Introduction
Tumors pose a serious threat to human health, and
their occurrence, progression and metastasis are influenced by
several factors. These include angiogenesis, which plays a crucial
role in tumor growth. The formation of new blood vessels leads to
the supply oxygen and nutrients to the tumor, contributing to its
growth and metastasis (1).
Consequently, inhibition of angiogenesis has emerged as a promising
approach for cancer treatment. At present, a number of
anti-angiogenic drugs, including bevacizumab and sorafenib are
available for clinical use (2).
However, the limited effectiveness and development of resistance in
certain patients have restricted the application of these drugs
(2). Therefore, novel targets are
being sought to identify an alternative strategy for the
development of more effective anti-angiogenic drugs.
Endothelial cell-specific molecule-1 (ESM1), also
known as endocan, is a proteoglycan that is released from cells and
circulates in the bloodstream (3).
Under normal physiological conditions, ESM1 is primarily expressed
in the bronchial epithelium, as well as in vascular endothelial
cells in the lung and kidneys (4).
However, under pathological conditions, ESM1 is aberrantly
expressed and interacts with its substrate proteins to promote
abnormal cell adhesion, proliferation and angiogenesis (5). During tumor development, ESM1 is
sporadically expressed in tumor cells and upregulated in tumor
vascular endothelial cells, thereby modulating angiogenesis
(6–10).
The present study is a comprehensive review of the
importance of ESM1 in tumor angiogenesis, which aims to summarize
the underlying mechanisms. By elucidating the role of ESM1 in tumor
angiogenesis, it is hoped that valuable ideas and directions will
be provided for the future development of more effective
anti-angiogenic therapies.
Structure and expression of ESM1
Structure of ESM1
ESM1 was initially isolated from a human umbilical
vein endothelial cell (HUVEC) cDNA library constructed by Lassalle
et al (11) in 1996. It is a
secreted proteoglycan composed of an 165-amino-acid polypeptide
covalently linked to a sulfated dermal polysaccharide chain
(11,12). The gene encoding human ESM1 is
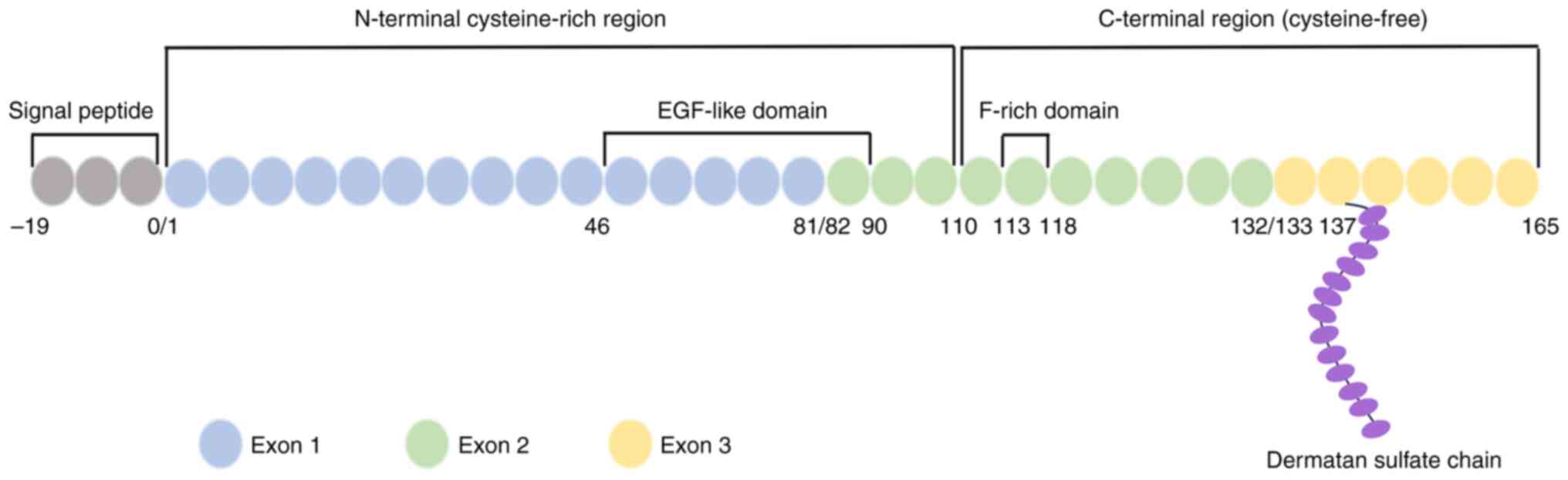
located on chromosome 5 at the q11.2 gene locus (13). This gene consists of three exons,
exons 1–3. Exon 1 encodes an N-terminal cysteine-rich region that
contains an epidermal growth factor (EGF)-like domain, while exon 2
encodes a phenylalanine-rich region that is considered to be
involved in ESM1 function. Exon 3 encodes a cysteine-free
C-terminal region of 33 amino acids, including a unique
O-glycosylation site at serine 137 (13,14).
The protein structure of ESM1 is illustrated in Fig. 1. The ESM1 protein detected in human
serum and HUVEC supernatants has a molecular weight of 50 kDa.
However, purified mature ESM1 obtained from the 293-ESM1 cell line
is a 165-amino-acid protein with a predicted molecular weight of
only 20 kDa, which indicates that post-translational modifications
occur when ESM1 is secreted (15).
The modification site has been identified to be serine 137, where a
single dermatan sulfate polysaccharide chain is bound (15).
Expression and regulation of ESM1
ESM1 is physiologically expressed in various tissues
with proliferative activity in the human body, including
gastrointestinal glandular, bronchial, pulmonary capillary,
glomerular and renal tubular tissues. Conversely, it is not
expressed in tissues without proliferative properties, including
large blood vessels and the spleen (5). However, expression and secretion of
ESM1 can become abnormal under pathological conditions. In diseases
associated with the endothelium, such as pneumonia, hypertension
and coronary heart disease, the serum levels of ESM1 can be
dysregulated; hence, ESM1 has been established as a potential
marker for endothelial dysfunction (4,16,17).
Furthermore, studies have revealed an association between aberrant
ESM1 expression and the development of various tumors. For example,
clinical investigations have observed increased ESM1 levels in
secretions or serum from patients with nasopharyngeal, endometrial
and ovarian carcinoma (18,19). In addition, elevated ESM1 expression
has been observed in cancer cells, including cervical cancer,
squamous cell cancer of the head and neck, esophageal cancer and
hepatocellular cancer cells (6,8,20,21).
Notably, ESM1 expression has been demonstrated to be increased in
the vascular endothelial cells of prolactinoma and gastric cancer,
indicating its involvement in tumor angiogenesis (22,23).
The expression of ESM1 is regulated by multiple
factors. Inflammatory factors such as tumor necrosis factor-α
(TNF-α), interleukin-1β (IL-1β) and lipopolysaccharide (LPS), as
well as hypoxia-inducible factor (HIF), vascular endothelial growth
factor (VEGF), high glucose, high salt and lead, can upregulate
ESM1 (24–26). By contrast, interferon-g,
platelet-derived growth factor, angiotensin 2, endothelin 1 and
insulin have inhibitory effects on ESM1 expression (27,28).
