Introduction
Maxillary sinus cancer is relatively rare among head
and neck cancers (1). If the tumor
extends superiorly, it can destroy the orbital floor and lead to
ocular symptoms such as double vision (2). The tumor that extends posteriorly may
destroy the pterygoid process, making it even more difficult to
open the mouth (3). The tumor that
spreads inward can fill the nasal cavity and cause symptoms such as
nasal obstruction (4). If tumor
expansion causes damage to the alveolar process, tumor may be
exposed in the oral cavity (5).
Treatment options for maxillary sinus cancer include surgery,
radiation therapy (RT), and drug therapy (6). However, there is little evidence
regarding the choice of treatment. Although surgery is often
selected as the initial treatment, surgery for advanced cases
significantly impacts the functional aspects and facial appearance
due to its location and significantly reduces the quality of life
(7,8).
Combination treatment with paclitaxel, carboplatin,
and cetuximab (PCE) as a new induction chemotherapy regimen for
advanced head and neck cancer has been recently reported (9). This treatment is characterized by a
high completion rate and low toxicity (10). Post-treatment options include RT
alone, chemoradiotherapy (CRT) with concurrent high-dose cisplatin
(CDDP), bioradiotherapy (BRT), and surgery; these can be selected
on a case-by-case basis, taking into account the patient's general
condition, the size and location of residual tumor, and the
effectiveness of PCE (11).
Although PCE is reported to be highly effective for head and neck
cancers, there are no detailed reports on the efficacy of PCE for
maxillary sinus cancer.
Herein, we report a case of a patient with highly
advanced maxillary sinus cancer with bilateral cervical metastatic
lymph nodes who was successfully treated with PCE followed by BRT.
The tumor was subsequently controlled via minimally invasive
surgery following recurrence.
Case report
A 69-year-old man presented to the Department of
Dentistry and Oral Surgery, Hokuto Hospital (Obihiro, Japan) in
June 2019 with left buccal swelling and an irregular mass on the
left maxillary gingiva that had gradually increased in size over
the past month. He had no extraordinary personal or family medical
history. Extraoral findings included left buccal swelling, trismus,
left nasal obstruction, and enlarged bilateral cervical lymph
nodes. Other findings, such as abnormal ocular position, diplopia,
or lacrimation, were not observed. A mass (diameter, 50 mm) in the
left palate, beyond the midline, and an ulcer on the left buccal
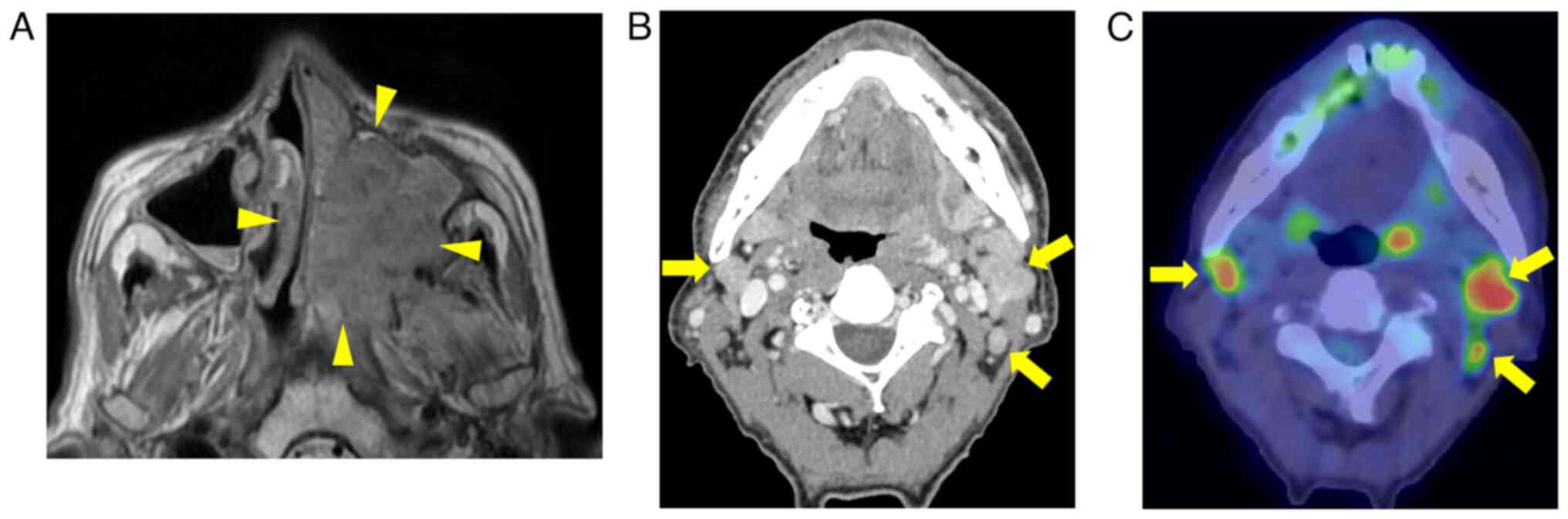
alveolar region were observed within the oral cavity (Fig. 1). Magnetic resonance imaging showed
that the lesion filled the ethmoid and maxillary sinus and
destroyed the pterygoid process (Fig.
