Introduction
Vascular endothelial cell growth factor 165 receptor
1 [neuropilin-1 (NRP-1)] is a protein-coding gene on human
chromosome 10p11.22 that codes the NRP-1 protein from 923 amino
acids (103 kDa), a cell surface receptor that contains protein
domains that allow their participation in different types of
signaling pathways that control cell migration (1). A family gene, NRP-2 (human
chromosome 2q33.3), encoding a novel member of the family protein
neuropilin-2 (NRP-2) that contains 931 amino acids with 104 kDa,
was identified as a high-affinity receptor for the Semaphorins
(2). Previous studies revealed that
NRP family proteins exert multiple functions as co-receptors for
vascular endothelial growth factor (VEGF) (3), transforming growth factor beta (TGF-β)
(4), hepatocyte growth factor
(5), fibroblast growth factor (FGF)
(6), and Semaphorin 3 (SEMA3)
(7). NRP family proteins are
involved in the interaction with multiple ligand receptors, thus
NRP family proteins may be involved in cancer occurrence and
development and might serve as therapeutic targets for gastric
cancer (8), glioma (9), endometrial cancer (10), bladder cancer (11), thyroid cancer (12), breast cancer (13), gallbladder cancer (14), colorectal cancer (15), and pancreatic adenocarcinoma (PDAC)
(16). Moreover, NRP signaling has
been associated with several biological processes, including
pro-tumorigenic cell proliferation, invasion, metastasis, and tumor
growth in PDAC (17). NRP signaling
can provide resistance to chemotherapeutic reagent exposure in
clinical settings by imitating the therapy-resistant cancer
stem-cell properties (18). Recent
advances in single-cell analysis (SCA) have revealed multiple
functional roles of cancer-related cellular proteins (19), but the roles of NRP remain poorly
understood. Here, we discuss recent advances in NRP biology in PDAC
based on the SCA-based precision study.
NRP signaling
NRP-1 contains a large N-terminal extracellular
domain, including complement-binding, coagulation factors V/VIII
(CF V/VIII), and meprin domains. The two NRP-1 (complement-binding)
CUB domains and the amino-terminal CF V/VIII domain are crucial for
SEMA3A binding. The amino-terminal NRP-1 CF V/VIII domain remains
the only required for binding to VEGF-165. Therefore, NRP-1 exerts
its biological functions by binding with Semaphorin ligands
(20). A previous study revealed
that SEMA3A can inhibit axonal growth and induce neuronal apoptosis
after binding to NRP-1, with the membrane-proximal meprin, A5/NRP1,
protein tyrosine-phosphatase µ (MAM) domain of NRP-1. NRP-1 is
involved in regulating cell survival by mediating the effects of
its ligands, such as VEGF. NRP-1 promotes survival in cancer cells
by activating signaling pathways, such as the PI3K/AKT pathway
(21). The activation of these
pathways helps in tumor progression and therapy resistance.
Additionally, NRP-1 plays an essential role in cell migration by
mediating the effects of Semaphorins and VEGF. NRP-1 can enhance
the invasive and migratory capabilities of cancer cells. NRP-1, by
interacting with its ligands, can activate downstream signaling
pathways, such as Src kinases, which modulate cytoskeletal dynamics
and cell adhesion, thereby promoting cell migration and invasion
(22). This causes tumor
metastasis, where cancer cells spread to other body parts. These
results indicate that the meprin domain is involved in forming a
higher-order receptor complex. NRP may play a key role in
cell-to-cell interaction via their responses to ligands (Fig. 1) (23). Moreover, a recent report indicated
the involvement of the NRP-1 signal in the symmetric cell division
to expand breast cancer stem-like cells (24). NRP-1 has been overexpressed in
various cancer types, including lung, breast, pancreatic, and
prostate cancers. NRP-1 affects cell survival, migration, and
attraction by binding to ligands and various co-receptors and may
serve as a cancer biomarker of refractory tumors (25).
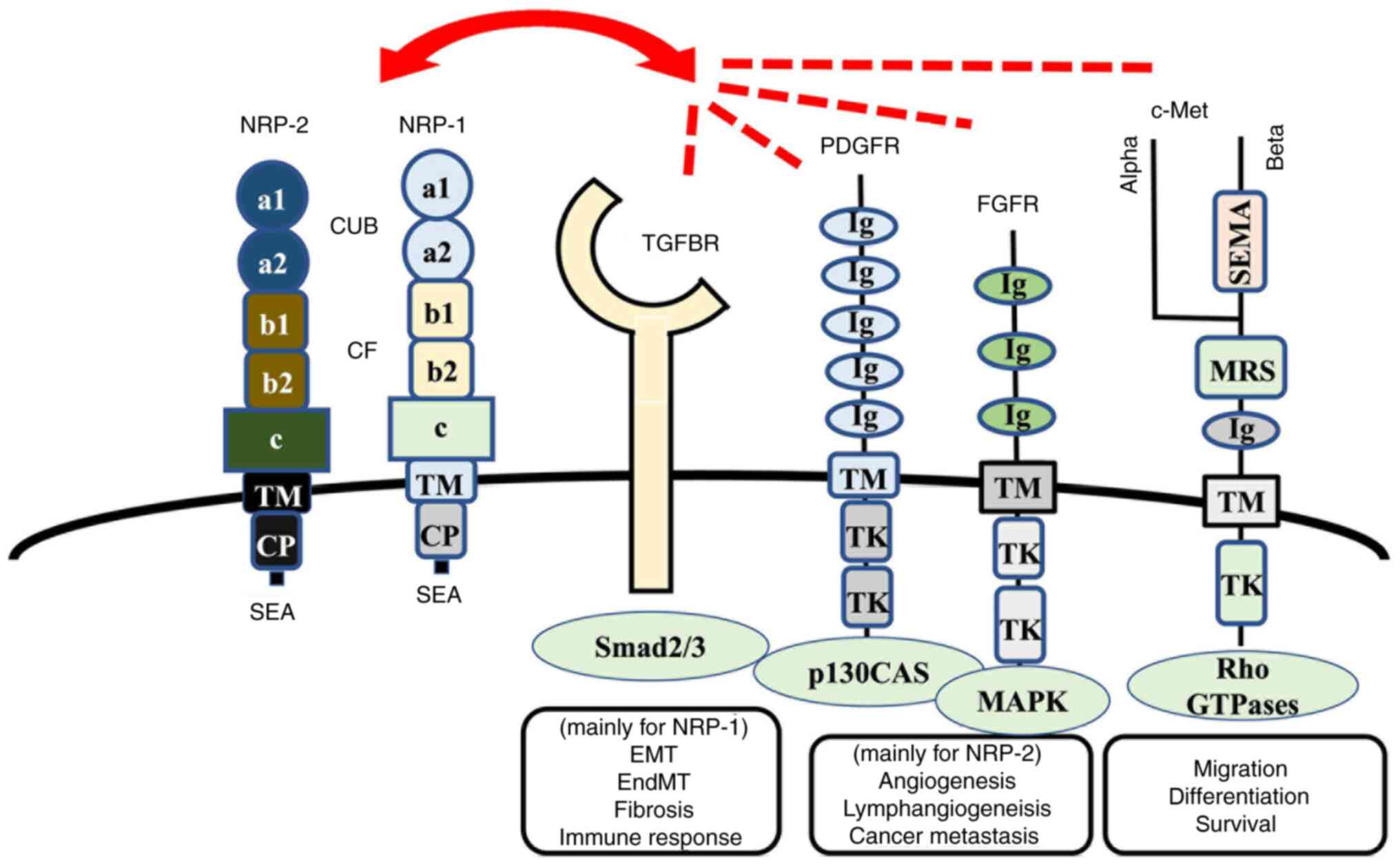 | Figure 1.NRP-1 and NRP-2 and their related
receptors. NRP-1 is a cell membrane-bound receptor that consists of
three extracellular domains: i) a1/a2 domain, homologous to the
complement proteins C1r/C1s, Uegf and Bmp-1 (referred to as the CUB
domain); ii) b1/b2 domain, which is homologous to the coagulation
factors V and VIII; and iii) c domain, which is homologous to
meprin, A5 protein and protein tyrosine phosphatase µ, as well as
TM and CP. NRP-1 contains an SEA sequence in the C-terminus that
represents a consensus binding motif for proteins that contain the
PDZ (PSD-95, Dlg, ZO-1) domain, such as synectin, which can act as
the docking site for interacting partners. The homologies between