Processes of tumor angiogenesis
Regulation and major processes of
tumor angiogenesis
Angiogenesis in tumors is regulated by a balance of
pro- and anti-angiogenic factors. Neovascularization occurs when
the levels of pro-angiogenic factors exceed those of
anti-angiogenic factors (29).
Among pro-angiogenic factors, the VEGF family and
its receptors have been extensively studied. The VEGF family
includes VEGF subtypes A-E and placental growth factor, among which
VEGF-A plays a major role in the regulation of angiogenesis
(30). VEGF-A interacts with its
receptors, primarily VEGF receptors 1 and 2 (VEGFR1/2), with a
stronger affinity for VEGFR1 (31).
However, the binding of VEGF-A to VEGFR-2 promotes mitosis and the
permeability of vascular endothelial cells, leading to
angiogenesis, and serves as the principal regulatory mechanism in
angiogenesis (31). In the context
of tumors, endothelial cells have been found to exhibit autocrine
production of VEGF-A, while tumor cells exhibit paracrine secretion
of VEGF-A (32). VEGF-A has been
identified to be one of the most influential factors in tumor
angiogenesis (32).
In addition to the VEGF/VEGFR-2 pathway,
angiopoietin-2 (Ang2) and its receptor, which is known as Ang1
receptor or tyrosine-protein kinase receptor TEK, are also involved
in tumor angiogenesis. Ang2 expression is normally low in dormant
endothelial cells but can be upregulated by hypoxia, endothelial
cell activation and VEGF-A (33).
The upregulation of Ang2 expression in tumor endothelial cells
increases vascular endothelial cell permeability, leading to tissue
hypoxia and the subsequent upregulation of VEGF-A, thereby
promoting angiogenesis (33).
Furthermore, tumor cells have been shown to secrete
fibroblast growth factor (FGF) to stimulate endothelial cell
proliferation (34). In addition,
tumor-associated macrophages and fibroblasts secrete matrix
metalloproteinases (MMPs) that degrade the extracellular matrix
(ECM) and thereby facilitate tumor angiogenesis (35). These factors collectively contribute
to the complex regulation of angiogenesis in tumors.
There are four main steps in the formation of tumor
vessels. First, angiogenesis is initiated in response to
stimulation from hypoxia, inflammation and reactive oxygen species
in the tumor microenvironment (TME). Tumor cells release factors
such as VEGF and FGF to induce angiogenesis through paracrine
signaling (32). Endothelial cells,
in turn, respond by autocrine signaling with increased VEGF
production and Ang2 upregulation (33). Inflammatory, endothelial and stromal
cells, among others, secrete MMPs, specifically MMP-2, −9 and −14
(36).
Secondly, vascular sprouting occurs as MMPs degrade
the basement membrane (BM) and ECM. This weakens the connections
between pericytes and endothelial cells, allowing endothelial cells
in dormant vessels to sense angiogenic signals (36). In response, the endothelial cells at
the leading edge of capillary growth are converted into tip cells,
which are highly proliferative and can migrate towards the
angiogenic signals (36,37).
Thirdly, vascular lumen formation occurs. Stalk
cells, located behind the tip cells, proliferate and modify their
morphology to establish adherent and tight junctions with
neighboring endothelial cells. This process results in the
formation of the vascular lumen (38,39).
Finally, vascular maturation occurs. Once the
vascular lumen is formed, neovascularization occurs through a
series of signaling events that recruit pericytes and facilitate
the deposition of BMs. These processes contribute to the maturation
of the newly formed vasculature. The various processes by which
angiogenesis occurs in the TME are shown in Fig. 2.
 | Figure 2.Process of tumor angiogenesis. (A) In
the TME, angiogenesis is initiated by the release of VEGF and FGF
from tumor cells, the increased production of VEGF and Ang2 by
endothelial cells, as well as the secretion of MMPs by
inflammatory, endothelial, stromal and other types of cells. (B)
MMPs degrade the BM and ECM. This degradation weakens the
connections between pericytes and endothelial cells, allowing
endothelial cells in dormant vessels to sense angiogenic signals.
(C) Endothelial cells in the leading edge of capillary growth are
converted into tip cells. Stalk cells, located behind the tip
cells, proliferate and modify their morphology to establish
adherent and tight junctions with neighboring endothelial cells.
This process results in the formation of the vessel lumen. (D)
Vascular maturation occurs. The permeability of the new blood
vessels is increased, due to a reduction in pericytes, disruptions
in intercellular adherent and tight junctions, and abnormalities in
BM structure. The increased permeability provides favorable
conditions for tumor metastasis. TME, tumor microenvironment; VEGF,
vascular endothelial growth factor; FGF, fibroblast growth factor;
Ang2, angiotensin 2; MMPs, matrix metalloproteinases; BM, basement
membrane; ECM, extracellular matrix. |
Characteristics of tumor
neovascularization
The distribution, morphology, structure and function
of neovascularization in tumor tissues differ significantly from
those of normal physiological blood vessels. In tumors, the
distribution of blood vessels is not uniform, as a higher
micro-vessel density is generally observed in the central region
compared with the surrounding and normal tissues outside the tumor
(40). However, certain studies
have indicated that the blood vessel density within the tumor is
lower than that in the surrounding tissue due to the production of
anti-angiogenic factors by tumor cells and excessive matrix
deposition, leading to hypoxia (40,41).
In addition to being unevenly distributed within the
tumor, tumor blood vessels have distinct characteristics. They
often exhibit a curved morphology, disorganized vascular networks
and increased vascular permeability. This increased permeability is
attributed to a reduction in pericytes, disruptions in
intercellular adherent junctions and tight junctions, and
abnormalities in BM structure, including uneven thicknesses and
perforations (42–45). These features provide favorable
conditions for tumor metastasis, as shown in Fig. 2.
Regulation of ESM1 in tumor
angiogenesis
Interaction of ESM1 with tumor
angiogenesis factors
The role of ESM1 in angiogenesis has been
established in various studies. For example, it has been revealed
that ESM1 is specifically expressed in retinal endothelial tip
cells during mouse development (46). In experiments with ESM1 knockout
mice, it was observed that these cells had fewer filopodia and
reduced phosphorylation of extracellular signal-regulated protein
kinase (ERK) compared with those in wild-type mice, leading to
delayed vascular development (46).
Furthermore, ESM1 expression was found to be upregulated in mouse
models of hypoxia-induced retinal vascular neovascularization and
laser-induced choroidal vascular neovascularization (47). In addition, the administration of an
intravitreal injection of ESM1 neutralizing antibody to the mice
resulted in the significant downregulation of several vascular
neovascularization-associated factors, including VEGFR1, VEGFR2,
placental growth factor and MMP-2 (47).
The role of ESM1 in tumor angiogenesis has been
confirmed through in vivo and in vitro experiments.
