Introduction
Neuroendocrine neoplasms (NEN) are rare and highly
heterogeneous, accounting for only ~2% of all malignant tumours
diagnosed in the Western world (1).
The pancreas is one of the common sites of occurrence. In 2019, the
World Health Organization (WHO) classified NENs into highly
differentiated neuroendocrine tumors (NETs), hypodifferentiated
neuroendocrine carcinoma (NEC) and mixed neuroendocrine non-NETs
(2). NENs can be further classified
into functional NEN and non-functional NEN according to whether
they have neuroendocrine function. pNEN, although relatively rare,
has shown an increasing incidence trend in recent years (3).
pNEC is highly aggressive and has a poor prognosis,
with survival times in patients with pNEC usually recorded as <1
year (3). Currently, whether to
perform surgery on patients with pNEC is still controversial, and
palliative surgery is generally performed only to prevent or treat
tumor-related complications. Systemic therapy is the primary
treatment for pNEC. The 2021 National Comprehensive Cancer Network
guidelines recommend platinum-based combination chemotherapy as the
first-line chemotherapy regimen for pNEC, including etoposide +
cisplatin (EP), etoposide + carboplatin, and irinotecan +
cisplatin. The EP protocol is most commonly used in pNEC, with an
objective response rate of ~30% and a median survival time of ~1
year. One study reported that the EP regimen has only marginal
antitumor activity and relatively heavy toxicity in pNEC compared
with the same regimen in extra-pulmonary NEC (4). After failure of the EP plan,
second-line chemotherapy options are limited and the overall
efficiency is low, not exceeding 18%. The immunotherapy in NENs is
still in the clinical exploratory phase, and its efficacy,
particularly with regard to immune checkpoint inhibitors, has shown
mixed results in NENs (5).
Similarly, targeted therapy has not entered the standard treatment
regimen. In conclusion, the treatment options for pNEC are minimal,
and the prognosis is unfavorable.
Case report
In January 2018, a 57-year-old man visited The 900th
Hospital of the Chinese People's Liberation Army Joint Logistic
Support Force (Fuzhou, China) for recurrent back pain that had
persisted for 6 months. The patient had not previously visited
other hospitals and was self-administering oral pain medication as
required. The patient had no specific past medical history. Bone
emission computed tomography suggested an abnormal radiological
concentration in the second and third lumbar spine, and further
examinations were recommended. However, the patient did not
continue the consultation for personal reasons.
In June 2018, the patient was hospitalized in the
Department of Oncology of The 900th Hospital of the Chinese
People's Liberation Army Joint Logistic Support Force due to
worsening back pain, and a whole-body 18F-fluorodeoxyglucose
positron emission tomography-CT scan revealed that the body and
tail of the pancreas were slightly thickened and hypermetabolic,
suggesting a pancreatic malignant tumor (Fig. 1). The scan also indicated multiple
bone destruction in the thoracic spine, lumbar spine and pelvis,
with hypermetabolism, which was considered tumor metastasis; and
multiple enlarged lymph node shadows in the bilateral
supraclavicular fossa, intra-mediastinum, both lung hila and the
retroperitoneum, with hypermetabolism, which was considered tumor
metastasis. An enhanced magnetic resonance imaging (MRI)
examination of the upper abdomen and the pelvic cavity also
suggested pancreatic malignancy with bone and multiple lymph node
metastases.
To clarify the diagnosis, the patient underwent a
bone marrow biopsy. Hematoxylin and eosin (H&E) and
immunohistochemical staining were performed and results were
examined using a light microscope. Tumor specimens were fixed in
10% neutral formalin for ~48 h at room temperature and embedded in
paraffin, and then cut into 4-µm thick sections for H&E
staining (hematoxylin for 5 min and eosin for 5 min at room
temperature). For immunohistochemistry, the tissue was fixed in 4%
formalin for 48 h at room temperature, embedded in paraffin and
then cut into 3-µm sections. These sections were then rehydrated in
a descending alcohol series (xylene, 100% ethanol, 95% ethanol, 85%
ethanol and ethanol-free water) and underwent antigen retrieval
using EDTA antigen retrieval treatment (cat. no. MVS0098; Fuzhou
Maixin Biotechnology Co., Ltd.) in a microwave on high heat for 2
min, followed by incubation at room temperature for 8 min.