NRP-1 and NRP-2 are 55% (in the a1/a2 domain), 48% (in the b1/b2
domain), 35% (in the c domain) and 49% (in the CP region) (75). TGF binds to cell membrane-bound
serine/threonine kinase receptors that belong to the TGF-β receptor
family. PDGFRs consist of extracellular five Ig-like domains and
intracellular tyrosine kinase domains, whereas FGFRs consist of
extracellular three Ig-like domains and intracellular tyrosine
kinase domains. NRP-1 and NRP-2 interact with those receptors and
modulate the biological function of cancer cells, vessel and
lymphatic endothelial cells, fibroblasts and immune cells, which
are components of architectures in tumor microenvironments. NRP,
Neuropilin; TM, transmembrane domain; CP, cytoplasmic region;
PDGFRs, platelet-derived growth factor receptors; FGFRs, fibroblast
growth factor receptors; SEMA, Semaphorins; MRS, Met-related
sequence; TK, tyrosine kinase; TGFBR, TGF-β receptor; EMT,
epithelial-to-mesenchymal transition; EndMT,
endothelial-to-mesenchymal transition; SEA, cytoplasmic domain. |
NRP-2 is characterized by a transmembrane protein
that binds to the SEMA domain, immunoglobulin domain (Ig),
Semaphorin 3C (SEMA3C), and Semaphorin 3F (SEMA3F) (26), and NRP-2 interacts with VEGF
(27). NRP-1 binds with high
affinity to the three structurally related Semaphorins, such as
SEMA3, SEMAE, and SEMA4, whereas NRP-2 shows high-affinity binding
to SEMAE and SEMA4, but not SEMA3 (2). NRP-2 is involved in cardiovascular
development, axon guidance, and tumorigenesis (28,29).
Neuropilins (NRPs) are transmembrane glycoproteins
that act as co-receptors for a variety of ligands, including
vascular endothelial growth factor (VEGF), semaphorins (SEMA), and
transforming growth factor-beta (TGF-β). These ligands bind to
NRPs, which then interact with and enhance the signaling of their
respective receptors, such as VEGF receptor (VEGFR) and TGF-β
receptor (TGFBR). VEGF is a key regulator of angiogenesis, and its
binding to NRP-1 enhances VEGFR-2 signaling, leading to endothelial
cell proliferation and migration. SEMA3s are involved in axon
guidance and immune regulation, and their binding to NRP-1 and
NRP-2 can activate downstream signaling pathways such as RhoA/ROCK
and PI3K/Akt. TGF-β is a multifunctional cytokine that plays a
critical role in cell growth, differentiation, and immune
regulation. Its binding to NRP-1 enhances TGFBR signaling, leading
to downstream activation of Smad2/3 and other signaling pathways.
NRPs have been reported to interact with various signaling
pathways, including TGF-β, PDGF, FGF, c-Met, and others (Fig. 1). Despite some controversy
surrounding these interactions, current knowledge suggests that
NRP-1 has been involved in cancer stem-cell maintenance and
progression through the Wnt/β-catenin signaling pathway (30) whereas NRP-2 has been associated with
lymphangiogenesis and lymphatic metastasis in certain cancer types
(31).
Intractable PDAC
Pancreatic cancer, also known as PDAC, is one of the
most aggressive cancers globally. A PDAC diagnosis carries a 5-year
survival rate of <10% (32,33).
PDAC's clinical aggressiveness has been attributed to the i) lack
of PDAC-specific symptoms (rendering early-stage detection
difficult) (34–36), ii) early metastases (typically
spreading to marginal tissues and distant organs, including the
liver) (34,35), and iii) chemo- and radiotherapy
resistance (34,37). Importantly, many other factors, such
as topographical, vascular, and ductal pancreatic anatomy (38), and the complex involvement of
stromal components of PDAC (39),
may be involved in high disease recurrence rates.
Studies of six cohorts, comprising 136,000 cells
from 71 cases with PDAC, indicated that PDAC contains various
cells, including cancer-associated fibroblasts (CAFs) to understand
the complexity of PDAC's cellular components. CAFs facilitate
cell-to-cell communication and are involved in PDAC spread and
therapeutic resistance (40,41).
They were classified into several subpopulations, including
inflammatory CAF (iCAF), myofibroblast CAF (myCAF), and
antigen-presenting CAF (apCAF), based on gene expression (41). Diverse CAF subpopulations were
reported for nine cancer types (42). PDAC that is characterized by iCAFs,
which express interleukin 6 (IL6), collagen type XIV alpha 1 chain
(COL14A1), lymphocyte antigen 6 complex locus C1 (LY6C), etc., was
classified as ‘classic-type’ with a strong inflammatory profile
(41). PDAC that is characterized
by myCAFs, which express actin alpha 2, smooth muscle (ACTA2/aSMA),
transgelin (TAGLN), thrombospondin 2 (THBS2), leucine-rich repeat
containing 15 (LRRC15), etc., was considered as ‘basal-type’ with a
strong myofibroblast profile (41).
