compression of the inferior vena cava
(Fig. 3). No other abnormalities
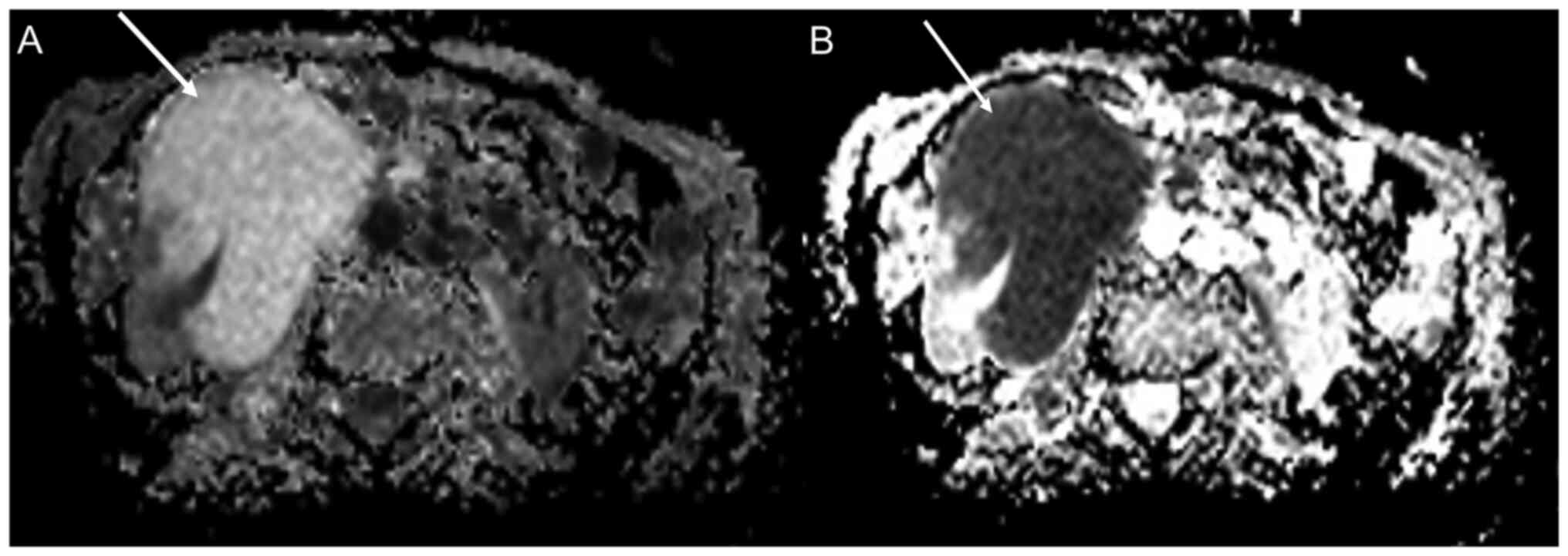
were found. Abdomen MRI confirmed the large renal formation
(Fig. 4) incorporating the pielic
sinus and the mesorenal parenchyma, indissociable from the duodenal
fold, despite no evident signs of invasion. No abdominopelvic
lymphadenopathies were described. Absence of a well-defined
capsule, preservation of the ‘reniform’ appearance and indistinct
margins, as well as high diffusion restriction were reported on
MRI. A transduodenal endoscopic ultrasound was performed to
evaluate infiltration of the duodenal wall by the neoplasm. This
indicated only duodenal compression and dislocation but no
infiltration (Fig. 5).
 | Table I.Laboratory analysis. |
Table I.