For instance, a study showed that lung adenocarcinoma tumors
subcutaneous transplanted in ESM1 knockout mice exhibited reduced
neovascularization compared with those in wild-type mice (46). Similarly, the volume of
subcutaneously transplanted tumors formed by ESM1 knockout ovarian
cancer A2780 cells was smaller compared with these formed by
control A2780 cells (48). In in
vitro experiments, the tube formation ability of HUVECs was
observed to decrease when they were incubated with supernatants
from ESM1 knockdown A2780 cells (48). These findings suggested that ESM1
promotes angiogenesis in vivo and in vitro.
Further investigations into the mechanism of action
of ESM1 have shown its involvement in the regulation of several
angiogenic factors and receptors. For example, the knockdown of
ESM1 in GH3 and MMQ rat prolactinoma cell lines led to the reduced
expression of genes associated with angiogenesis, such as VEGFR2,
von Willebrand factor and EGF receptor (EGFR) (22). Similar results were obtained when
ESM1 was silenced in AN3CA and RL95-2 endometrial cancer cells,
which resulted in the downregulation of the angiogenic factor
receptors VEGFR1, VEGFR2 and EGFR, ultimately leading to reduced
angiogenesis, while ESM1 overexpression had the opposite effects
(49). ESM1 has been shown to
influence the major angiogenic factor VEGF, as the knockdown of
ESM1 decreased VEGF expression in human cervical cancer squamous
cell carcinoma cells (50).
Conversely, VEGF is able to regulate ESM1; in vivo
experiments with VEGF-A knockout mice or mice injected with VEGFR2
blockers showed a reduction in the ESM1 expression of the mice
(47). Studies have revealed that
the binding of VEGF-A to VEGFR2 leads to the phosphorylation of
VEGFR2 and the subsequent activation of downstream signaling
pathways that regulate ESM1 expression (47,51).
The interactions of ESM1 with factors associated with tumor
angiogenesis are shown in Table I
and Fig. 3.
 | Figure 3.Roles and mechanisms of ESM1 in tumor
angiogenesis. Solid lines indicate demonstrated mechanisms and
dashed lines indicate possible mechanisms. ESM1 activates the NF-κB
pathway in two ways: Via the upregulation of TNF-α and IL-8, and
via promotion of binding between VEGF and VEGFR2. The activated
NF-κB upregulates VEGF via the transcription factor HIF-1α, thereby
promoting angiogenesis. ESM1 also activates the PI3K/AKT/mTOR
pathway to upregulate MMPs, VEGF and HIF-1α by facilitating the
binding of VEGF with VEGFR2. Regarding EGF and its receptor EGFR,
ESM1 not only directly binds to EGFR but also facilitates the
binding of EGF to EGFR, leading to activation of the EGFR signaling
pathway. The activated EGFR, in turn, increases ESM1 expression
through the JAK/STAT3 and ERK/ELK pathways, establishing a
regulatory loop between ESM1 and EGFR. Increased ESM1 also
upregulates angiogenesis-related factors such as VEGFR1, VEGFR2,
EGFR and VWF, thereby promoting angiogenesis. In addition,
activated EGFR can upregulate HIF-1α and MMPs through the
PI3K/AKT/mTOR pathway. In the MAPK pathway, ESM1 increases the
expression of VEGF through ERK, JNK and p38. As for the Notch
pathway, the ligand DLL4 is upregulated by ESM1, thereby inhibiting
VEGF expression. ESM1, endothelial cell specific molecule-1; NF-κB,
nuclear factor-κB; TNF-α, tumor necrosis factor-α; IL-8,
interleukin-8; VEGF, vascular endothelial growth factor; VEGFR1/2,
VEGF receptor 1/2; PI3K, phosphoinositide 3-kinase; mTOR, mammalian
target of rapamycin; MMPs, matrix metalloproteinases; HIF-1α,
hypoxia-inducible factor-1α; EGF, epidermal growth factor; EGFR,
EGF receptor; JAK, Janus kinase; STAT3, signal transducer and
activator of transcription 3; ERK, extracellular signal-regulated
kinase; ELK, ETS-like kinase; JNK, c-Jun N-terminal kinase; VWF,
von Willebrand factor; DLL4, d-like ligand 4; P, phosphorylated;
TAB1-3, TAK1-binding protein 1–3; TAK1, transforming growth
factor-b-activated kinase 1; RAS, rat sarcoma; RAF, rapidly
accelerated fibrosarcoma; MEK/MKK, mitogen-activated protein kinase
kinase; NICD, Notch intracellular domain. |
 | Table I.Tumor angiogenesis factors and
signaling pathways regulated by ESM1. |
Table I.
Tumor angiogenesis factors and
signaling pathways regulated by ESM1.
| First author/s,
year | In vivo or
in vitro | ESM1
expression | Biological
importance | ESM1-regulated
genes/proteins/pathway | (Refs.) |
|---|
| Cai et al,
2016 | In
vitro | Down | Inhibits the
viability of GH3 and MMQ rat prolactinoma cell lines | VEGFR2; VWF | (22) |
| Rocha et al,
2014 | In vivo and
in vitro | Down | Inhibits retinal
vascular outgrowth | VEGF; ERK | (46) |
| Su et al,
2018 | In
vitro | Up | Promotes retinal
neovascularization | VEGFR1, VEGFR2,
PIGF, MMP-2; ERK; P38 | (47) |
| Li et al,
2023 | In vivo and
in vitro | Down | Inhibits ovarian
cancer angiogenesis | VEGF;
PI3K/Akt/mTOR | (48) |
| He et al,
2022 | In
vitro | Up | Promotes
endometrial cancer angiogenesis | VEGFR1, VEGFR2,
EGFR | (49) |
| Li, 2023 | In
vitro | Down | Inhibits
proliferation, invasion and apoptosis of SiHa and ME-180 cervical
squamous cell carcinoma cell lines | VEGF | (50) |
| Yang et al,
2023 | In vivo and
in vitro | Up | Promotes colorectal
cancer angiogenesis | MMP-2, MMP-3,
MMP-9, VEGF, COX-2, HIF-1α; PI3K/Akt/mTOR | (53) |
| Yang et al,
2020 | In vitro and
in vivo | Up | Promotes non-small
cell lung cancer growth | EGF; EGFR;
JAK/STAT3; ERK/ELK | (55) |
| Lee et al,
2014 | In vitro and
in vivo | Up | Promotes vascular
cell permeability | VEGFR1, VEGFR2,
MAPK (ERK, JNK, P38) | (58) |
| Kang et al,
2022 | In vivo and
n vitro | Up/down | Promotes/inhibits
breast cancer angiogenesis | VEGF; DLL4 | (63) |
| Huang et al,
2021 | In
vitro | Down | Inhibits SW13 human
adrenocortical carcinoma cell line growth | DLL4 | (64) |
| Kumar and Mani,
2021 | In
vitro | Up | Promotes NO and ROS
in endothelial cells | NF-κB | (68) |
In summary, ESM1 promotes angiogenesis via the
regulation of angiogenic factors, and there is a reciprocal
relationship between ESM1 and angiogenic factors, which form a
positive feedback loop.
Role of ESM1 in the signaling pathway
of tumor angiogenesis
Limited research has been conducted on the specific
signaling pathways by which ESM1 regulates tumor angiogenesis.
However, existing studies suggest the involvement of several
signaling pathways, including the phosphoinositide 3-kinase
(PI3K)/AKT, mitogen-activated protein kinase (MAPK), Notch and
nuclear factor-κB (NF-κB) pathways.
