Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors worldwide. About 20% of patients with CRC have
already distant metastases at presentation (1) and 50% of patients with CRC develop
metastatic disease (2).
Furthermore, a Norwegian study showed that 15.6% of patients with
CRC who were considered surgically cured had recurrent cancers,
including distant metastases, during a 5-year follow-up (3). Common sites of metastases from CRC
include the liver and lungs, while metachronous bone metastasis
(MBM) occurs infrequently (4).
Multiple myeloma (MM) is a neoplastic plasma-cell
disorder that is characterized by clonal proliferation of malignant
plasma cells in the bone marrow microenvironment, monoclonal
protein in the blood or urine, and associated organ dysfunction. It
accounts for ~1% of neoplastic diseases and 13% of hematologic
cancers (5). Clinical
manifestations of MM include bone pain, anemia, bleeding and
hypercalcemia. These lesions can affect the spine, ribs, sternum,
pelvis and other body parts (6–8).
Multiple primary neoplasms, defined as the presence
of two or more histologically distinct neoplasms, are grouped into
two large categories, namely synchronous and metachronous neoplasms
(9). The two primary tumors of the
patient reported in the present study are asynchronous and the
medical history of this patient is particularly distinctive. During
a 6-year follow-up after rectal cancer surgery, the patient did not
experience any bone pain, anemia, proteinuria or abnormalities in
coagulation function associated with MM. Prior to hernia surgery,
the patient did not have any anemia or coagulation dysfunction.
Following hernia surgery near the stoma, the patient developed
refractory anemia and coagulation dysfunction. It was hypothesized
that, if the patient had not undergone the hernia surgery,
refractory anemia and coagulation dysfunction may not have
occurred. Bone marrow puncture smear was also not performed to
discover MM. Therefore, this case is considered to be unique and
worthy of a case report.
Case report
The patient is a 65-year-old male who was diagnosed
with rectal cancer in October 2013 at Southwest Hospital
(Chongqing, China). The patient underwent laparoscopic
abdominoperineal resection (Miles procedure) for rectal cancer at
Chongqing Southwest Hospital. It was not possible to obtain