Laboratory analysis.
| Parameter | Patient value | Normal value |
|---|
| WBC,
×103/µl | 7.60 | 4.50–11.00 |
| Neutrophils,
×103/µl | 4.72 | 2.20–7.50 |
| Lymphocytes,
×103/µl | 2.23 | 1.00–5.00 |
| Monocytes,
×103/µl | 0.70 | 0.16–1.00 |
| Eosinophils,
×103/µl | 0.12 | 0.0–0.60 |
| Basophils,
×103/µl | 0.04 | 0.0–0.20 |
| RBC,
×106/µl | 4.39 | 4.50–6.00 |
| HGB, g/dl | 13.92 | 13.00–17.50 |
| HCT, % | 39.84 | 42-51 |
| MCV, fL | 90.70 | 82-96 |
| MCH, pg | 31.70 | 27-31 |
| MCHC, g/dl | 34.92 | 31-36 |
| PLT,
×103/µl | 121 | 150-450 |
| MPV, fL | 11.80 | 7.40–12.50 |
| PCT, % | 0.14 | 0.19–0.38 |
| Glucose, mg/dl | 70 | 70-100 |
| Urea, mg/dl | 40 | 10-50 |
| Creatinine,
mg/dl | 1.13 | 0.67–1.17 |
| Uric acid, mg/dl | 5.80 | 3.40–7.00 |
| Sodium, mEq/l | 142 | 135-146 |
| Potassium, mEq/l | 3.90 | 3.50–5.30 |
| Chlorine, mEq/l | 106 | 98-111 |
| Albumin, g/dl | 4.50 | 3.50–5.50 |
| Total protein,
g/dl | 6.90 | 6.60–8.70 |
| Cholesterol tot.,
mg/dl | 124 | 60-200 |
| Cholesterol HDL,
mg/dl | 52 | >35 |
| Cholesterol LDL,
mg/dl | 56 | 10-129 |
| Triglycerides,
mg/dl | 80 | 20-175 |
| Cholinesterase,
U/l | 7498 | 5320-12920 |
| GGT, U/l | 15 | 8-61 |
| ALT-GPT, U/l | 15 | 5-41 |
| AST-GOT, U/l | 20 | 0-34 |
| Bilirubin tot.,
mg/dl | 1.49 | <1.2 |
| Bilirubin dir.,
mg/dl | 0.56 | <0.30 |
| Bilirubin indir.,
mg/dl | 0.93 | <0.75 |
| Phosphorus,
mg/dl | 2.50 | 2.70–4.50 |
| Calcium, mg/dl | 9.50 | 8.60–10.20 |
| Amylase, U/l | 72 | 28-100 |
| Lipase, U/l | 46 | 13-78 |
| Alkaline
phosphatase, U/l | 128 | 40-129 |
| Creatine
phosphokinase, U/l | 43 | 60-190 |
| LDH, U/l | 198 | 120-240 |
| Iron, µg/ml | 107 | 59-158 |
| Ferritin,
ng/ml | 430 | 30-400 |
| Transferrin,
mg/dl | 190 | 191-337 |
| PT, sec | 10.50 | 9-13 |
| INR | 0.91 | 0.80–1.20 |
| aPTT, sec | 33.40 | 24-38 |
| Fibrinogen,
mg/dl | 236 | 200-400 |
The patient underwent complete excision of the right
axillary lymphadenopathy and ultrasound-guided percutaneous biopsy
of the right kidney expansive lesion. The procedures were performed
without complications. The samples were formalin fixed and paraffin
embedded, fixation was obtained by leaving the sample in formalin
(concentration 10%) for 48 h at room temperature. 7 µm-thick slides
were cut and strained by hematoxylin and eosin (40 min at room
temperature). A light microscope was used for histological
examination. Histological examination showed renal parenchyma
partially effaced by a lymphoid population (Fig. 6A) with nuclear crushing, mainly
constituted by small lymphocytes (Fig.
6B). Immunohistochemistry was performed on formalin fixed and
paraffin embedded 4µm thick tissue sections (fixative
concentration: 10% formalin; fixative temperature: room
temperature; duration: 48 h). Immunohistochemistry was performed
automatically on BenchMark Ultra platform (Ventana Medical
Systems), according to the manufacturer's instructions. Antigen
retrieval was performed by using Ultra CC1 for 36 min at 95°C.
Endogenous peroxides and proteins blocking was done in the platform
using Inhibitor CM, ready to use dilution, 37°C, 4 min. Tissues
sections were incubated with the monoclonal antibodies (Ventana
Medical Systems) for 32 min at 37°C (dilution: ready to use).