PI3K/AKT signaling pathway
PI3K and AKT play a critical role in various
processes, including tumor growth, metabolism and angiogenesis
(52). ESM1 has been found to act
through the PI3K/AKT pathway during angiogenesis. For example, it
was found that when HUVECs were cultured with the supernatants of
SW480 and SW620 colorectal cancer cells transfected with ESM1
mimic, their tube-forming ability was increased (53). Further experiments demonstrated that
the transfection of these cancer cells with ESM1 mimic upregulated
the expression of MMP-2, MMP-3, MMP-9, VEGF, cyclooxygenase-2 and
HIF-1α through the PI3K/AKT/mammalian target of rapamycin (mTOR)
signaling pathway (53).
Considering that ESM1 increases the binding of VEGF to VEGFR2
(46), and PI3K/AKT/mTOR is
downstream of the VEGF/VEGFR2 pathway (54), this suggests that ESM1 activates the
PI3K/AKT/mTOR pathway via the phosphorylation of VEGFR2,
consequently upregulating the aforementioned angiogenesis-related
factors. In a study of non-small cell lung cancer cells, it was
observed that recombinant ESM1 directly bound to EGFR and
facilitated the binding of EGF to EGFR, leading to activation of
the EGFR signaling pathway (55).
The activated EGFR, in turn, was indicated to upregulate ESM1 via
Janus kinase/signal transducer and activator of transcription 3
signaling and ERK/ETS-like kinase, thereby establishing a
regulatory loop between ESM1 and EGFR (55). In addition, another study revealed
that the activated EGFR pathway sustains angiogenesis in solid
tumors through the downstream PI3K/AKT/mTOR signaling pathway
(56). Summaries of the mechanism
by which ESM1 regulates tumor angiogenesis via the PI3K/AKT
signaling pathway are shown in Table
I and Fig. 3.
MAPK signaling pathway
The MAPK pathway, which includes the ERK, c-Jun
N-terminal kinase (JNK) and p38 signaling pathways, plays a crucial
role in a number of cellular processes, including cell
proliferation, differentiation and apoptosis, angiogenesis and
tumor metastasis (57). In
vivo and in vitro experiments have confirmed that ESM1
regulates angiogenesis through the MAPK pathway. For example, in
vivo experiments conducted using ESM1 knockout mice revealed a
reduction in phosphorylated ERK in the retina, which led to delayed
vascular development (46). In
addition, in vitro experiments involving the stimulation of
human retinal endothelial cells and HUVECs with recombinant ESM1
demonstrated the activation of MAPK (ERK, JNK and p38) signaling
pathways associated with angiogenesis (47,58),
which further confirms the relationship between ESM1 and the MAPK
signaling pathway. Phosphorylated ERK, JNK and p38 can translocate
into the nucleus and induce VEGF production, thereby regulating
angiogenesis (57,59,60).
Furthermore, since ESM1 maintains tumor angiogenesis through
regulation of the EGFR signaling pathway, and rat sarcoma/rapidly
accelerated fibrosarcoma/mitogen-activated protein kinase
kinase/ERK and PI3K/AKT are the main signaling pathways downstream
of EGFR (55,61), ESM1 can also regulate these
pathways. Summaries of how ESM1 regulates tumor angiogenesis
through the MAPK signaling pathway are shown in Table I and Fig. 3.
Notch signaling pathway
The Notch signaling pathway plays a critical role in
normal cell differentiation, proliferation, apoptosis and
angiogenesis under physiological conditions (62). In pathological conditions, it also
regulates angiogenesis. For instance, ESM1 overexpression in the
MDA-MB-231 human breast cancer cell line and mice enhances
angiogenesis via the upregulation of VEGF and d-like ligand 4
(DLL4, a ligand of Notch1 in the Notch signaling) (63). In addition, ESM1 activates the
DLL4/Notch signaling pathway in adrenocortical carcinoma cells
(64) (Table I and Fig. 3). It is worth noting that activation
of the DLL4/Notch pathway inhibits VEGF-induced neovascularization
(65). This may appear
contradictory to the angiogenic role of ESM1; however, it has been
suggested that activation of the DLL4/Notch pathway contributes to
vessel sprouting (66). Since the
number of studies investigating the regulatory mechanisms of ESM1
in the DLL4/Notch pathway is limited, the precise role of the
DLL4/Notch pathway in ESM1-mediated angiogenesis regulation remains
unclear, emphasizing that further research is necessary.
NF-κB signaling pathway
The NF-κB pathway is crucial in tumor angiogenesis
(67). ESM1 has been shown to
regulate tumor angiogenesis through the NF-κB pathway. Recombinant
ESM1 has been shown to activate NF-κB in HUVECs (68), and it is known that NF-κB is
downstream of PI3K/AKT (69).
Therefore, it is speculated that ESM1 activates NF-κB through the
PI3K/AKT pathway. Furthermore, ESM1 upregulates inflammatory
factors such as TNF-α and IL-8, both of which can activate NF-κB
(58,70,71).
Furthermore, NF-κB upregulates VEGF via HIF-1α, leading to
angiogenesis (72). The regulation
of tumor angiogenesis by ESM1 through the NF-κB signaling pathway
is summarized in Table I and
Fig. 3.
Interaction of ESM1 with the TME
Mutual reinforcement between hypoxia
and ESM1
In addition to tumor cells, the TME comprises
various components, including fibroblasts, endothelial cells,
immune cells and ECM (73). Rapid
and uncontrolled tumor growth, coupled with inadequate blood
supply, leads to the TME typically being hypoxic (74). Hypoxia is mainly characterized by
the production of HIF (75). Among
the HIF family, HIF-1α is the primary transcription factor
associated with hypoxia (76).
Studies have confirmed the existence of a regulatory relationship
between HIF-1α and ESM1. For example, when HUVECs were exposed to
intermittent hypoxia, ESM1 expression was significantly increased
in a time-dependent manner. However, the knockdown of HIF-1α
expression led to a reduction in ESM1 levels under these conditions
(77). The regulation of ESM1
expression by HIF-1α was shown to be mediated VEGF (77), as shown in Fig. 4. In addition, in human umbilical
artery endothelial cells, HIF was found to facilitate the
translocation of the transcription factor Forkhead box O1 (FoxO1)
from the cytoplasm to the nucleus in response to hypoxia, thereby
upregulating ESM1 levels (78), as
shown in Fig. 4. These findings
indicate that HIF-1α indirectly regulates ESM1 through VEGF and
FoxO1. Moreover, ESM1 has been found to regulate HIF-1α. In
vitro experiments demonstrated that ESM1 promoted drug
resistance, proliferation, migration, invasion and
epithelial-mesenchymal transition through HIF-1α in A549 and PC-9
non-small cell lung cancer cells following intermittent hypoxia
(79). Similar results were
observed in a mouse model of lung cancer in which the mice were
exposed to chronic intermittent hypoxia and a small interfering RNA
targeting ESM1 was delivered using a lentivirus (79), as shown in Fig. 4. It is evident that HIF-1α and ESM1
mutually regulate each other, forming a positive feedback loop. In
summary, hypoxia induces the expression of ESM1 in the TME, and the
elevation of ESM1 further exacerbates tissue hypoxia, ultimately
promoting angiogenesis.
 | Figure 4.Interaction of ESM1 with hypoxia and
inflammation in the tumor microenvironment. Under hypoxia, HIF-1α
upregulates ESM1 by promoting VEGF expression and facilitating the
entry of the transcription factor FoxO1 into the nucleus; Notably,
ESM1 can also accelerate hypoxia via the upregulation of HIF-1α.