ApCAFs, which represent a distinct subset of CAFs expressing major
histocompatibility complex class II (MHC II) and CD74, possess
antigen-presenting capabilities. However, they notably lack the
expression of co-stimulatory molecules, such as CD40, CD80, and
CD86, resulting in the inability to initiate the typical activation
response in CD4+ T cells. The specific role of apCAFs
remains unclear, but a widely accepted hypothesis indicates that
they might attract CD4+ T cells by expressing MHCII and
subsequently interfering with their normal functionality. This
interference causes CD4+ T cell inactivation or
differentiation into regulatory T cells, thereby potentially
contributing to the development of an immunosuppressive tumor
microenvironment (43,44). Inter-cellular communication via
ligands and their receptors indicated that sonic hedgehog
(Shh)-mediated signals in CAFs suppressed cancer cell proliferation
and progression in a PDAC model (45).
NRP expression in single PDAC cells
Few reports have focused on NRP expression at the
single-cell level in PDAC, thus we used a published single-cell
database (https://zenodo.org/record/6024273#.Y7T3tNXP1D8) to
examine 136,000 cells from 71 patients with PDAC (41). We revealed various NRP expressions
in human PDAC cells, which expressed both NRP-1 and NRP-2. In
contrast, ductal cell type 1, another cell cluster, was positive
for NRP-1 but not NRP-2. Thus, NRP-1 and NRP-2 appear to allow
ductal cells in the pancreas to fulfill different functions. NRP-1
expression, in stellate cells, was higher than NRP-2. Fibroblasts,
macrophages, and endothelial cells expressed substantial amounts of
both NRP-1 and NRP-2. Endocrine cells featured very few NRP-1 or
NRP-2 expressions, indicating that cases with aggressive phenotypes
demonstrate fewer endocrine cells. MyCAF cells tend to express both
NRP-1 and NRP-2 at high levels, while iCAF cells express only NRP-1
at high levels. Additionally, SEMA3A expression was similar in
myCAF and iCAF, but the number of FGF1-expressing cells appeared
slightly higher in myCAF. Targeting NRP signaling may represent a
potential PDAC therapy approach, considering the high expression of
NRP-1 and NRP-2 in ductal cells and fibroblasts.
Therapeutic targeting of NRP-1-positive cells in
PDAC can regulate endothelial-to-mesenchymal transition (EndMT),
which is an important source of fibroblasts in pathological
disorders, thereby reducing tumor fibrosis and PDAC progression
(46). The tumor-penetrating
peptide was reported via a transcytosis transport pathway that is
regulated by NRP-1. This system enhances the transcytosis of
silicasome-based chemotherapy for PDAC in NRP-1-positive cells
(47). Chimeric antigen receptor T
cell (CAR-T) immunotherapy allows T cells to recognize an antigen
and attach to antigen-positive cells, thus CAR-T targeting NRPs
might be a potential PDAC therapy (8).
Innovative therapeutic approaches against
NRPs-positive PDAC cells
EndMT
Previous studies revealed the epithelial-mesenchymal
transition (EMT) mechanism by which epithelial cells lose their
polarity and cell-cell adhesion and epithelial cells acquire
mesenchymal features and obtain invasive phenotypes. These
characterize mesenchymal stem cells, chemotherapy-resistant cells,
and cancer metastasis (48,49). Extensive transcriptional
reprogramming occurs during the EMT process, and this mechanism is
useful for determining the presence of metastases and circulating
tumor cells, as well as developing therapies against metastasizing
cancer cells (50–52). In particular, high expression of
zinc finger E-box binding homeobox 1, Yes-associated
transcriptional regulator, FOS like 1, AP-1 transcription factor
subunit (FOSL1), and the Jun proto-oncogene, AP-1 transcription
factor subunit, indicate the presence of an aggressive, breast
cancer subtype. These findings confirm the translational importance
of the EMT process (50).
However, the EndMT process was reported to involve
extensive transcriptional reprogramming in endothelial cells,
shifting them toward mesenchymal phenotypes and functional
responses. These processes were previously studied in
cardiovascular tissues. Sirtuin 1 (SIRT1), activated by
resveratrol, attenuated isoproterenol-induced cardiac fibrosis by
regulating EndMT via the TGF-β1/Mothers against Decapentaplegic
Homolog 2/3 (Smad2/3) pathway. Thus, TGF-β1 strongly induces EndMT.
Further, SIRT1 may be involved in cardiac fibrosis under the EndMT
(53–55). Tumor necrosis factor-α in cancer
enhances TGF-β-induced EndMT via TGF-β signal augmentation
(55).
PDAC characterizes an intense fibrotic reaction
(i.e., desmoplasia) which is partly responsible for its
aggressiveness, thus NRP-1 could be used to regulate TGF-β1-induced
EndMT and fibrosis. Some researchers have promoted NRP-1 as a
therapeutic target to reduce tumor fibrosis and slow disease
progression in patients with PDAC (46). NRP interacts with many receptors and
aggregates signals from other individual receptors, thereby
executing EMT and EndMT (Fig. 2).
Precision medicines that target NRP-1 and NRP-2 could be specified
to a patient's genetic profile. Precision PDAC medicine may use
drugs that target genetic mutations, such as KRAS Proto-Oncogene,
GTPase, and Tumor Protein P53, and drugs that target the pathways
and processes that are altered in PDAC (e.g., cell death, survival,
migration, adhesion) (40,56).