Immunoreactivity was visualized using an OptiView DAB IHC detection
kit (Ventana Medical Systems, cat. no. 760-700), incubation at 37°C
for 8 min, and then counterstained with Hematoxylin II and Bluing
Reagent for 8 min and 4 min respectively, at the room temperature.
We used the following antibodies: CD20 (Ventana - Roche, cat. no.
760-2531), Bcl6 (Ventana-Roche, cat. no. 760-4241), Bcl2
(Ventana-Roche, cat. no. 790-4464), CD3 (Ventana-Roche, cat. no.
790-4341), CD10 (Ventana-Roche, cat. no. 790-4506), Cyclin D1
(Ventana-Roche, cat. no. 790-4508), IgD (Ventana - Roche, cat. no.
760-4444), CD21 (Ventana-Roche, cat. no. 760-4438), CD23
(Ventana-Roche, cat. no. 790-4408), Ki67 (Ventana-Roche, cat. no.
790-4286). Secondary antibody (ready to use, Ventana-Roche, cat.
no. 760-099, conjugate HRP multimer, temperature 36°C, incubation 8
min. A light microscope was used for the interpretation.
Immunohistochemistry indicated positivity for CD20
(Fig. 6C) and Bcl6 (Fig. 6D). A diagnosis of non-Hodgkin's
B-cell lymphoma consistent with grade G1/G2 follicular lymphoma was
made according to World Health Organization10). Definitive
histological assessment of the axillary lymph node also revealed a
follicular arranged lymphoid population (Fig. 7A) constituted by centrocytes and
centroblasts (Fig. 7B), positive
staining for CD20 (Fig. 7C) and
Bcl6 (Fig. 7D) in
immunohistochemistry, and negative staining for Bcl2 (Fig. 7E), CD3, CD10, CD5, Cyclin D1, IgD,
CD21 and CD23 (data not shown). The proliferation index (Ki67) was
15% (Fig. 7F). Therefore, a
conclusive diagnosis of World Health Organization grade G1/G2
follicular lymphoma with follicular pattern was made (10). Fluorescence in situ
hybridization did not detect BCL2 genetic rearrangements in either
biopsy (data not shown). FISH assay was performed on 4-µm-thick
sections cut from each of the formalin-fixed paraffin-embedded
biopsy specimens using the BOND FISH kit (Leica Biosystems,
Newcastle Upon Tyne, UK) on the automated BOND system (Leica
Biosystems) according to the manufacturer's instructions. FISH
automatic protocol includes the following steps: deparaffinization
of sections by Bond dewax solution at 96°C for 1 min; pretreatment
by BOND Epitope Retrieval Solution at 76°C for 25 min and at 37°C
for 12 min, and enzymatic pretreatment by BOND Enzyme Pre-treatment
Kit (enzyme dilution 1:100) at 37°C for 35 min. Denaturation and
hybridization of the probe were performed on the automated BOND
system (Leica Biosystems): 75°C for 5 min for the denaturation