Similarly, following stimulation by inflammatory factors, such as
LPS, TNF-α, IL-1β, IL-8 and MCP-1, the level of ESM1 is increased.
ESM1 also increases the expression of TNF-α, IL-8, MCP-1, VCAM-1
and ICAM-1. ESM1, endothelial cell specific molecule-1; HIF-1α,
hypoxia-inducible factor-1α; VEGF, vascular endothelial growth
factor; FoxO1, Forkhead box O1; LPS, lipopolysaccharide; TNF-α,
tumor necrosis factor-α; IL, interleukin; MCP-1, monocyte
chemotactic protein-1; VCAM-1, vascular cell adhesion molecule-1;
ICAM-1, intercellular cell adhesion molecule-1. |
Mutual reinforcement between
inflammation and ESM1
Inflammation is a prominent feature of the TME.
Within the TME, inflammatory cells play a pivotal role in tumor
development, growth and metastasis through the secretion of
chemokines, inflammatory factors and growth factors (80,81). A
link between ESM1 and inflammation has been shown in several
studies. Clinical investigations have identified that ESM1 levels
are elevated in the plasma or tissue of patients afflicted with
various inflammatory diseases, including acute respiratory distress
syndrome, sepsis and rheumatoid arthritis (82–84).
In addition, experimental research has substantiated the
association between high ESM1 levels and inflammatory responses,
since in vitro assays using HUVECs and in vivo
experiments using mice revealed increased levels of ESM1 following
treatment with LPS (58).
ESM1 levels have also been found to increase in
HUVECs following stimulation with IL-8, monocyte chemotactic
protein-1 (MCP-1) and TNF-α (58),
as depicted in Fig. 4. As
aforementioned and illustrated in Fig.
3, ESM1 activates HUVECs through the MAPK pathways, namely ERK,
JNK and p38, and the NF-κB pathway (58). Furthermore, the treatment of HUVECs
with IL-1β results in the induction of ESM1 expression (11), as shown in Fig. 4. It has also been shown that ESM1
can influence the expression of inflammatory factors, chemokines
and adhesion molecules. Upon stimulation with LPS and IL-1β, HUVECs
and trophoblast cells exhibited significantly increased expression
of IL-6, IL-8 and MCP-1, but when ESM1 was knocked down using small
interfering RNA, the stimulatory effect of IL-6, IL-8 and MCP-1
expression was diminished in both cell types (85), as depicted in Fig. 4.
In another study, the culture of HUVECs with ESM1
for 3 h led to the upregulation of IL-8, MCP-1 and TNF-α expression
(58). Furthermore, ESM1 also
upregulated vascular cell adhesion molecule-1 (VCAM-1) and
intercellular cell adhesion molecule-1 (ICAM-1) (58), as shown in Fig. 4. Conversely, ESM1 knockdown reduced
VCAM-1 and ICAM-1 protein expression in mouse aortic vascular
smooth muscle cells (26). These
findings indicate that a reciprocal regulatory relationship exists
between specific inflammatory factors and ESM1; inflammatory
factors upregulate ESM1 expression, while ESM1 induces the
expression of factors associated with inflammation, thereby
exacerbating inflammation within the TME and promoting
angiogenesis.
Value of ESM1 as a future therapeutic target
for cancer
Current research progress of ESM1
As a novel marker of cancer, ESM1 has been studied
extensively. Research has shown that ESM1 is involved in the
process of tumor development in two ways: Firstly, it is abnormally
expressed in cancer cells and participates in tumor cell
proliferation, migration and invasion (6,8,20,21);
secondly, its expression is increased in vascular endothelial
cells, which is associated with the promotion of cancer
angiogenesis (22,23). Most of these findings have been
obtained from experiments using cells or animals. However, only the
abnormal expression of ESM1 in tumor tissues has been confirmed in
clinical trials (18,19). Although ESM1 has been shown to be
closely associated with tumorigenesis, no interventional agents
targeting ESM1 are currently being used in the clinic.
Based on the association between ESM1 and tumors,
and the lack of clarity regarding the mechanism of ESM1 in the
development of cancer, it is necessary to further clarify its
mechanism through various studies in the future, particularly in
clinical research. Following this, the development of novel
antitumor drugs that target ESM1 may be possible.
Value of ESM1 as a prognostic and
predictive indicator of cancer
The relationship of ESM1 with tumor growth,
metastasis and patient prognosis has garnered significant attention
within the medical community. Clinical investigations have
consistently confirmed the abnormal expression of ESM1 in the blood
or tumor tissues of patients with diverse cancers. Notably,
elevated ESM1 levels have been observed in the circulatory systems
of patients diagnosed with endometrial, cervical, renal, breast and
lung cancer, multiple myeloma and hepatocellular carcinoma
(19,86–90).
Furthermore, upregulated ESM1 expression has been detected in tumor
samples collected from patients with pancreatic neuroendocrine
tumors and glioblastoma (91,92).
A meta-analysis exploring the association between
ESM1 expression and the prognosis of patients with cancer has
unveiled a significant negative association between ESM1 levels and
patient survival rate (93).
Consequently, ESM1 has emerged as a promising novel tumor marker
for the evaluation of treatment efficacy, monitoring of disease
progression and assessment of prognosis in patients with cancer.
The practicality of using ESM1 in clinical settings is bolstered by
its status as a secreted protein readily detectable in blood
samples. In fact, commercial enzyme-linked immunosorbent assay kits
designed for the detection of ESM1 have already entered the market,
which further substantiates the feasibility of using ESM1 as a
cancer marker in future clinical practice.
Therapeutic strategies and drug
development for ESM1
Given the pivotal role of ESM1 in tumor development
and metastasis, it has emerged as a promising therapeutic target
for cancer. Future therapeutic strategies for ESM1 may include the
following: i) Inhibiting the synthesis and secretion of ESM1; ii)
neutralizing the already-produced ESM1, and iii) blocking the
downstream signaling pathways activated by ESM1.
Research has clearly shown that factors such as
inflammation and hypoxia can stimulate the expression and release
of ESM1 (58,77). Consequently, the use of
anti-inflammatory drugs and inhibitors targeting HIF-1α is a viable
approach for impeding the synthesis and secretion of ESM1.
Monoclonal antibodies designed for the neutralization of ESM1 have
not yet been developed; however, the search for ESM1-neutralizing
antibodies is a promising direction for future drug development
efforts. Furthermore, ESM1 has been shown to activate downstream
signaling pathways, including the PI3K/AKT, MAPK and NF-κB pathways
(53,58,68).
Inhibitors targeting these pathways may serve as an additional
means for blocking the downstream effects mediated by ESM1.