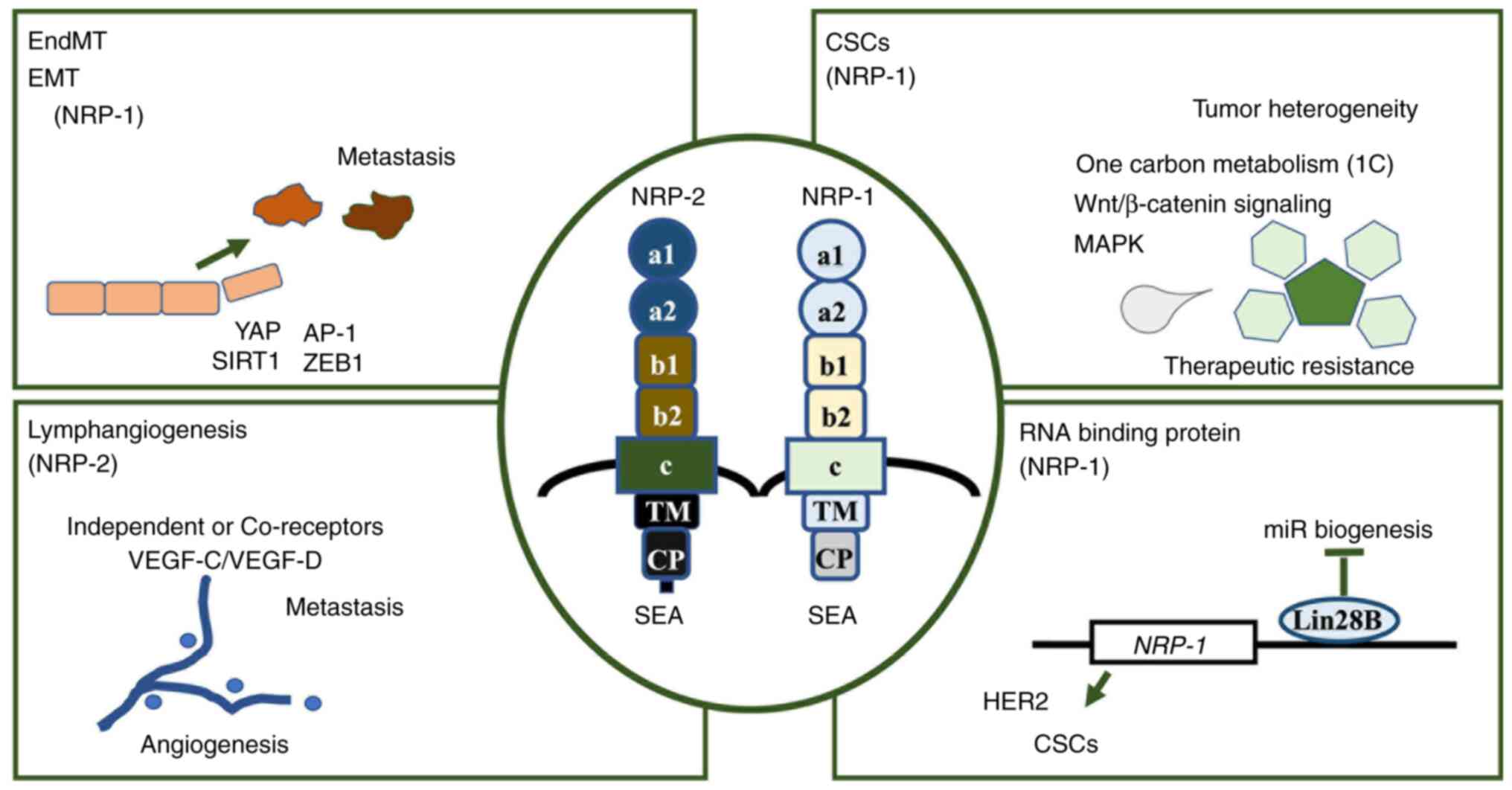 | Figure 2.Biological roles of NRPs exerting
various aspects. Therapeutic approaches against NRPs can be based
on their involvement in various processes. NRP-1 primarily
participates in the activity of EndMT and EMT, CSCs and RNA binding
protein-mediated gene expression regulation, while NRP-2 is
predominantly associated with lymphangiogenesis. NRP, neuropilin;
EndMT, endothelial-to-mesenchymal transition; EMT,
epithelial-to-mesenchymal transition; CSCs, cancer stem cells; SEA,
cytoplasmic domain; VEGF-C, vascular endothelial growth factor C;
VEGF-D, vascular endothelial growth factor D; YAP, yes-associated
protein; AP-1, activator protein 1; SIRT1, sirtuin 1; ZEB1, zinc
finger E-box-binding homeobox 1; Lin28B, Lin-28 homolog B; TM,
transmembrane domain; CP, cytoplasmic region. |
Cancer stem cells (CSCs)
CSCs help in therapeutic resistance and tumor
heterogeneity (57,58) (Fig.
2). A study investigated the multipotent characteristics of
CSCs in patients with PDAC (59).
NRP signaling contributes to CSC maintenance and development
(18). The VEGF/NRP signaling axis
is a prime therapeutic target because of its ability to confer
resistance to standard chemotherapies (18). NRP-1 interacts with PDZ (also known
as disks-large homologous regions) domain-containing protein GIPC1
and PH domain-containing family G member 5 to activate p38
mitogen-activated protein kinase signaling and CSC survival
(60). Targeting either NRP-1 or
NRP-2 can inhibit tumor initiation and decrease therapeutic
resistance in patients with cancer (18).
The increasing evidence for the NRP-1 involvement in
cancer has led many studies to investigate its potential as a
therapeutic target. Previous studies have focused on the anticancer
effects of targeting NRP-1, but little is known about the
potentially adverse effects associated with such targeting. Further
studies are needed to understand the full spectrum of effects
associated with targeting NRP-1 in patients with cancer, including
an investigation of potentially adverse events. Such studies should
include both in vitro and in vivo cases and clinical
trials. NRP-1 targeting-related adverse effects are important
because they influence the safety and efficacy of potential future
therapeutic targets.
Co-receptor targeting
Cancer cells in the tumor microenvironment produce
multiple growth factors that promote lymphangiogenesis from
initially enlarged lymphatics to collection within lymphatic
vessels (61). Lymphatic
enlargement may involve the remodeling of lymphatic vessels with
smooth muscle cells (61). Several
lymphangiogenic factors, such as VEGF-C/VEGF-D, can promote tumor
metastasis (Fig. 2) (61).
NRP-2 acts as an independent or co-receptor for
tumor lymphangiogenesis and lymphatic metastasis (Fig. 2) (62). During tumor progression, NRP-2 binds
to the ligands VEGF-C/VEGF-D and activates the VEGF-C/VEGF-D/NRP-2
signaling axis, which stimulates lymphangiogenesis regulation in
lymphatic endothelial cells and tumor cells (62). A 131I-labeled monoclonal antibody
targeting NRP-2 for single photon emission computed tomography
imaging allows lymphangiogenesis and tumor angiogenesis
visualization in clinical settings (63). Reportedly, mice lacking the
transmembrane receptor NRP1, also known as NRP KO mice, exhibit
reduced glioma volume and decreased neoangiogenesis, while showing
an increased anti-tumorigenic macrophage infiltration (64). Recent studies revealed that NRP-2
may regulate tumor progression through multiple, concurrent
mechanisms (i.e., angiogenesis, lymphangiogenesis, EMT, and
metastasis). These results indicate that NRP could serve as a
therapeutic target for innovative antitumor therapies (62,65).