process and 37°C for 17 h for the hybridization of BCL2 probes. The
used probe was the commercially available ready-for-use probe
ZytoLight SPEC BCL2 Dual Color Break Apart Probe (ZytoVision) that
is composed as follows: ZyGreen (excitation 503 nm/emission 528 nm)
labeled polynucleotides (~10 ng/µl), which target sequences mapping
in 18q21.3 (chr18:60,046,152-60,589,273) proximal to the BCL2
breakpoint region and ZyOrange (excitation 547 nm/emission 572 nm)
labeled polynucleotides (~4.5 ng/µl), which target sequences
mapping in 18q21.33-q22.1 (chr18:60,994,528-61,658,503) distal to
the BCL2 breakpoint region. The slides were then washed to reduce
non-specific hybridization of nucleic acid probes by Post
Hybridization Wash containing formamide (<50%) at 48°C for 4
min. The slides were washed with purified water, air-dried, and
dehydrated in ascending grades of alcohol. Lastly, 10 µl of DAPI
was applied on the slides. FISH interpretation was performed with
the automated fluorescence microscope Leica DM5500 B (Leica
Biosystems) using the filter ET-D/O/G for double Spectrum Green
plus Spectrum Orange. FISH for BCL2 locus rearrangements is
considered positive in relation to the presence of a break-apart
pattern with one fusion signal and two separated orange and green
signals in more than 15% of the cells analyzed. In our case, FISH
signals were counted in ≥100 nonoverlapping intact nuclei and no
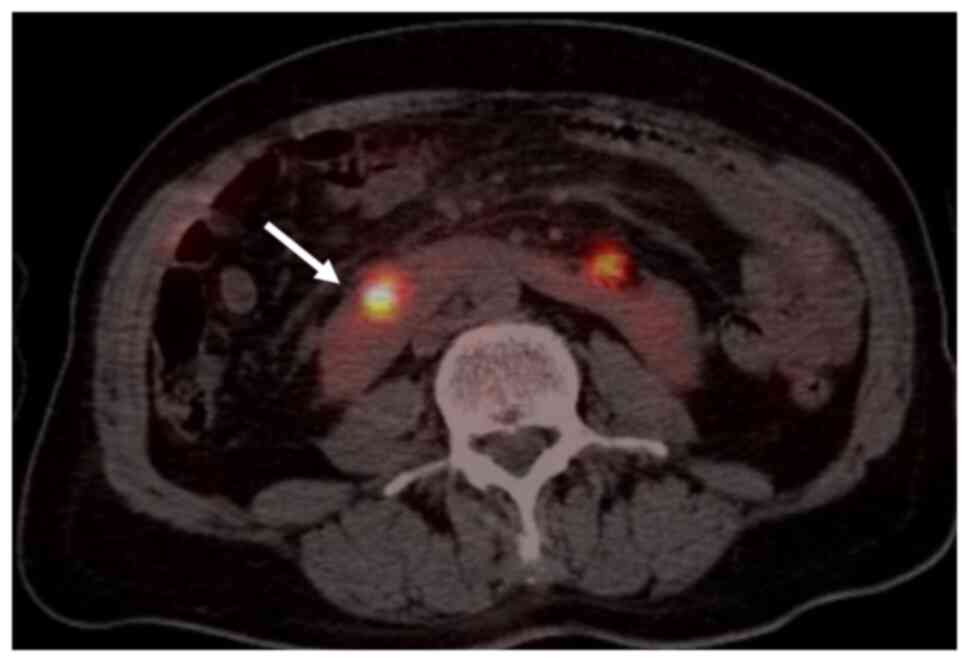
break-apart pattern was observed. Finally, 18-fluorodeoxyglucose
(18-FDG) positron emission tomography (PET)-CT showed an isolated,
large area of tracer accumulation (maximum standardized uptake
value, 20.1) in the right HSK section (Fig. 8). The patient was started on
chemo-immunotherapy, according to a standard protocol (1,000 mg
Gazyvaro administered over day 1, 8, 15 of cycle 1 + 90 mg/mq
bendamustine on day 1, 2 of the same cycle; the planned dose of
1,000 mg Gazyvaro on day 1 + 90 mg/mq bendamustine on day 1,2 had
been administered for additional five cycles every 28 days;
followed by 1,000 mg Gazyvaro maintenance every 2 months for 2
years or until disease progression). At the end of the treatment,
in May 2023, 18-FDG PET-CT was performed, demonstrating a complete
remission of the disease in the right kidney without any other
suspicion of disease (Fig. 9).
Currently, the patient is in good clinical condition, undergoing
maintenance therapy with Gazyvaro and follow-up with 18-FDG PET-CT
every six months for two years, and then once a year for five
years.