Endogenous peroxidase activity was quenched with 3% hydrogen
peroxide in methanol before incubation with primary antibodies.
Immunohistochemical staining was performed overnight at 4°C using
the following primary antibodies (prediluted; Fuzhou Maixin
Biotechnology Co., Ltd.): Synaptophysin (Syn; cat. no. MAB0742),
chromogranin A (CgA; cat. no. RMA0548), neuron-specific enolase
(NSE; cat. no. MAB0791) and Ki-67 (cat. no. RMA0731). The secondary
antibody was obtained from the M&R HRP/DAB Detection IHC Kit
(prediluted; cat. no. HC301-01; Vazyme Biotech Co., Ltd.) and was
used to treat sections at room temperature for 30 min.
Subsequently, a chromogen detection reagent was applied (M&R
HRP/DAB Detection IHC Kit; cat. no. HC301-01; Vazyme Biotech Co.,
Ltd.). The IHC staining results demonstrated that the bone marrow
tissue expressed Syn, CgA, NSE and Ki-67 (60%). The H&E and
immunohistochemistry examinations (Fig.
2) were suggestive of pNEC invading the bone marrow. Since The
900th Hospital of the Chinese People's Liberation Army Joint
Logistic Support Force was unable to conduct second-generation
genetic testing, after full communication with the patient's
family, the patient's family sent the patient's venous blood
samples at their own expense to Shanghai Yikang Medical Laboratory
Co., Ltd. The result showed that tumor mutation burden was 1.44
mutations/Mb. A total of 25 mutations in 23 genes were detected in
the sample, of which no variants were detected that could be
associated with clinical use.
Combining the results, the patient was diagnosed
with pNEC, T2N1M1 stage IV, according to the American Joint
Committee on Cancer 8th edition (6). In July 2018, the patient started to
receive intravenous chemotherapy with the EP regimen (160 mg
etoposide on days 1–3; 40 mg cisplatin on day 1) for 3-week cycles.
In August 2018, after two cycles of chemotherapy, the patient's
white blood cell count dropped to 2.3×109/l (normal
range, 3.5–9.5×109/l) [Common Terminology Criteria for
Adverse Events (CTCAE) version 5.0 grade 2; https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdf],
before returning to normal after symptomatic management, so the
dose of etoposide was adjusted to 140 mg starting with the third
cycle. However, at the end of the third cycle of chemotherapy, the
patient's white blood cell count dropped to 1.5×109/l
(CTCAE version 5.0 grade 3), so the dose of etoposide was adjusted
again to 100 mg. In total, eight cycles of therapy were
administered. During chemotherapy, CT of the upper abdomen was
performed twice and the efficacy was assessed as stable. Following
the completion of chemotherapy, the patient received maintenance
treatment with oral anlotinib (12 mg on days 1–14 every 3 weeks) in
January 2019 and remained on this regimen until July 2022, when the
patient experienced disease progression. To prevent severe bone
destruction, the patient was intravenously administered 4 mg
ibandronate every 4 weeks. Between 2019 and 2022, the patient
underwent multiple MRI examinations of the upper abdomen, and the
efficacy was assessed as stable disease. Until July 2022, the CT
scan of the chest and upper abdomen was repeated, and the disease
was considered to be progressive, with imaging suggestive of
adrenal, lung, pleural and liver metastases, and a progression-free
survival time of 48 months.