First, NRPs tend to promote cell adhesion, cell-matrix
interactions, cell motility, tumor angiogenesis, cell
proliferation, and invasion (62,65).
Second, NRPs are expressed in a range of cancer cells, including
PDAC, as discussed above. Third, NRPs are amenable to targeted
inhibition by inhibiting co-receptors or downstream signaling
pathways (18). NRPs-ligand
interaction inhibitors render NRPs an attractive target for novel
therapeutic strategies (66).
Preclinical studies revealed NRPs-targeting strategies to be safe,
thereby further strengthening the case for their use as innovative
antitumor therapies (62,65).
NRP mRNA binding protein
Recent studies open a new era of diagnostic and
therapeutic that target RNA binding mechanisms of NRP transcripts.
RNA binding protein Lin28B can directly bind to the 3′ untranslated
region (UTR) of the NRP-1 transcript, thereby increasing NRP-1 mRNA
stability and NRP-1 expression (67,68).
This interaction has been suggested to activate Wnt/β-catenin
signaling downstream that is involved in CSC or CSC-like cell
maintenance and progression in gastric cancer (Fig. 2) (68). It's worth noting that the regulation
of Wnt/β-catenin signaling remains a subject of ongoing debate and
investigation. While existing literature suggests an association
between Lin28B-binding NRPs and Wnt/β-catenin signaling, further
research is needed to fully elucidate the complexities of this
relationship. Lin28B can exert multiple functions in cancer
development by suppressing the biogenesis of several microRNAs,
including let-7 and (possibly) miR107, miR-143, and miR-200c
(69,70). Overexpressed Lin28B can recruits
terminal uridylyl transferase 4 (TUT4/ZCCHC11) to pre-let-7
transcripts, leading to their terminal uridylation and degradation
(71). Lin28B in cancer is
indicated to be related to let-7 family derepression, which can
facilitate cellular transformation with stemness. These insights
contribute to the development of new strategies for cancer therapy
(Fig. 3).
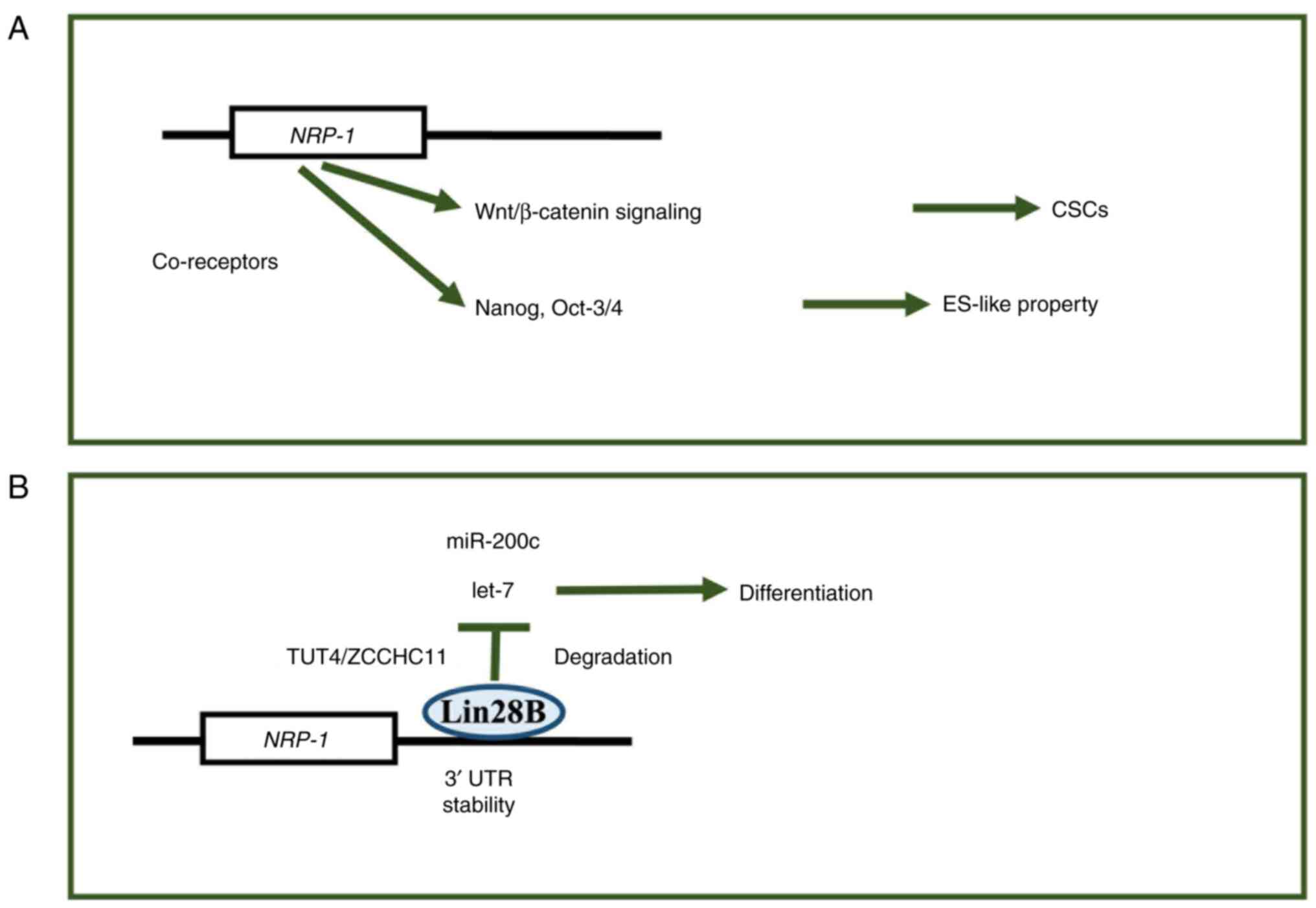 | Figure 3.NRP-1 gene expression generating axis
of stem-cell properties. (A) NRP-1 was involved in the
Wnt/β-catenin-signaling-dependent CSC generation and the Nanog and
Oct-3/4 signaling pathway mechanisms, endowing cells with
biological properties resembling those of ESCs (ES-like property),
such as self-renewal, pluripotency and the reversibility of
cellular states, which are hallmarks of ESCs. (B) RNA binding
protein, Lin28B, is involved in the control of let-7 and miRNA
stability, which plays a role in controlling cell differentiation
and cell stemness. NRP, neuropilin; CSCs, cancer stem cells; ESC,
embryonic stem cells; miR/miRNA, microRNA; UTR, untranslated
region; let-7, let-7 microRNA; TUT4, Terminal uridylyltransferase
4; ZCCHC11, zinc-finger, CCHC domain-containing protein 11; Lin28B,
Lin-28 homolog B. |
Another study of RNA immunoprecipitation and
luciferase reporter analysis indicated that RNA binding protein
PUM2 competitively bound to NRP-1 3′UTR with a microRNA, miR-376a,
which can suppress breast cancer cell stemness and increase NRP-1
mRNA stability and expression in breast cancer (72).