postoperative pathological images from the hospital. The
postoperative pathological result was poorly differentiated
adenocarcinoma (stage T3N1M0) with a moderate risk of recurrence.
Following surgery, the patient received standard chemotherapy
according to the FOLFOX regimen (oxaliplatin, calcium folinate and
5-fluorouracil). The patient underwent regular follow-ups every
year through abdominal computerized tomography (CT), colonoscopy
and assessment of serum carcinoembryonic antigen (CEA) levels after
surgery. No tumor recurrence or metastasis was observed during the
6-year follow-up period. In addition, in October 2019, the patient
visited our hospital (Affiliated Hospital of Zunyi Medical
University, Zunyi, China) due to ‘right-sided back pain’. Chest CT
revealed bone destruction of the seventh posterior rib on the right
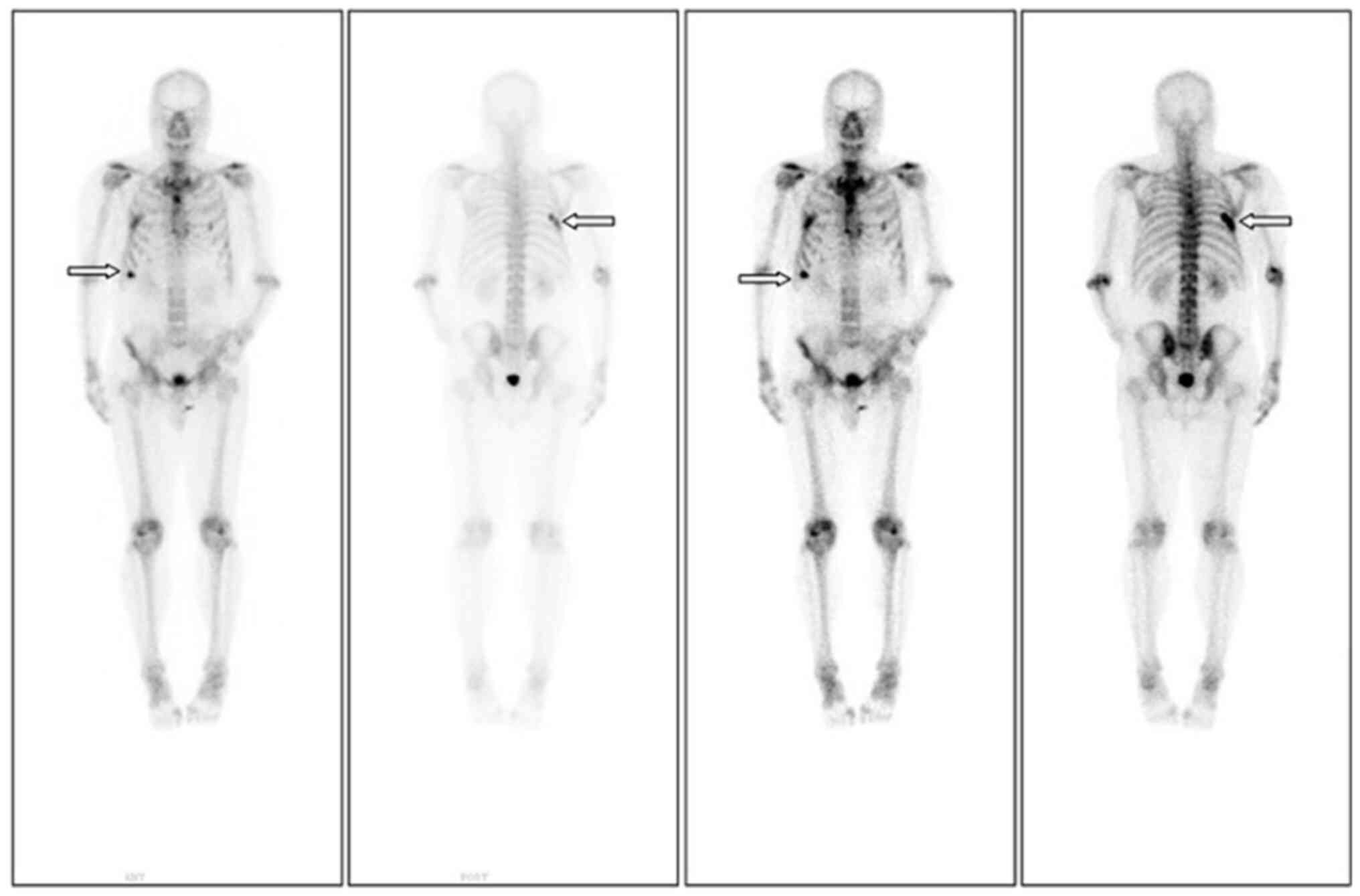
side. An emission CT (ECT) was conducted to further evaluate the
overall condition. ECT showed increased metabolic activity in the
right seventh rib, indicating the possibility of bone metastasis
(Fig. 1). Abdominal CT and
colonoscopy revealed no local tumor recurrence or peritoneal
metastasis, and CEA levels were within the normal range. Based on
his medical history, the patient was considered to have developed
postoperative bone metastasis from rectal cancer. The patient then
sought medical care at Southwest Hospital (Chongqing, China).
Spinal magnetic resonance imaging (MRI) was performed at Southwest
Hospital, revealing multiple vertebral body lesions involving the
thoracic, lumbar and sacral regions, as well as abnormal
enhancement in the appendages and bilateral iliac bones. Positron
emission tomography (PET)/CT has high specificity and sensitivity;
therefore, the patient underwent a PET/CT examination at Southwest
Hospital. The sternum, multiple vertebrae and sixth/seventh rib on
the right side showed bone destruction and slightly increased
glucose metabolism (images not available, as only retrievable by
the patient). Clinicians at that hospital also assumed that the
patient had developed bone metastasis following rectal cancer
treatment. They considered that the previous FOLFOX chemotherapy
regimen was effective and its use could be continued.
After clarifying the condition, the patient returned
to our hospital for chemotherapy according to the FOLFOX regimen.