Conclusions and prospects
The present review reveals the multifaceted roles of
ESM1 and the underlying mechanisms by which ESM1 promotes tumor
angiogenesis. It also comprehensively summarizes the regulatory
mechanisms governing ESM1 within the TME. In summary, it describes
how ESM1 expression is increased in response to hypoxic and
inflammatory conditions within the TME. This heightened ESM1
expression, in turn, orchestrates the expression of pro-angiogenic
factors through the PI3K/AKT, MAPK, NF-κB and Notch signaling
pathways, thereby inducing angiogenesis.
Furthermore, the review introduces the concept that
ESM1 actively engages with the TME. ESM1 expression is increased
within the TME and, notably, ESM1 reciprocally promotes the
progression and maintenance of the TME via the regulation of
inflammatory factors, thereby establishing a positive feedback loop
between ESM1 and the TME.
As an emerging tumor marker, ESM1 plays a pivotal
role in tumor growth, metastasis, invasion and angiogenesis.
Consequently, it is a promising biomarker for disease monitoring
and a potential therapeutic target for clinical practice in the
future. However, it is worth noting that the research on the role
of ESM1 in angiogenesis remains limited, and the precise mechanisms
by which ESM1 regulates angiogenesis require further exploration in
future studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JZ and PZ were responsible for drafting and revising
the manuscript, and creating the figures. JW and JS revised the
manuscript. Data authentication is not applicable. All authors have
read and approved the final version of the manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qi S, Deng S, Lian Z and Yu K: Novel drugs
with high efficacy against tumor angiogenesis. Int J Mol Sci.
23:69342022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan KF, Yang YC, Lee WJ, Hua KT and Chien
MH: Proteoglycan endocan: A multifaceted therapeutic target in
Cancer. Biochim Biophys Acta Rev Cancer. 1877:1886722022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Jiang L, Yu XH, Hu M, Zhang YK,
Liu X, He P and Ouyang X: Endocan: A key player of cardiovascular
disease. Front Cardiovasc Med. 8:7986992022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang SM, Zuo L, Zhou Q, Gui SY, Shi R, Wu
Q, Wei W and Wang Y: Expression and distribution of endocan in
human tissues. Biotech Histochem. 87:172–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu J, Liu Q, Zhu L, Liu Y, Zhu X, Peng S,
Chen M and Li P: Endothelial cell-specific molecule 1 drives
cervical cancer progression. Cell Death Dis. 13:10432022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu W, Yang Y, He B, Ma F, Sun F, Guo M,
Zhang M and Dong Z: ESM1 promotes triple-negative breast cancer
cell proliferation through activating AKT/NF-κB/Cyclin D1 pathway.
Ann Transl Med. 9:5332021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Yang D, Zhang C, Wei S, Zhao R, Dai
S and Shan B: ESM1 is a promising therapeutic target and prognostic
indicator for esophageal Carcinogenesis/Esophageal squamous cell
carcinoma. Biomed Res Int. 2022:53281922022.PubMed/NCBI
|
|
9
|
Abid MR, Yi X, Yano K, Shih SC and Aird
WC: Vascular endocan is preferentially expressed in tumor
endothelium. Microvasc Res. 72:136–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen LY, Liu X, Wang SL and Qin CY:
Over-expression of the Endocan gene in endothelial cells from
hepatocellular carcinoma is associated with angiogenesis and tumour
invasion. J Int Med Res. 38:498–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lassalle P, Molet S, Janin A, Heyden JV,
Tavernier J, Fiers W, Devos R and Tonnel AB: ESM-1 is a novel human
endothelial cell-specific molecule expressed in lung and regulated
by cytokines. J Biol Chem. 271:20458–20464. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kali A and Shetty KS: Endocan: A novel
circulating proteoglycan. Indian J Pharmacol. 46:579–583. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarrazin S, Adam E, Lyon M, Depontieu F,
Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P and
Delehedde M: Endocan or endothelial cell specific molecule-1
(ESM-1): A potential novel endothelial cell marker and a new target
for cancer therapy. Biochim Biophys Acta. 1765:25–37.
2006.PubMed/NCBI
|
|
14
|
Delehedde M, Devenyns L, Maurage CA and
Vivès RR: Endocan in cancers: A lesson from a circulating dermatan
sulfate proteoglycan. Int J Cell Biol. 2013:7050272013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Béchard D, Gentina T, Delehedde M,
Scherpereel A, Lyon M, Aumercier M, Vazeux R, Richet C, Degand P,
Jude B, et al: Endocan is a novel chondroitin sulfate/dermatan
sulfate proteoglycan that promotes hepatocyte growth factor/scatter
factor mitogenic activity. J Biol Chem. 276:48341–48349. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Freitas Caires N, Gaudet A, Portier L,
Tsicopoulos A, Mathieu D and Lassalle P: Endocan, sepsis,
pneumonia, and acute respiratory distress syndrome. Crit Care.
22:2802018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leite AR, Borges-Canha M, Cardoso R, Neves
JS, Castro-Ferreira R and Leite-Moreira A: Novel biomarkers for
evaluation of endothelial dysfunction. Angiology. 71:397–410. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu PH, Chou SF, Chen CL, Hung H, Lai CY,
Yang PM, Jeng YM, Liaw SF, Kuo HH, Hsu HC, et al: Upregulation of
endocan by Epstein-Barr virus latent membrane protein 1 and its
clinical significance in nasopharyngeal carcinoma. PLoS One.
8:e822542013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laloglu E, Kumtepe Y, Aksoy H and Topdagi
Yilmaz EP: Serum endocan levels in endometrial and ovarian cancers.
J Clin Lab Anal. 31:e220792017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Chen X and Huang Z: Identification
of ESM1 overexpressed in head and neck squamous cell carcinoma.
Cancer Cell Int. 19:1182019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baghy K, Tátrai P, Regős E and Kovalszky
I: Proteoglycans in liver cancer. World J Gastroenterol.
22:379–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai L, Leng ZG, Guo YH, Lin SJ, Wu ZR, Su
ZP, Lu JL, Wei LF, Zhuge QC, Jin K and Wu ZB: Dopamine agonist
resistance-related endocan promotes angiogenesis and cells
viability of prolactinomas. Endocrine. 52:641–651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang Y, Niu W, Lian PL, Wang XQ, Meng ZX,
Liu Y and Zhao R: Endocan-expressing microvessel density as a
prognostic factor for survival in human gastric cancer. World J
Gastroenterol. 22:5422–5429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Shen YW, Zhang LJ, Chen JJ, Bian
HT, Gu WJ, Zhang H, Chen HZ, Zhang WD and Luan X: Targeting
endothelial cell-specific molecule 1 protein in cancer: A promising
therapeutic approach. Front Oncol. 11:6871202021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zolali E, Rezabakhsh A, Nabat E, Jaberi H,
Rahbarghazi R and Garjani A: Metformin effect on Endocan biogenesis
in human endothelial cells under diabetic condition. Arch Med Res.