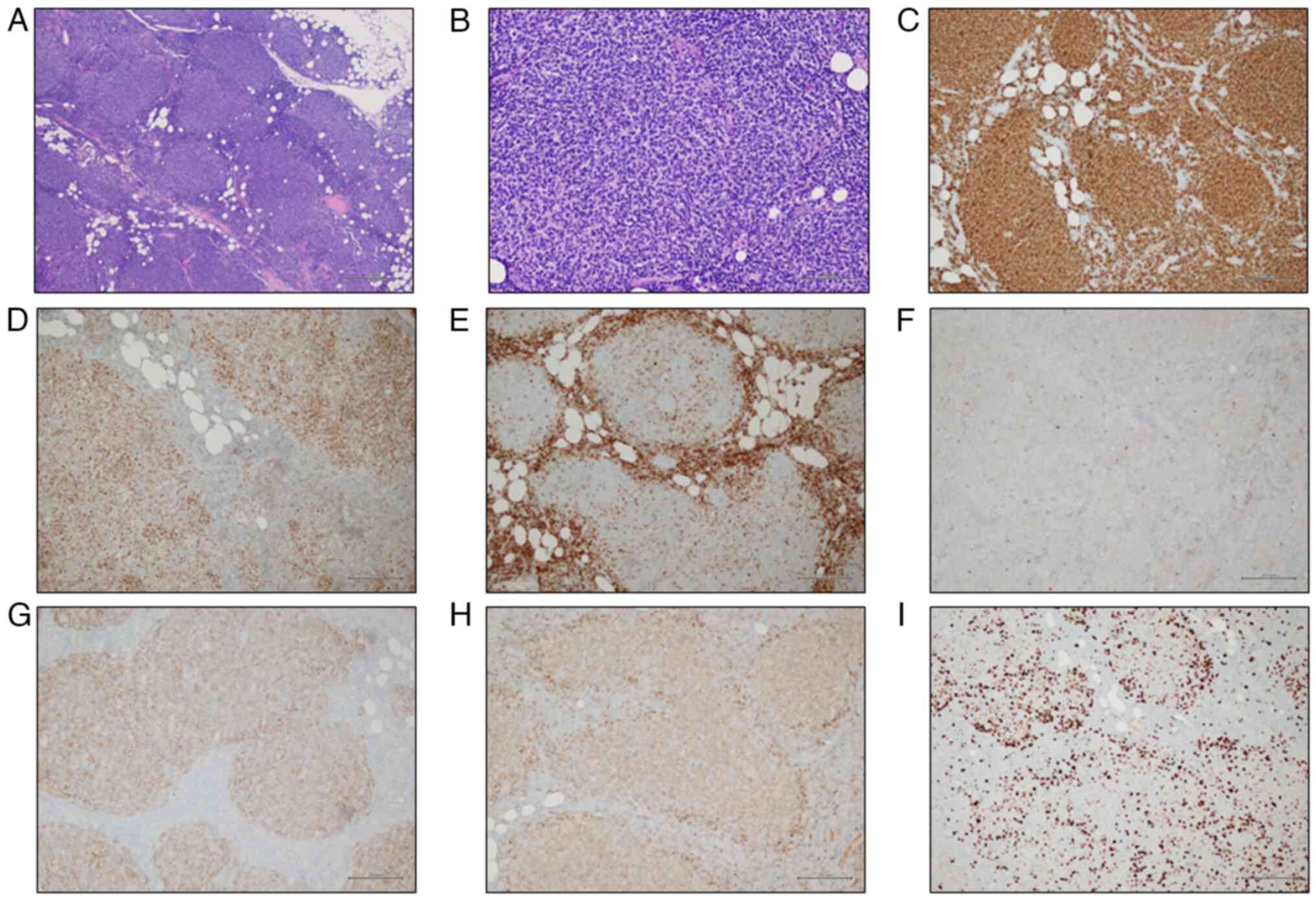 | Figure 7.Histological findings of lymph node
biopsy. (A) The lymph node parenchyma was replaced by a lymphoid
population arranged in nodules (H&E stain; original
magnification, ×40). (B) The lymphoid population was composed of
many centrocytes and few centroblasts (H&E stain; original
magnification, ×200). Immunohistochemically, the population was
positive for (C) CD20 and (D) Bcl6 (Bcl6 immunostain; original
magnification, ×100), and negative for (E) Bcl2 (Bcl2 immunostain;
original magnification, ×100) and (F) CD10 (CD10 immunostain;
original magnification, ×100). The neoplastic follicles were (G)
partially supported by a meshwork of CD21-positive follicular
dendritic cells (CD21 immunostain; original magnification, ×100),
and (H) partially surrounded by a thin IgD-positive mantle cell
layer (IgD immunostain; original magnification, ×100). (I) The
proliferation index (Ki67) was ~15% (magnification, ×100). |
Discussion
Primary renal non-Hodgkin's lymphoma is a rare
disease and only a few cases have been reported in the literature
(6,11–15).