The patient was not treated further due to financial
reasons. In January 2023, the patient was again hospitalized in the
Department of Oncology of The 900th Hospital of the Chinese
People's Liberation Army Joint Logistic Support Force and underwent
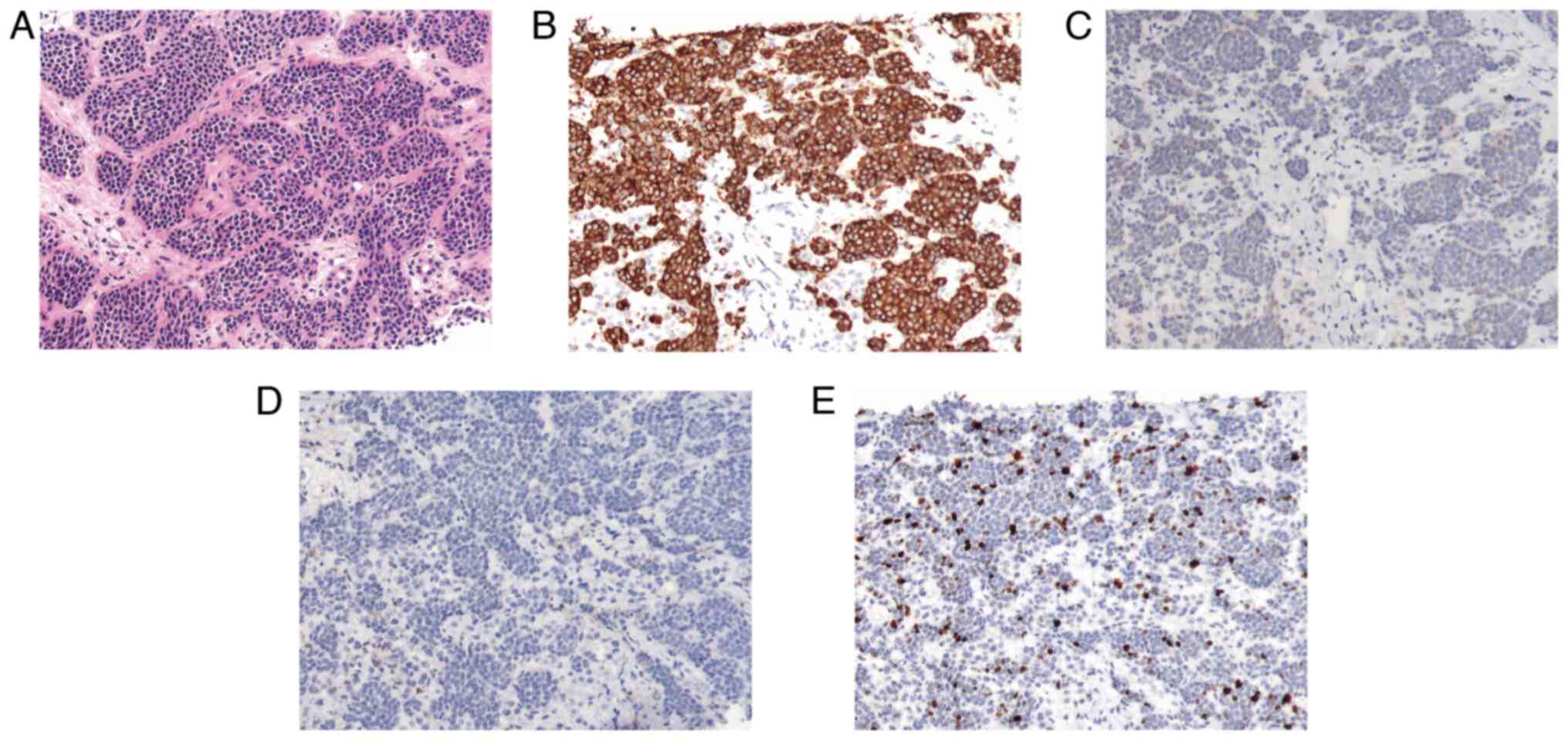
a liver puncture. The H&E and immunohistochemistry examinations
(Fig. 3) showed the following
results: Syn(++++), CgA(+), somatostatin receptor 2(−) (prediluted;
cat. no. RMA0867; Fuzhou Maixin Biotechnology Co., Ltd.) and Ki-67
(15%). This was suggestive of a pancreatic (p)NET (grade 2 in the
WHO grading system) (2). As the
patient's disease continued to progress, treatment regimens were
changed several times, including the use of irinotecan (200-mg
intravenous drip on day 1 every 14 days) for 1 cycle, sunitinib
(37.5 mg orally each day) in combination with mitotane (2,000 mg
orally each day) for 1 month, and capecitabine (1 g orally twice a
day on days 1–14 every 3 weeks) in combination with temozolomide
(200 mg orally on days 1–5 every 3 weeks) for 1 cycle. However, the
treatment was not as effective as it could have been.