After six rounds of chemotherapy, the patient's bone pain symptoms
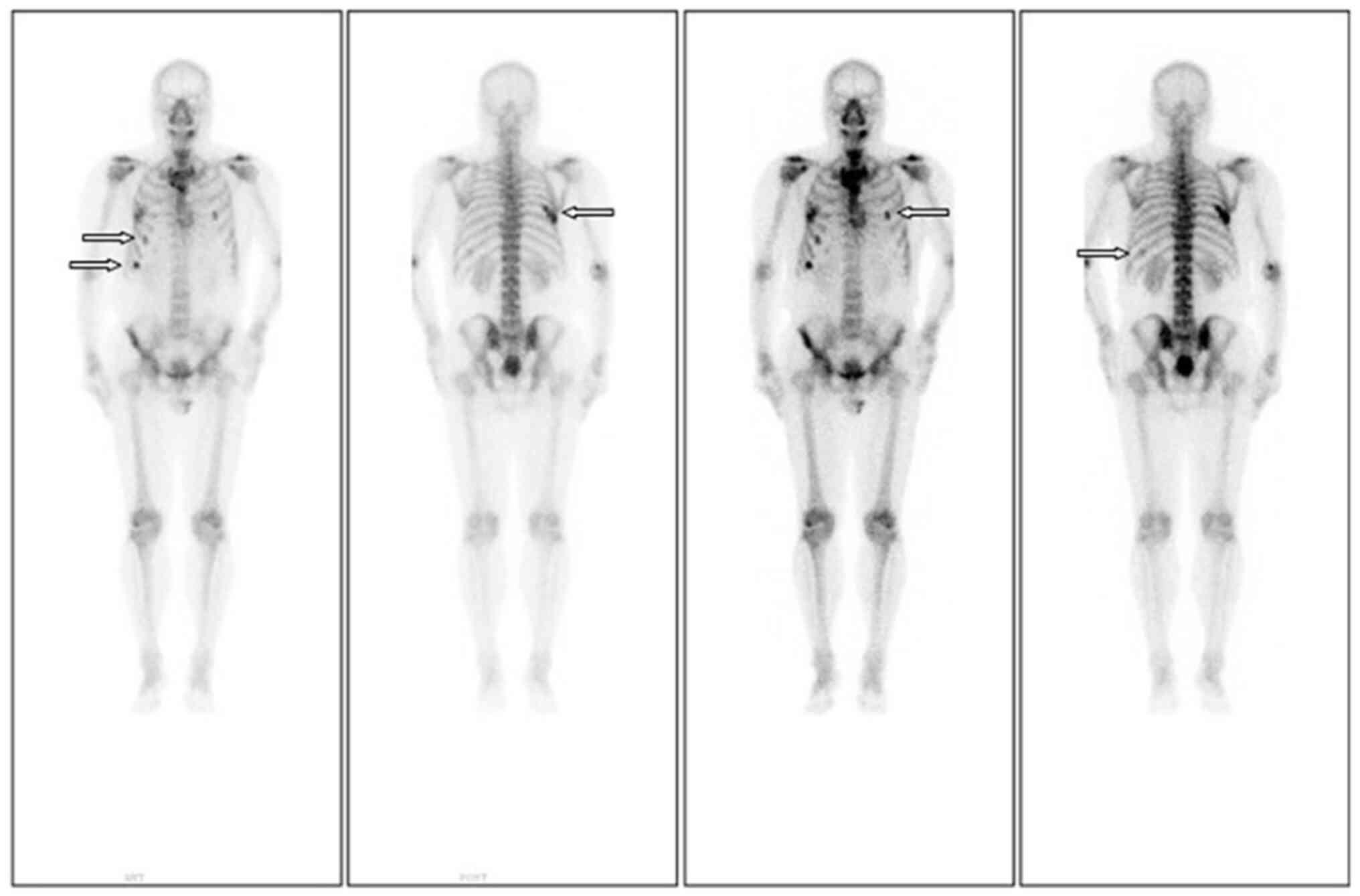
did not improve significantly. Follow-up ECT revealed the emergence
of a new lesion on the left tenth rib, compared with the
pre-chemotherapy image (Fig. 2).