Furthermore, no case reports describing the association between HSK
and renal lymphoma have been documented.
Mendelson et al (16) reported a case of HSK anomaly
mimicking an isolated preaortic lymphadenopathy on ultrasonographic
imaging; in case of diagnostic doubt, an urogram or uro-CT scan
should be considered in order to avoid misdiagnosis and to improve
the resolution of the anatomical structures.
A higher incidence of renal cancer has been reported
in individuals with a HSK than in the general population (4–6). The
main cause is considered to be chronic inflammation resulting from
difficulty urinating due to renal abnormalities, such as
ureteropelvic obstruction, lithiasis and infections (17). The present patient had no clinical
and radiological signs of urinary output impairment or urinary
infection despite the presence of a large tumor. Therefore, we
hypothesized that the pathogenetic mechanism and growth modality of
lymphoma are different from those of renal cancer. When considering
solid renal masses or cystic masses with solid components, the
initial assumption often is that it may be a malignant neoplasm of
the kidney (18). In the past, the
treatment of choice usually involved radical nephrectomy; however,
over the years, there has been a shift towards partial nephrectomy
or a more conservative approach (19). In the present case, due to the
careful use of imaging techniques (mainly CT and MRI),
characteristics of a possible renal lymphoma were recognized in the
mass, including the absence of a well-defined capsule, preservation
of the ‘reniform’ appearance and indistinct margins (20), as well as high diffusion restriction
on MRI. These features, combined with the presence of the right
axillary bulky lymphadenopathy, raised suspicion of renal lymphoma
as the primary hypothesis, and thus, a biopsy was performed,
leading to a definitive diagnosis, while preserving the kidney. We
hypothesized that renal biopsy should always be recommended when
its outcome can impact clinical decision-making, especially because
HSK surgery is not easy and without complications. Consequently,
biopsy is warranted to distinguish between transitional cell
carcinoma and RCC, to discriminate RCC from lymphoma or metastases,
to differentiate malignancy from inflammatory conditions, and to
acquire histological information in patients with metastatic
disease (21).
In conclusion, HSK can be associated with several
renal tumors, such as transitional cell carcinoma, Wilms' tumor,
nephroblastoma, carcinoid tumors, sarcoma and oncocytoma (6). To the best of our knowledge, B cell
non-Hodgkin's lymphoma in a HSK has never been described in the
literature. The present experience suggests that primary renal
lymphoma should also be included among the possible neoplasms of
the HSK. A renal biopsy should be performed before surgery in cases
where atypical findings are obtained from imaging techniques. In
the present case, biopsy was performed, and thus, nephrectomy was
avoided and specific medical therapy was quickly initiated.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FI, FMM, ARo, GB conceived and design the study. FI,
ARe, RR, FSL, LD, MGF, CG, SP, ST participated in the analysis and
interpretation of the data. FI, FMM, CG participated in the
drafting and editing of the manuscript. GB, SP, FSL, ST constructed
figures and tables. FI, FMM, GB, Are, Aro, MGF, SP, CG, FSL, ST, RR
and LD confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schiappacasse G, Aguirre J, Soffia P,
Silva CS and Zilleruelo N: CT findings of the main pathological
conditions associated with horseshoe kidneys. Br J Radiol.