Starting in April 2023, the patient developed
pneumonia, which did not improve with antibiotics. After
consultation with respiratory physicians, it was considered that
the patient's pneumonia may be related to tumor invasion of the
lungs, which resulted in destruction of the lung structure, and
that the patient's pneumonia might continue to progress if the
tumor progression could not be controlled. After fully
communicating with the patient's family, the patient's family
decided to discontinue antitumor treatment and chose to return to
the local hospital for supportive care. The patient passed away a
month after being discharged from the hospital.
Discussion
In the present case, the patient had multiple bone
metastases at the time of the initial diagnosis. For pNEN with
distant metastasis, the value and significance of surgery should be
comprehensively evaluated by considering the age of the patient,
their general condition, the functional characteristics of the
tumor, the pathological grade, and the number and distribution of
the metastases. In the present case, the NEC was non-functional,
and the patient had already developed multiple bone metastases
throughout the body, so the significance of local surgery was not
great. Furthermore, after informing them about the situation, the
patient and their family wanted to continue conservative treatment.
Therefore, after eight cycles of chemotherapy, the patient was not
treated with surgery. However, there is no standard recommendation
on whether to continue treatment after first-line chemotherapy and
which plan to use. At present, the lack of sizeable genetic mapping
studies of pNEC, the limited number of patients with pNEC and the
few clinical trials on targeted therapy in pNEC have all limited
the application of targeted therapy in pNEC. The patient's genetic
test results also did not identify clinically significant
mutations.
Sunitinib is primarily recommended for advanced,
well-differentiated pNETs (7).
However, no studies have reported the efficacy of sunitinib in the
treatment of pNEC. In the present study, the attempt at second-line
treatment with anlotinib monotherapy was based on the ALTER 1202
study (8). This was a randomized,
double-blind, placebo-controlled, multicenter phase II study that
enrolled patients aged 18–75 years with histologically confirmed
small cell lung cancer. The study also required that enrolled
patients had received at least second-line chemotherapy in the
past. Enrolled patients were randomized 2:1 to receive either
anlotinib or a placebo. At the 2018 World Lung Cancer Congress,
Professor Ying Cheng orally reported the PFS results of the ALTER
1202 study. Data was officially available as of June 2018, and the
median PFS time in the anlotinib group was 4.1 months, which was
significantly higher than the 0.7 months in the placebo group. The
study was ultimately published in the British Journal of Cancer
(8). Given that small cell lung
cancer and pNEC are both NENs, after group discussion, second-line
treatment with anlotinib monotherapy was eventually attempted in
the present case.
Anlotinib is a multi-targeted oral small molecule
tyrosine kinase receptor inhibitor that targets vascular
endothelial growth factor receptor-1 (VEGFR1), VEGFR2/KDR, VEGFR3,
stem cell factor receptor, platelet-derived growth factor β,
fibroblast growth factor receptor-1 (FGFR1), FGFR2 and FGFR3, and
also inhibits tumor angiogenesis and tumor cell proliferation
(9,10). The anti-angiogenic activity of
anlotinib is more potent than that of the other three
anti-angiogenic drugs, including sunitinib, sorafenib and
nintedanib (11). Compared with
sunitinib, anlotinib has a broader and better antitumor effect.
Furthermore, anlotinib is well tolerated and most adverse effects
can be managed with medical intervention. Clinical trials of
anlotinib in pNEC have not yet been conducted, and to the best of
our knowledge, no case using anlotinib for pNEC has previously been
reported.