Understanding the role of RNA binding proteins
(RBPs) in cancer stemness is improving. The NRP axis is crucial for
regulating key pathways that are involved in cancer progression.
First, NRP-1 helps regulate the Wnt/β-catenin signaling (67,68),
which is important for maintaining cancer stem-cell populations.
Second, NRPs help regulate tumorigenesis and metastasis by
modulating oncogenic and metastasis-associated gene expression.
This is particularly true for NRP-2 and tumor lymphangiogenesis and
lymphatic metastasis mechanisms (62). Third, NRP-1 promotes
stem-cell-associated induced pluripotent stem gene expression,
including homeobox transcription factor Nanog and POU Class 5
Homeobox 1 (Oct-3/4) (73). RBPs
help regulate pre- and post-transcriptional processes, such as
splicing, mRNA stability, and translation (74), thus they may contribute to cancer
aggressiveness via gene expression regulation in the NRP axis.
Conclusions
Precision medicines that target the NRP axis might
improve the diagnoses and treatment of patients with PDAC. The NRP
axis contains potential therapeutic targets that could be used to
develop new and individualized PDAC treatments.
Various approaches have been used to target the
NRP-1 and NRP-2 axes, including gene editing, small molecule
inhibitors, and monoclonal antibodies. These approaches help
identify novel therapeutic targets that may improve patient
outcomes and biomarkers for risk-based patient stratification, as
well as the selection of the most effective treatment for each
patient. Precision medicines that target the NRP axis are leading
the field in an exciting new direction that may revolutionize our
ability to treat this deadly disease.
Acknowledgements
Not applicable.
Funding
This work was partly supported by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science
and Technology [grant nos. 17cm0106414h0002, JP21lm0203007,
18KK0251, 19K22658, 20H00541, 21K19526, 22H03146, 22K19559,
23K19505 and 16H06279 (PAGS)]. In addition, partial support was
offered by the Mitsubishi Foundation (grant no. 2021-48).
Availability of data and materials
Not applicable.
Authors' contributions
HI conceived the study objectives and obtained the
funding. SM, TH, YT, YS, YH, YA, NG, KO, and HI contributed to
creating the figures, collecting information and writing the
manuscript. SM, TH, HS, ST, NG, KO, and HI constructed the study
design and outlined the content. All authors have read and approved
the final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen H, Chédotal A, He Z, Goodman CS and
Tessier-Lavigne M: Neuropilin-2, a novel member of the neuropilin
family, is a high affinity receptor for the semaphorins Sema E and
Sema IV but not Sema III. Neuron. 19:547–559. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu C and Jiang X: Role of NRP-1 in
VEGF-VEGFR2-independent tumorigenesis. Target Oncol. 11:501–505.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kofler N and Simons M: The expanding role
of neuropilin: Regulation of transforming growth factor-β and
platelet-derived growth factor signaling in the vasculature. Curr
Opin Hematol. 23:260–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klotz DM, Kuhlmann JD, Link T, Goeckenjan
M, Hofbauer LC, Göbel A, Rachner TD and Wimberger P: Clinical
impact of soluble neuropilin-1 in ovarian cancer patients and its
association with its circulating ligands of the HGF/c-MET axis.
Front Oncol. 12:9748852022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu W, Parikh AA, Stoeltzing O, Fan F,
McCarty MF, Wey J, Hicklin DJ and Ellis LM: Upregulation of
neuropilin-1 by basic fibroblast growth factor enhances vascular
smooth muscle cell migration in response to VEGF. Cytokine.
32:206–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leclerc M, Voilin E, Gros G, Corgnac S, de
Montpréville V, Validire P, Bismuth G and Mami-Chouaib F:
Regulation of antitumour CD8 T-cell immunity and checkpoint
blockade immunotherapy by neuropilin-1. Nat Commun. 10:33452019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bębnowska D, Grywalska E,
Niedźwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J,
Pasiarski M, Góźdź S, Roliński J and Polkowski W: CAR-T cell
therapy-an overview of targets in gastric cancer. J Clin Med.
9:18942020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Zhang G, Shi Y, Qiu R and Khan AA:
Neuropilin-1 (NRP-1) and magnetic nanoparticles, a potential
combination for diagnosis and therapy of gliomas. Curr Pharm Des.
21:5434–5449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oplawski M, Dziobek K, Grabarek B, Zmarzły
N, Dąbruś D, Januszyk P, Brus R, Tomala B and Boroń D: Expression
of NRP-1 and NRP-2 in endometrial cancer. Curr Pharm Biotechnol.
20:254–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Förster S, Givehchi M, Nitschke K, Mayr T,
Kilian K, Dutta S, Datta K, Nuhn P, Popovic Z, Muders MH and Erben
P: Neuropilin-2 and Its transcript variants correlate with clinical
outcome in bladder cancer. Genes (Basel). 12:5502021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu DG, Chang WW, Jan MS, Tu CW, Lu YC and
Tai CK: Promotion of metastasis of thyroid cancer cells via
NRP-2-mediated induction. Oncol Lett. 12:4224–4230. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Wang H, Li C, Zhao Y, Wu L, Du X
and Han Z: VEGF-A/neuropilin 1 pathway confers cancer stemness via
activating Wnt/β-catenin axis in breast cancer cells. Cell Physiol
Biochem. 44:1251–1262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Zhang R, Ma L, Li Q, Zhao YL,
Zhang GJ, Zhang D, Li WZ, Cao S, Wang L and Geng ZM: Neuropilin-1
is up-regulated by cancer-associated fibroblast-secreted IL-8 and
associated with cell proliferation of gallbladder cancer. J Cell
Mol Med. 24:12608–12618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lungulescu C, Ghimpau V, Gheonea DI, Sur D
and Lungulescu CV: The role of neuropilin-2 in the epithelial to
mesenchymal transition of colorectal cancer: A systematic review.
Biomedicines. 10:1722022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma L, Zhai B, Zhu H, Li W, Jiang W, Lei L,
Zhang S, Qiao H, Jiang X and Sun X: The miR-141/neuropilin-1 axis
is associated with the clinicopathology and contributes to the
growth and metastasis of pancreatic cancer. Cancer Cell Int.