50:304–314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng M, Xie Z, Zhang J, Li S, Wu Y and Yan
X: Arctigenin attenuates vascular inflammation induced by high salt
through TMEM16A/ESM1/VCAM-1 pathway. Biomedicines. 10:27602022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hortal J, Giannella M, Pérez MJ, Barrio
JM, Desco M, Bouza E and Muñoz P: Incidence and risk factors for
ventilator-associated pneumonia after major heart surgery.
Intensive Care Med. 35:1518–1525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daly C, Pasnikowski E, Burova E, Wong V,
Aldrich TH, Griffiths J, Ioffe E, Daly TJ, Fandl JP, Papadopoulos
N, et al: Angiopoietin-2 functions as an autocrine protective
factor in stressed endothelial cells. Proc Natl Acad Sci USA.
103:15491–15496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baeriswyl V and Christofori G: The
angiogenic switch in carcinogenesis. Semin Cancer Biol. 19:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferrara N and Henzel WJ: Pituitary
follicular cells secrete a novel heparin-binding growth factor
specific for vascular endothelial cells. Biochem Biophys Res
Commun. 161:851–858. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duran CL, Borriello L, Karagiannis GS,
Entenberg D, Oktay MH and Condeelis JS: Targeting Tie2 in the tumor
microenvironment: From Angiogenesis to Dissemination. Cancers
(Basel). 13:57302021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: The prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:32492018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rust R, Gantner C and Schwab ME: Pro- and
antiangiogenic therapies: Current status and clinical implications.
FASEB J. 33:34–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Phng LK, Stanchi F and Gerhardt H:
Filopodia are dispensable for endothelial tip cell guidance.
Development. 140:4031–4040. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barău A, Ruiz-Sauri A, Valencia G,
Gómez-Mateo Mdel C, Sabater L, Ferrandez A and Llombart-Bosch A:
High microvessel density in pancreatic ductal adenocarcinoma is
associated with high grade. Virchows Arch. 462:541–546. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Di Maggio F, Arumugam P, Delvecchio FR,
Batista S, Lechertier T, Hodivala-Dilke K and Kocher HM: Pancreatic
stellate cells regulate blood vessel density in the stroma of
pancreatic ductal adenocarcinoma. Pancreatology. 16:995–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morikawa S, Baluk P, Kaidoh T, Haskell A,
Jain RK and McDonald DM: Abnormalities in pericytes on blood
vessels and endothelial sprouts in tumors. Am J Pathol.
160:985–1000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dejana E, Tournier-Lasserve E and
Weinstein BM: The control of vascular integrity by endothelial cell
junctions: Molecular basis and pathological implications. Dev Cell.
16:209–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baluk P, Morikawa S, Haskell A, Mancuso M
and McDonald DM: Abnormalities of basement membrane on blood
vessels and endothelial sprouts in tumors. Am J Pathol.
163:1801–1815. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rocha SF, Schiller M, Jing D, Li H, Butz
S, Vestweber D, Biljes D, Drexler HC, Nieminen-Kelhä M, Vajkoczy P,
et al: Esm1 modulates endothelial tip cell behavior and vascular
permeability by enhancing VEGF bioavailability. Circ Res.
115:581–590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Su T, Zhong Y, Demetriades AM, Shen J, Sui
A, Yao Y, Gao Y, Zhu Y, Shen X and Xie B: Endocan blockade
suppresses experimental ocular neovascularization in mice. Invest
Ophthalmol Vis Sci. 59:930–939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li YK, Zeng T, Guan Y, Liu J, Liao NC,
Wang MJ, Chen KX, Luo XY, Chen CY, Quan FF, et al: Validation of
ESM1 related to ovarian cancer and the biological function and
prognostic significance. Int J Biol Sci. 19:258–280. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He Y, Lin L, Ou Y, Hu X, Xu C and Wang C:
Endothelial cell-specific molecule 1 (ESM1) promoted by
transcription factor SPI1 acts as an oncogene to modulate the
malignant phenotype of endometrial cancer. Open Med (Wars).
17:1376–1389. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li D, Su X, Xue S, Yao L, Yu D, Tang X and
Huang Y: Targeting ESM1/VEGFα signaling axis: A promising
therapeutic avenue for angiogenesis in cervical squamous cell
carcinoma. J Cancer. 14:1725–1735. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roudnicky F, Poyet C, Wild P, Krampitz S,
Negrini F, Huggenberger R, Rogler A, Stöhr R, Hartmann A,
Provenzano M, et al: Endocan is upregulated on tumor vessels in
invasive bladder cancer where it mediates VEGF-A-induced
angiogenesis. Cancer Res. 73:1097–1106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shahcheraghi SH, Tchokonte-Nana V, Lotfi
M, Lotfi M, Ghorbani A and Sadeghnia HR: Wnt/beta-catenin and
PI3K/Akt/mTOR signaling pathways in Glioblastoma: Two main targets
for drug design: A Review. Curr Pharm Des. 26:1729–1741. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang L, Dong Z, Li S and Chen T: ESM1
promotes angiogenesis in colorectal cancer by activating
PI3K/Akt/mTOR pathway, thus accelerating tumor progression. Aging
(Albany NY). 15:2920–2936. 2023.PubMed/NCBI
|
|
54
|
Namjoo M, Ghafouri H, Assareh E, Aref AR,
Mostafavi E, Hamrahi Mohsen A, Balalaie S, Broussy S and Asghari
SM: A VEGFB-based peptidomimetic inhibits VEGFR2-Mediated
PI3K/Akt/mTOR and PLCγ/ERK signaling and elicits apoptotic,
antiangiogenic, and antitumor activities. Pharmaceuticals (Basel).
16:9062023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang YC, Pan KF, Lee WJ, Chang JH, Tan P,
Gu CC, Chang WM, Yang SF, Hsiao M, Hua KT and Chien MH: Circulating
proteoglycan endocan mediates EGFR-Driven progression of non-small
cell lung cancer. Cancer Res. 80:3292–3304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rao L, Giannico D, Leone P, Solimando AG,
Maiorano E, Caporusso C, Duda L, Tamma R, Mallamaci R, Susca N, et
al: HB-EGF-EGFR signaling in bone marrow endothelial cells mediates
angiogenesis associated with multiple myeloma. Cancers (Basel).
12:1732020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
58
|
Lee W, Ku SK, Kim SW and Bae JS: Endocan
elicits severe vascular inflammatory responses in vitro and in
vivo. J Cell Physiol. 229:620–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guma M, Rius J, Duong-Polk KX, Haddad GG,
Lindsey JD and Karin M: Genetic and pharmacological inhibition of
JNK ameliorates hypoxia-induced retinopathy through interference
with VEGF expression. Proc Natl Acad Sci USA. 106:8760–8765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Volpi G, Facchinetti F, Moretto N, Civelli
M and Patacchini R: Cigarette smoke and α,β-unsaturated aldehydes
elicit VEGF release through the p38 MAPK pathway in human airway
smooth muscle cells and lung fibroblasts. Br J Pharmacol.
163:649–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou J, Ji Q and Li Q: Resistance to
anti-EGFR therapies in metastatic colorectal cancer: Underlying
mechanisms and reversal strategies. J Exp Clin Cancer Res.
40:3282021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang N, Hu Y, Wang M, Zhao Z and Li M:
The notch signaling pathway contributes to angiogenesis and tumor
immunity in breast cancer. Breast Cancer (Dove Med Press).