2A). Contrast-enhanced computed tomography and
18-fluoro-2-deoxyglucose positron emission tomography (FDG-PET)
revealed multiple cervical lymph node metastases extending
distally, including level V on the left side and level III on the
right side (Fig. 2B and C). There
was no evidence of distant metastasis. Biopsy revealed features of
squamous cell carcinoma (SCC), and the patient was diagnosed with
the left maxillary sinus cancer T4aN2cM0. Although the tumor was
not considered unresectable, PCE treatment was provided as initial
treatment owing to the invasive nature and postoperative functional
implications of the surgery. The PCE regimen consisted of
paclitaxel (100 mg/m2 on Days 1, 8), carboplatin (AUC
2.5 on Days 1, 8), and cetuximab (400 mg/m2 on Days 1,
250 mg/m2 on Days 8, 15) for 3 weeks for a maximum of
six cycles. At the end of the 1st cycle, the tumor in the maxilla
had collapsed and opened widely in the maxillary sinus. By the end
of the 6th cycle, the tumor had markedly shrunk and remained only
partially anterior to the pterygoid process (Fig. 3A and B). No signs of metastasis were
seen in the cervical lymph nodes. During the course of PCE
treatment, the patient was diagnosed with neutropenia (Grade 2–4)
and treated with cetuximab only. The residual tumor was treated by
BRT (66 Gy/33 Fr) with weekly doses of cetuximab (250
mg/m2). Subsequently, the lesion disappeared, and a
complete response to the treatment was achieved. Cetuximab was
continued weekly after BRT. Ten months after completion of BRT, an
erosive lesion was detected on the anterior wall of the left
maxillary sinus via PET (Fig. 4A and
B). Following a biopsy which revealed SCC, cetuximab was
discontinued, and partial maxillectomy along with split-thickness
skin grafting was performed under general anesthesia. An incision
was made along the nasal wing, and the superficial layer of the
maxilla was lifted up to the zygomatic bone; the anterior and
lateral walls of the maxillary sinus and the sinus mucosa-like scar
containing the recurrent tumor were resected (Fig. 5A and B). The pathological diagnosis
was SCC, with negative margins and no bone invasion. The patient
was treated with the anticancer drug S-1 (120 mg/day, 2-week
administration followed by one week of rest) for 1 year as adjuvant
therapy. The patient could survive with minimal functional
disability and a dento-maxillary prosthesis. No local or cervical
recurrence was observed at the two-year follow-up after
surgery.
Discussion
According to the National Comprehensive Cancer
Network guidelines (12), surgical
treatment involves total maxillectomy and bilateral neck
dissection, which are invasive and cause significant postoperative
functional impairment. In addition, the patient was diagnosed with
numerous distal lymph node metastases in the bilateral cervical
regions, and there was a high possibility of distant metastasis.
Arterial injection chemotherapy was one option, but bilateral neck
dissection could not be avoided; therefore, PCE was selected as the
initial treatment. The conventional induction chemotherapy, which
comprised the combination of docetaxel, cisplatin, and
5-fluorouracil (TPF), had significant toxicity issues. In 2010, PCE
was introduced as a novel induction chemotherapy regimen for
advanced head and neck cancer (9).