19:2482019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matkar PN, Jong ED, Ariyagunarajah R,
Prud'homme GJ, Singh KK and Leong-Poi H: Jack of many trades:
Multifaceted role of neuropilins in pancreatic cancer. Cancer Med.
7:5036–5046. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mercurio AM: VEGF/neuropilin signaling in
cancer stem cells. Int J Mol Sci. 20:4902019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gohil SH, Iorgulescu JB, Braun DA, Keskin
DB and Livak KJ: Applying high-dimensional single-cell technologies
to the analysis of cancer immunotherapy. Nat Rev Clin Oncol.
18:244–256. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu C, Limberg BJ, Whitaker GB, Perman B,
Leahy DJ, Rosenbaum JS, Ginty DD and Kolodkin AL: Characterization
of neuropilin-1 structural features that confer binding to
semaphorin 3A and vascular endothelial growth factor 165. J Biol
Chem. 277:18069–18076. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Feng Y, Xie X, Wu H, Su XN, Qi J,
Xin W, Gao L, Zhang Y, Shah VH and Zhu Q: Neuropilin-1 aggravates
liver cirrhosis by promoting angiogenesis via VEGFR2-dependent
PI3K/Akt pathway in hepatic sinusoidal endothelial cells.
EBioMedicine. 43:525–536. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Timoshenko AV, Rastogi S and Lala PK:
Migration-promoting role of VEGF-C and VEGF-C binding receptors in
human breast cancer cells. Br J Cancer. 97:1090–1098. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams G, Eickholt BJ, Maison P, Prinjha
R, Walsh FS and Doherty P: A complementary peptide approach applied
to the design of novel semaphorin/neuropilin antagonists. J
Neurochem. 92:1180–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tominaga K, Minato H, Murayama T, Sasahara
A, Nishimura T, Kiyokawa E, Kanauchi H, Shimizu S, Sato A, Nishioka
K, et al: Semaphorin signaling via MICAL3 induces symmetric cell
division to expand breast cancer stem-like cells. Proc Natl Acad
Sci USA. 116:625–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernández-Palanca P, Payo-Serafín T,
Fondevila F, Méndez-Blanco C, San-Miguel B, Romero MR, Tuñón MJ,
Marin JJG, González-Gallego J and Mauriz JL: Neuropilin-1 as a
potential biomarker of prognosis and invasive-related parameters in
liver and colorectal cancer: A systematic review and meta-analysis
of human studies. Cancers (Basel). 14:34552022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Curreli S, Wong BS, Latinovic O,
Konstantopoulos K and Stamatos NM: Class 3 semaphorins induce
F-actin reorganization in human dendritic cells: Role in cell
migration. J Leukoc Biol. 100:1323–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang X, Yang Q, Zhang C, Zhang Y, Liang
X, Liu Y and Xu G: Roles for VEGF-C/NRP-2 axis in regulating renal
tubular epithelial cell survival and autophagy during serum
deprivation. Cell Biochem Funct. 37:290–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reichert S, Scheid S, Roth T, Herkel M,
Petrova D, Linden A, Weberbauer M, Esser J, Diehl P, Grundmann S,
et al: Semaphorin 3F promotes transendothelial migration of
leukocytes in the inflammatory response after survived cardiac
arrest. Inflammation. 42:1252–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bollard J, Patte C, Radkova K, Massoma P,
Chardon L, Valantin J, Gadot N, Goddard I, Vercherat C, Hervieu V,
et al: Neuropilin-2 contributes to tumor progression in preclinical
models of small intestinal neuroendocrine tumors. J Pathol.
249:343–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu W, Wu T, Dong X and Zeng YA:
Neuropilin-1 is upregulated by Wnt/β-catenin signaling and is
important for mammary stem cells. Sci Rep. 7:109412017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Huang Y, Zhang J, Wei Y, Mahoud S,
Bakheet AM, Wang L, Zhou S and Tang J: Pathway-related molecules of
VEGFC/D-VEGFR3/NRP2 axis in tumor lymphangiogenesis and lymphatic
metastasis. Clin Chim Acta. 461:165–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoon SJ, Shin SH, Yoon SK, Jung JH, You Y,
Han IW, Choi DW and Heo JS: Appraisal of 5-year recurrence-free
survival after surgery in pancreatic ductal adenocarcinoma. J
Hepatobiliary Pancreat Sci. 28:287–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Belfiori G, Crippa S, Francesca A,
Pagnanelli M, Tamburrino D, Gasparini G, Partelli S, Andreasi V,
Rubini C, Zamboni G and Falconi M: Long-term survivors after
upfront resection for pancreatic ductal adenocarcinoma: An actual
5-year analysis of disease-specific and post-recurrence survival.
Ann Surg Oncol. 28:8249–8260. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Dosso S, Siebenhüner AR, Winder T,
Meisel A, Fritsch R, Astaras C, Szturz P and Borner M: Treatment
landscape of metastatic pancreatic cancer. Cancer Treat Rev.
96:1021802021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang JC and Kundranda M: Novel diagnostic
and predictive biomarkers in pancreatic adenocarcinoma. Int J Mol
Sci. 18:6672017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Long J, Zhang Y, Yu X, Yang J, LeBrun DG,
Chen C, Yao Q and Li M: Overcoming drug resistance in pancreatic
cancer. Expert Opin Ther Targets. 15:817–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bazira PJ and Mahadevan V: Anatomy of the
pancreas and spleen. Surgery (Oxford). 40:213–218. 2022. View Article : Google Scholar
|
|
39
|
Neesse A, Michl P, Frese KK, Feig C, Cook
N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM and
Tuveson DA: Stromal biology and therapy in pancreatic cancer. Gut.
60:861–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Connor AA and Gallinger S: Pancreatic
cancer evolution and heterogeneity: Integrating omics and clinical
data. Nat Rev Cancer. 22:131–142. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chijimatsu R, Kobayashi S, Takeda Y,
Kitakaze M, Tatekawa S, Arao Y, Nakayama M, Tachibana N, Saito T,
Ennishi D, et al: Establishment of a reference single-cell RNA
sequencing dataset for human pancreatic adenocarcinoma. iScience.