The particularity of the present patient was that
bone destruction had already developed in 2018, and the biological
behavior of the tumor was relatively inert during a period of
almost 4 years. In 2022, the disease progressed rapidly, with
multiple metastases in the adrenal glands, lungs and liver. Unlike
other tumors, while the pathological findings in the bone marrow
suggested pNEC, the result in the liver was pNET. A limitation to
the study was that no biopsies were performed on the different
sites of metastases in the liver. Furthermore, the biopsied tissue
from the liver was not genetically tested to investigate whether
disease progression was associated with emerging genetic
mutations.
In conclusion, as a relatively rare, highly
aggressive malignancy with a poor prognosis, pNEC currently has
limited therapeutic options, with only platinum-containing
chemotherapy as the standard treatment option, and the therapeutic
outcome is poor. The application of targeted therapy or
immunotherapy in pNEC is still pending the results a series of
clinical studies and trials. The present case report, as the first
case of EP chemotherapy followed by targeted therapy with
anlotinib, with a survival time of almost 60 months, may provide
some ideas for the development of clinical trials related to
pNEC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ, YW, XC and JL were responsible for study
conception and design. Administrative support was provided by XC
and JL. YZ, YW and JW provided study materials or patients.
Collection and assembly of data was performed by JW and ZZ. Data
analysis and interpretation was performed by ZZ and JL. All authors
were involved in manuscript writing. All authors read and approved
the final manuscript. YZ, YW, JW, ZZ, JL and XC confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The study was conducted in accordance with the
ethical standards from the 1964 Declaration of Helsinki and its
later amendments. Local ethical approval was obtained from the
Ethics Committee of the 900th Hospital of the Chinese People's
Liberation Army Joint Logistic Support Force (Fuzhou, China;
approval no. 2023-062).
Patient consent for publication
Written informed consent was obtained from the
patient for the case information and images to be published in this
case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basu B, Sirohi B and Corrie P: Systemic
therapy for neuroendocrine tumours of gastroenteropancreatic
origin. Endocr Relat Cancer. 17:R75–R90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Onco. l3:1335–1342. 2017. View Article : Google Scholar
|
|
4
|
Iwasa S, Morizane C, Okusaka T, Ueno H,
Ikeda M, Kondo S, Tanaka T, Nakachi K, Mitsunaga S, Kojima Y, et
al: Cisplatin and etoposide as first-line chemotherapy for poorly
differentiated neuroendocrine carcinoma of the hepatobiliary tract
and pancreas. Jpn J Clin Oncol. l40:313–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mosalem O, Sonbol MB, Halfdanarson TR and
Starr JS: Tyrosine kinase inhibitors and immunotherapy updates in
neuroendocrine neoplasms. Best Pract Res Clin Endocrinol Metab.
37:1017962023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao X and Zhang D: The 8th Edition
American Joint Committee on cancer staging for
hepato-pancreato-biliary cancer: A review and update. Arch Pathol
Lab Med. 145:543–553. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang
SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ and Hou BH:
Pancreatic neuroendocrine tumors: A review of serum biomarkers,
staging, and management. World J Gastroenterol. 26:2305–2322. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L,
Han B, Chen G, He J, Wang J, et al: Anlotinib vs placebo as third-
or further-line treatment for patients with small cell lung cancer:
A randomised, double-blind, placebo-controlled phase 2 study. Br J
Cancer. 125:366–371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y
and Lou L: Preclinical characterization of anlotinib, a highly
potent and selective vascular endothelial growth factor receptor-2
inhibitor. Cancer Sci. 109:1207–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taurin S, Yang CH, Reyes M, Cho S, Coombs
DM, Jarboe EA, Werner TL, Peterson CM and Janát-Amsbury MM:
Endometrial cancers harboring mutated fibroblast growth factor
receptor 2 protein are successfully treated with a new small
tyrosine kinase inhibitor in an orthotopic mouse model. Int J
Gynecol Cancer. 28:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin B, Song X, Yang D, Bai D, Yao Y and Lu
N: Anlotinib inhibits angiogenesis via suppressing the activation
of VEGFR2, PDGFRβ and FGFR1. Gene. 654:77–86. 2018. View Article : Google Scholar : PubMed/NCBI
|