It is characterized by high completion and response rates and low
toxicity. Additionally, it has the advantages of being administered
in an outpatient setting and allowing the use of chemoradiotherapy
(CRT) as post-treatment. In this study, six weeks of PCE treatment
resulted in an objective response rate (ORR) of 96% (complete
response, 19%; partial response, 77%), indicating a very good
response rate. This regimen has been reported to have a high
response rate of 65% to 97% as induction chemotherapy for locally
advanced head and neck cancer (11,13–17).
However, only one recent study by Takenaka et al (11) reported using this regimen as
induction chemotherapy for sinonasal carcinoma; two cycles of PCE
were used as preoperative chemotherapy, and two out of four
patients with sinonasal sinus cancer responded to the treatment
(ORR, 50%).
This case demonstrated the possibility of treating
maxillary sinus cancer using a minimally invasive method, which may
be applied to other head and neck cancers. Post-treatment after a
PCE regimen may include RT alone, CRT with CDDP, BRT, or surgery.
The TREMPLIN trial (18) showed
that the efficacy of CRT was similar to that of BRT after TPF.
However, no such comparative study has been conducted for PCE, and
there are no criteria for selecting the appropriate post-treatment
method at present. Enokida et al (14) performed PCE for eight weeks,
followed by CRT with CDDP, and showed that the completion rate of
CRT was 97% and the response rate was 93.8%. Takenaka et al
(11) performed two cycles of PCE
followed by RT, CRT, or surgery, depending on the tumor site and
condition. The post-treatment method for laryngeal and
hypopharyngeal carcinoma was selected based on a chemoselection
strategy. Therefore, CRT and BRT may be effective for the
post-treatment of maxillary sinus cancer that has responded well to
PCE. BRT is more manageable than CRT due to the possibility of
developing myelosuppression and renal dysfunction by CDDP. However,
BRT may cause severe infusion reactions and interstitial pneumonia.
In the current case report, the tumor continued to shrink during
PCE, and continuing cetuximab was considered considerably
beneficial; therefore, BRT was chosen as post-PCE therapy, which
resulted in a good outcome.
This report demonstrates the effectiveness of PCE
and BRT as post-treatment methods for advanced maxillary sinus
cancer. In patients initially treated with PCE, careful imaging
evaluations, assessment of the treatment efficacy, and selection of
the appropriate post-treatment method will lead to improved
outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM, MT, TS and SM discussed the treatment strategy.
MT and SM treated the patient with PCE. MM, MT and TS performed
surgery for the recurrent lesion. MM, MT, TS and SM performed
follow-up observations. MM, MT and SM drafted the manuscript. All
authors approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ORR
|
objective response rate
|
|
SCC
|
squamous cell carcinoma
|
References
|
1
|
Dulguerov P, Jacobsen MS, Allal AS,
Lehmann W and Calcaterra T: Nasal and paranasal sinus carcinoma:
Are we making progress?: A series of 220 patients and a systematic
review. Cancer. 92:3012–3029. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ono T, Tanaka N, Umeno H, Chitose S, Shin
B, Aso T, On K, Hattori C, Etoh H, Kakuma T and Abe T: Treatment
outcomes of locally advanced squamous cell carcinoma of the
maxillary sinus treated with chemoradioselection using
superselective intra-arterial cisplatin and concomitant radiation:
Implications for prognostic factors. J Craniomaxillofac Surg.
45:2128–2134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeremic B, Nguyen-Tan P and Bamberg M:
Elective neck irradiation in locally advanced squamous cell
carcinoma of the maxillary sinus: A review. J Cancer Res Clin
Oncol. 128:235–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mundy EA, Neiders ME, Sako K and Greene
GW: Maxillary sinus cancer: A study of 33 cases. J Oral Pathol Med.
14:27–36. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cengiz AB, Uyar M, Comert E, Dursun E and
Eryilmaz A: Sinonasal tract malignancies: Prognostic factors and
surgery outcomes. Iran Red Crescent Med J. 15:e141182013.PubMed/NCBI
|
|
6
|
Won HS, Chun SH, Kim BS, Chung SR, Yoo IR,
Jung CK, Kim YS, Sun DI, Kim MS and Kang JH: Treatment outcome of
maxillary sinus cancer. Rare Tumors. 1:110–114. 2009. View Article : Google Scholar
|
|
7
|
Arosio AD, Turri-Zanoni M, Sileo G,
Tirloni M, Volpi L, Lambertoni A, Margherini S, Mercuri A,
Battaglia P, Cherubino M, et al: Maxillary sinus floor
infiltration: Results from a series of 118 maxillary sinus cancers.