14:291–309. 2022.PubMed/NCBI
|
|
63
|
Kang N, Liang X, Fan B, Zhao C, Shen B, Ji
X and Liu Y: Endothelial-Specific Molecule 1 inhibition lessens
productive angiogenesis and tumor metastasis to overcome
bevacizumab resistance. Cancers (Basel). 14:56812022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang YG, Wang Y, Zhu RJ, Tang K, Tang XB
and Su XM: EMS1/DLL4-Notch signaling axis augments cell
cycle-mediated tumorigenesis and progress in human adrenocortical
carcinoma. Front Oncol. 11:7715792021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yan M and Plowman GD: Delta-like 4/Notch
signaling and its therapeutic implications. Clin Cancer Res.
13:7243–7246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pitulescu ME, Schmidt I, Giaimo BD,
Antoine T, Berkenfeld F, Ferrante F, Park H, Ehling M, Biljes D,
Rocha SF, et al: Dll4 and Notch signalling couples sprouting
angiogenesis and artery formation. Nat Cell Biol. 19:915–927. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gilmore TD: NF-κB and human cancer: What
have we learned over the Past 35 Years? Biomedicines. 9:8892021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kumar SK and Mani KP: Endocan alters
nitric oxide production in endothelial cells by targeting AKT/eNOS
and NFkB/iNOS signaling. Nitric Oxide. 117:26–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yu M, Qi B, Xiaoxiang W, Xu J and Liu X:
Baicalein increases cisplatin sensitivity of A549 lung
adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed
Pharmacother. 90:677–685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang D, Li H, Luo X, Liu D, Wei Q and Ye
X: Integrated 16S rDNA, metabolomics, and TNF-α/NF-κB signaling
pathway analyses to explain the modulatory effect of Poria cocos
aqueous extract on anxiety-like behavior. Phytomedicine.
104:1543002022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiong H, Ye J, Xie K, Hu W, Xu N and Yang
H: Exosomal IL-8 derived from Lung Cancer and Colon Cancer cells
induced adipocyte atrophy via NF-κB signaling pathway. Lipids
Health Dis. 21:1472022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Szade A, Grochot-Przeczek A, Florczyk U,
Jozkowicz A and Dulak J: Cellular and molecular mechanisms of
inflammation-induced angiogenesis. IUBMB Life. 67:145–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther. 221:1077532021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shao C, Yang F, Miao S, Liu W, Wang C, Shu
Y and Shen H: Role of hypoxia-induced exosomes in tumor biology.
Mol Cancer. 17:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lv X, Li J, Zhang C, Hu T, Li S, He S, Yan
H, Tan Y, Lei M, Wen M and Zuo J: The role of hypoxia-inducible
factors in tumor angiogenesis and cell metabolism. Genes Dis.
4:19–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sun H, Zhang H, Li K, Wu H, Zhan X, Fang
F, Qin Y and Wei Y: ESM-1 promotes adhesion between monocytes and
endothelial cells under intermittent hypoxia. J Cell Physiol.
234:1512–1521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fukumoto M, Kondo K, Uni K, Ishiguro T,
Hayashi M, Ueda S, Mori I, Niimi K, Tashiro F, Miyazaki S, et al:
Tip-cell behavior is regulated by transcription factor FoxO1 under
hypoxic conditions in developing mouse retinas. Angiogenesis.
21:203–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gu X, Zhang J, Shi Y, Shen H, Li Y, Chen Y
and Liang L: ESM1/HIF-1α pathway modulates chronic intermittent
hypoxia-induced non-small-cell lung cancer proliferation, stemness
and epithelial-mesenchymal transition. Oncol Rep. 45:1226–1234.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wigner P, Grębowski R, Bijak M,
Saluk-Bijak J and Szemraj J: The interplay between oxidative
stress, inflammation and angiogenesis in bladder cancer
development. Int J Mol Sci. 22:44832021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wu L, Saxena S and Singh RK: Neutrophils
in the tumor microenvironment. Adv Exp Med Biol. 1224:1–20. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tang L, Zhao Y, Wang D, Deng W, Li C, Li
Q, Huang S and Shu C: Endocan levels in peripheral blood predict
outcomes of acute respiratory distress syndrome. Mediators Inflamm.
2014:6251802014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Reinhart K, Meisner M and Brunkhorst FM:
Markers for sepsis diagnosis: What is useful? Crit Care Clin.
22:503–519. ix–x. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kim KS, Lee YA, Ji HI, Song R, Kim JY, Lee
SH, Hong SJ, Yoo MC and Yang HI: Increased expression of endocan in
arthritic synovial tissues: Effects of adiponectin on the
expression of endocan in fibroblast-like synoviocytes. Mol Med Rep.
11:2695–2702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Murthi P, Sarkis R, Lim R, Nguyen-Ngo C,
Pratt A, Liong S and Lappas M: Endocan expression is increased in
the placenta from obese women with gestational diabetes mellitus.
Placenta. 48:38–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Leroy X, Aubert S, Zini L, Franquet H,
Kervoaze G, Villers A, Delehedde M, Copin MC and Lassalle P:
Vascular endocan (ESM-1) is markedly overexpressed in clear cell
renal cell carcinoma. Histopathology. 56:180–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Basim P and Argun D: A comparison of the
circulating endocan levels between the inflammatory and malignant
diseases of the same organ: The breast. J Invest Surg.
34:1207–1213. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Scherpereel A, Gentina T, Grigoriu B,
Sénéchal S, Janin A, Tsicopoulos A, Plénat F, Béchard D, Tonnel AB
and Lassalle P: Overexpression of endocan induces tumor formation.
Cancer Res. 63:6084–6089. 2003.PubMed/NCBI
|
|
89
|
Steiner N, Hajek R, Sevcikova S, Borjan B,
Untergasser G, Göbel G and Gunsilius E: The plasma levels of the
angiogenic cytokine endocan are elevated in patients with multiple
myeloma. Anticancer Res. 38:5087–5092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ozaki K, Toshikuni N, George J, Minato T,
Matsue Y, Arisawa T and Tsutsumi M: Serum endocan as a novel
prognostic biomarker in patients with hepatocellular carcinoma. J
Cancer. 5:221–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lin LY, Yeh YC, Chu CH, Won JGS, Shyr YM,
Chao Y, Li CP, Wang SE and Chen MH: Endocan expression is
correlated with poor progression-free survival in patients with
pancreatic neuroendocrine tumors. Medicine (Baltimore).
96:e82622017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Maurage CA, Adam E, Minéo JF, Sarrazin S,
Debunne M, Siminski RM, Baroncini M, Lassalle P, Blond S and
Delehedde M: Endocan expression and localization in human
glioblastomas. J Neuropathol Exp Neurol. 68:633–641. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Huang X, Chen C, Wang X, Zhang JY, Ren BH,
Ma DW, Xia L, Xu XY and Xu L: Prognostic value of endocan
expression in cancers: Evidence from meta-analysis. Onco Targets
Ther. 9:6297–6304. 2016. View Article : Google Scholar : PubMed/NCBI
|