88:201404562015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Humphries A, Speroni S, Eden K, Nolan M,
Gilbert C and McNamara J: Horseshoe kidney: Morphologic features,
embryologic and genetic etiologies, and surgical implications. Clin
Anat. 36:1081–1088. 2023. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang M, Kim YC, Lee H, Kim DK, Oh KH, Joo
KW, Kim YS, Chin HJ and Han SS: Renal outcomes in adult patients
with horseshoe kidney. Nephrol Dial Transplant. 36:498–503. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roussel E, Tasso G, Campi R, Kriegmair MC,
Kara Ö, Klatte T, Capitanio U, Bertolo R, Ingels A, Erdem S, et al:
Surgical management and outcomes of renal tumors arising from
horseshoe kidneys: Results from an international multicenter
collaboration. Eur Urol. 79:133–140. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rivera F, Caparrós G, Vozmediano C,
Bennouna M, Anaya S, Sánchez de la Nieta MD, García Rojo M and
Blanco J: Riñón «en herradura», adenocarcinoma renal y síndrome
nefrótico (‘Horseshoe kidney’, renal adenocarcinoma and nephrotic
syndrome). Nefrologia. 30:596–598. 2010.(In Spanish). PubMed/NCBI
|
|
6
|
Rubio Briones J, Regalado Pareja R,
Sánchez Martín F, Chéchile Toniolo G, Huguet Pérez J and
Villavicencio Mavrich H: Incidence of tumoral pathology in
horseshoe kidneys. Eur Urol. 33:175–179. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
https://pubmed.ncbi.nlm.nih.gov/
|
|
8
|
https://scholar.google.com/
|
|
9
|
Kandel LB, McCullough DL, Harrison LH,
Woodruff RD, Ahl ET Jr and Munitz HA: Primary renal lymphoma: Does
it exist? Cancer. 60:386–391. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turner JJ, Hughes AM, Kricker A, Milliken
S, Grulich A, Kaldor J and Armstrong B: WHO non-Hodgkin's lymphoma
classification by criterion-based report review followed by
targeted pathology review: An effective strategy for epidemiology
studies. Cancer Epidemiol Biomarkers Prev. 14:2213–2219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasunaga Y, Hoshida Y, Hashimoto M, Miki
T, Okuyama A and Aozasa K: Malignant lymphoma of the kidney. J Surg
Oncol. 64:207–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farrow GM, Harrison EG and Utz DC:
Sarcomas and sarcomatoid and mixed malignant tumors of the kidney
in adults-part 11. Cancer. 22:551–555. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Freeman C, Berg JW and Cutler SJ:
Occurrence and prognosis of extranodal lymphomas. Cancer.
29:252–260. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bokhari SRA, Inayat F, Bokhari MR and
Mansoor A: Primary renal lymphoma: A comprehensive review of the
pathophysiology, clinical presentation, imaging features,
management and prognosis. BMJ Case Rep. 13:e2350762020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parsonnet J, Hansen S, Rodriguez L, Gelb
AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH and Friedman GD:
Helicobacter pylori infection and gastric lymphoma. N Engl Med.
330:1267–1271. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mendelson DS, Mitty HA, Janus C and Cohen
BA: Horseshoe kidney mimicking adenopathy. Urol Radiol. 5:121–122.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawada S, Ichikawa T, Koizumi J, Hashimoto
J, Endo J, Hashida K, Yamamuro H, Nomoto T, Sakamoto Y and Imai Y:
Assessment of renal shape of horseshoe kidney with multidetector
row CT in adult patients: Relationship between urolithiasis and
renal isthmus. Tokai J Exp Clin Med. 38:159–166. 2013.PubMed/NCBI
|
|
18
|
Pierorazio PM, Johnson MH, Patel HD, Sozio
SM, Sharma R, Iyoha E, Bass EB and Allaf ME: Management of renal
masses and localized renal cancer: Systematic review and
meta-analysis. J Urol. 196:989–999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morrison JC, Launer BM, Barqawi ZA and Kim
SP: Surgical management of the localized renal mass: Risk and
benefit trade-offs and surgical approach considerations. AME Med J.
6:2020.
|
|
20
|
Nicolau C, Antunes N, Paño B and Sebastia
C: Imaging characterization of renal masses. Medicina (Kaunas).
57:512021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ljungberg B, Albiges L, Abu-Ghanem Y,
Bensalah K, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F,
Hora M, Kuczyk MA, et al: European association of urology
guidelines on renal cell carcinoma: The 2019 update. Eur Urol.
75:799–810. 2019. View Article : Google Scholar : PubMed/NCBI
|