Laryngoscope. 132:26–35. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Homma A, Oridate N, Suzuki F, Taki S,
Asano T, Yoshida D, Onimaru R, Nishioka T, Shirato H and Fukuda S:
Superselective high-dose cisplatin infusion with concomitant
radiotherapy in patients with advanced cancer of the nasal cavity
and paranasal sinuses: A single institution experience. Cancer.
115:4705–4714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kies MS, Holsinger FC, Lee JJ, William WN
Jr, Glisson BS, Lin HY, Lewin JS, Ginsberg LE, Gillaspy KA,
Massarelli E, et al: Induction chemotherapy and cetuximab for
locally advanced squamous cell carcinoma of the head and neck:
Results from a phase II prospective trial. J Clin Oncol. 28:8–14.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tahara M, Kiyota N, Yokota T, Hasegawa Y,
Muro K, Takahashi S, Onoe T, Homma A, Taguchi J, Suzuki M, et al:
Phase II trial of combination treatment with paclitaxel,
carboplatin and cetuximab (PCE) as first-line treatment in patients
with recurrent and/or metastatic squamous cell carcinoma of the
head and neck (CSPOR-HN02). Ann Oncol. 29:1004–1009. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takenaka M, Arai A, Yoshizawa K, Yoshimura
K, Mitsuda J, Saburi S, Tsujikawa T, Sugiyama Y and Hirano S:
Feasibility of combination of paclitaxel, carboplatin, and
cetuximab as induction chemotherapy for advanced head and neck
squamous cell carcinoma. Clin Oncol. 3:13302019.
|
|
12
|
Pfister DG, Spencer S, Adelstein D, Adkins
D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ,
et al: Head and neck cancers, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
18:873–898. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haddad RI, Massarelli E, Lee JJ, Lin HY,
Hutcheson K, Lewis J, Garden AS, Blumenschein GR, William WN,
Pharaon RR, et al: Weekly paclitaxel, carboplatin, cetuximab, and
cetuximab, docetaxel, cisplatin, and fluorouracil, followed by
local therapy in previously untreated, locally advanced head and
neck squamous cell carcinoma. Ann Oncol. 30:471–477. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Enokida T, Ogawa T, Homma A, Okami K,
Minami S, Nakanome A, Shimizu Y, Maki D, Ueda Y, Fujisawa T, et al:
A multicenter phase II trial of paclitaxel, carboplatin, and
cetuximab followed by chemoradiotherapy in patients with
unresectable locally advanced squamous cell carcinoma of the head
and neck. Cancer Med. 9:1671–1682. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bauman J, Langer C, Quon H, Algazy K, Lin
A, Desai A, Mutale F and Weiss J: Induction chemotherapy with
cetuximab, carboplatin and paclitaxel for the treatment of locally
advanced squamous cell carcinoma of the head and neck. Exp Ther
Med. 5:1247–1253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forman R, Bhatia AK and Burtness B:
Efficacy and toxicity of weekly paclitaxel, carboplatin, and
cetuximab as induction chemotherapy or in cases of metastases or
relapse for head and neck cancer in elderly or frail patients. J
Clin Oncol. 39:6042. 2021. View Article : Google Scholar
|
|
17
|
Shirasu H, Yokota T, Kawakami T, Hamauchi
S, Onozawa Y, Ogawa H, Onoe T, Mori K and Onitsuka T: Efficacy and
feasibility of induction chemotherapy with paclitaxel, carboplatin
and cetuximab for locally advanced unresectable head and neck
cancer patients ineligible for combination treatment with
docetaxel, cisplatin, and 5-fluorouracil. Int J Clin Oncol.
25:1914–1920. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lefebvre JL, Pointreau Y, Rolland F,
Alfonsi M, Baudoux A, Sire C, de Raucourt D, Malard O, Degardin M,
Tuchais C, et al: Induction chemotherapy followed by either
chemoradiotherapy or bioradiotherapy for larynx preservation: The
TREMPLIN randomized phase II study. J Clin Oncol. 31:853–859. 2013.
View Article : Google Scholar : PubMed/NCBI
|