25:1046592022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, McAndrews KM and Kalluri R:
Clinical and therapeutic relevance of cancer-associated
fibroblasts. Nat Rev Clin Oncol. 18:792–804. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Elyada E, Bolisetty M, Laise P, Flynn WF,
Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS,
et al: Cross-species single-cell analysis of pancreatic ductal
adenocarcinoma reveals antigen-presenting cancer-associated
fibroblasts. Cancer Discov. 9:1102–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang H, Wang Z, Zhang Y, Pradhan RN,
Ganguly D, Chandra R, Murimwa G, Wright S, Gu X, Maddipati R, et
al: Mesothelial cell-derived antigen-presenting cancer-associated
fibroblasts induce expansion of regulatory T cells in pancreatic
cancer. Cancer Cell. 40:656–673.e7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rhim AD, Oberstein PE, Thomas DH, Mirek
ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP,
Tattersall IW, et al: Stromal elements act to restrain, rather than
support, pancreatic ductal adenocarcinoma. Cancer Cell. 25:735–747.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matkar PN, Singh KK, Rudenko D, Kim YJ,
Kuliszewski MA, Prud'homme GJ, Hedley DW and Leong-Poi H: Novel
regulatory role of neuropilin-1 in endothelial-to-mesenchymal
transition and fibrosis in pancreatic ductal adenocarcinoma.
Oncotarget. 7:69489–69506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu X, Lin P, Perrett I, Lin J, Liao YP,
Chang CH, Jiang J, Wu N, Donahue T, Wainberg Z, et al:
Tumor-penetrating peptide enhances transcytosis of silicasome-based
chemotherapy for pancreatic cancer. J Clin Invest. 127:2007–2018.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:9652016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mak MP, Tong P, Diao L, Cardnell RJ,
Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J,
Wistuba II, et al: A patient-derived, pan-cancer EMT signature
identifies global molecular alterations and immune target
enrichment following epithelial-to-mesenchymal transition. Clin
Cancer Res. 22:609–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Feldker N, Ferrazzi F, Schuhwerk H,
Widholz SA, Guenther K, Frisch I, Jakob K, Kleemann J, Riegel D,
Bönisch U, et al: Genome-wide cooperation of EMT transcription
factor ZEB1 with YAP and AP-1 in breast cancer. EMBO J.
39:e1032092020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Lui KO and Zhou B: Reassessing
endothelial-to-mesenchymal transition in cardiovascular diseases.
Nat Rev Cardiol. 15:445–456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gorelova A, Berman M and Al Ghouleh I:
Endothelial-to-mesenchymal transition in pulmonary arterial
hypertension. Antioxid Redox Signal. 34:891–914. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu ZH, Zhang Y, Wang X, Fan XF, Zhang Y,
Li X, Gong YS and Han LP: SIRT1 activation attenuates cardiac
fibrosis by endothelial-to-mesenchymal transition. Biomed
Pharmacother. 118:1092272019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cancer Genome Atlas Research Network.
Electronic address, . simpleandrew_aguirre@dfci.harvard.edu;
Cancer Genome Atlas Research Network: Integrated genomic
characterization of pancreatic ductal adenocarcinoma. Cancer Cell.
32:185–203.e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ishii H, Iwatsuki M, Ieta K, Ohta D,
Haraguchi N, Mimori K and Mori M: Cancer stem cells and
chemoradiation resistance. Cancer Sci. 99:1871–1877. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Noguchi K, Eguchi H, Konno M, Kawamoto K,
Nishida N, Koseki J, Wada H, Marubashi S, Nagano H, Doki Y, et al:
Susceptibility of pancreatic cancer stem cells to reprogramming.
Cancer Sci. 106:1182–1187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Grun D, Adhikary G and Eckert RL: NRP-1
interacts with GIPC1 and SYX to activate p38 MAPK signaling and
cancer stem cell survival. Mol Carcinog. 58:488–499. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang J, Huang Y, Zhang J, Xing B, Xuan W,
Wang H, Huang H, Yang J and Tang J: NRP-2 in tumor
lymphangiogenesis and lymphatic metastasis. Cancer Lett.
418:176–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen L, Wang L, Yan J, Ma C, Lu J, Chen G,
Chen S, Su F, Wang W and Su X: 131I-labeled monoclonal antibody
targeting neuropilin receptor type-2 for tumor SPECT imaging. Int J
Oncol. 50:649–659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Miyauchi JT, Caponegro MD, Chen D, Choi
MK, Li M and Tsirka SE: Deletion of neuropilin 1 from microglia or
bone marrow-derived macrophages slows glioma progression. Cancer
Res. 78:685–694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Grandclement C and Borg C: Neuropilins: A
new target for cancer therapy. Cancers (Basel). 3:1899–1928. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peng K, Bai Y, Zhu Q, Hu B and Xu Y:
Targeting VEGF-neuropilin interactions: A promising antitumor
strategy. Drug Discov Today. 24:656–664. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang S, Zhang Z and Gao Q: Transfer of
microRNA-25 by colorectal cancer cell-derived extracellular
vesicles facilitates colorectal cancer development and metastasis.
Mol Ther Nucleic Acids. 23:552–564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang X, Hu H and Liu H: RNA binding
protein Lin28B confers gastric cancer cells stemness via directly
binding to NRP-1. Biomed Pharmacother. 104:383–389. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Piskounova E, Polytarchou C, Thornton JE,
LaPierre RJ, Pothoulakis C, Hagan JP, Iliopoulos D and Gregory RI:
Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct
mechanisms. Cell. 147:1066–1079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Y, Wang D, Zhou M, Chen H, Wang H, Min
J, Chen J, Wu S, Ni X, Zhang Y, et al: The KRAS/Lin28B axis
maintains stemness of pancreatic cancer cells via the let-7i/TET3
pathway. Mol Oncol. 15:262–278. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho
J, Yeom KH, Han J and Kim VN: TUT4 in concert with Lin28 suppresses
microRNA biogenesis through pre-microRNA uridylation. Cell.
138:696–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang L, Chen Y, Li C, Liu J, Ren H, Li L,
Zheng X, Wang H and Han Z: RNA binding protein PUM2 promotes the
stemness of breast cancer cells via competitively binding to
neuropilin-1 (NRP-1) mRNA with miR-376a. Biomed Pharmacother.
114:1087722019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jimenez-Hernandez LE, Vazquez-Santillan K,
Castro-Oropeza R, Martinez-Ruiz G, Muñoz-Galindo L, Gonzalez-Torres
C, Cortes-Gonzalez CC, Victoria-Acosta G, Melendez-Zajgla J and
Maldonado V: NRP1-positive lung cancer cells possess
tumor-initiating properties. Oncol Rep. 39:349–357. 2018.PubMed/NCBI
|
|
74
|
Gerstberger S, Hafner M and Tuschl T: A
census of human RNA-binding proteins. Nat Rev Genet. 15:829–845.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Uniewicz KA, Cross MJ and Fernig DG:
Exogenous recombinant dimeric neuropilin-1 is sufficient to drive
angiogenesis. J Biol Chem. 286:12–23. 2011. View Article : Google Scholar : PubMed/NCBI
|

