Owing to significant bone pain, orthopedic experts recommended
using zoledronic acid to inhibit osteoclasts. Although the patient
experienced temporary pain relief with this treatment, the pain
returned and worsened over time. Subsequently, the patient's
treatment was changed to dinozumab and he received eight courses of
treatment. However, the patient's bone pain still did not show any
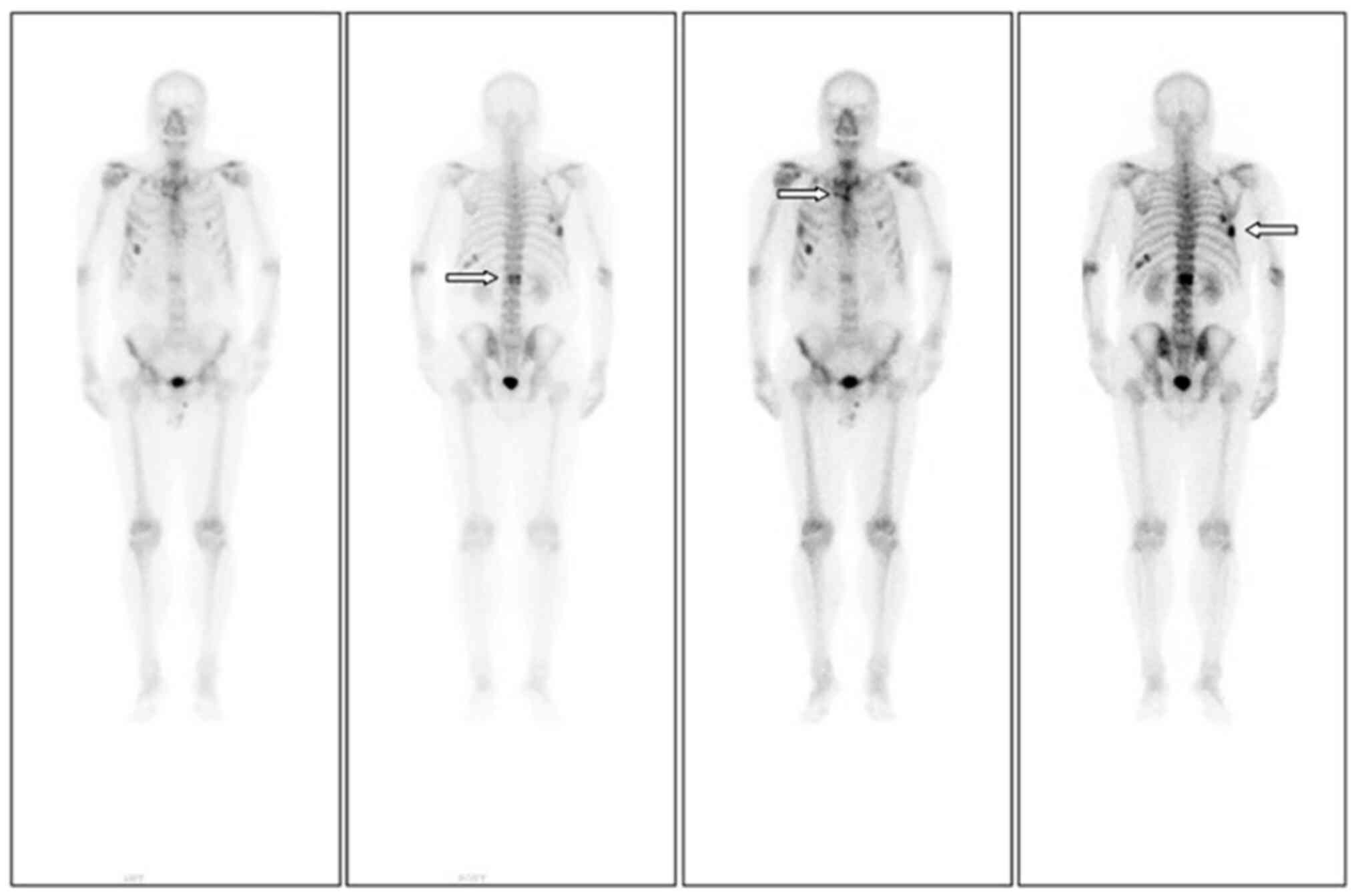
significant improvement. Reexamination with ECT (Fig. 3) indicated active bone metabolism in
the right seventh posterior rib, left tenth posterior ribs, upper
sternum and first lumbar spine.
Subsequently, the patient presented with a lump
around the colostomy stoma site and intermittent abdominal pain in
November 2020. Physical examination revealed a lump measuring
~10×10 cm around the stoma, which did not reduce in size when the
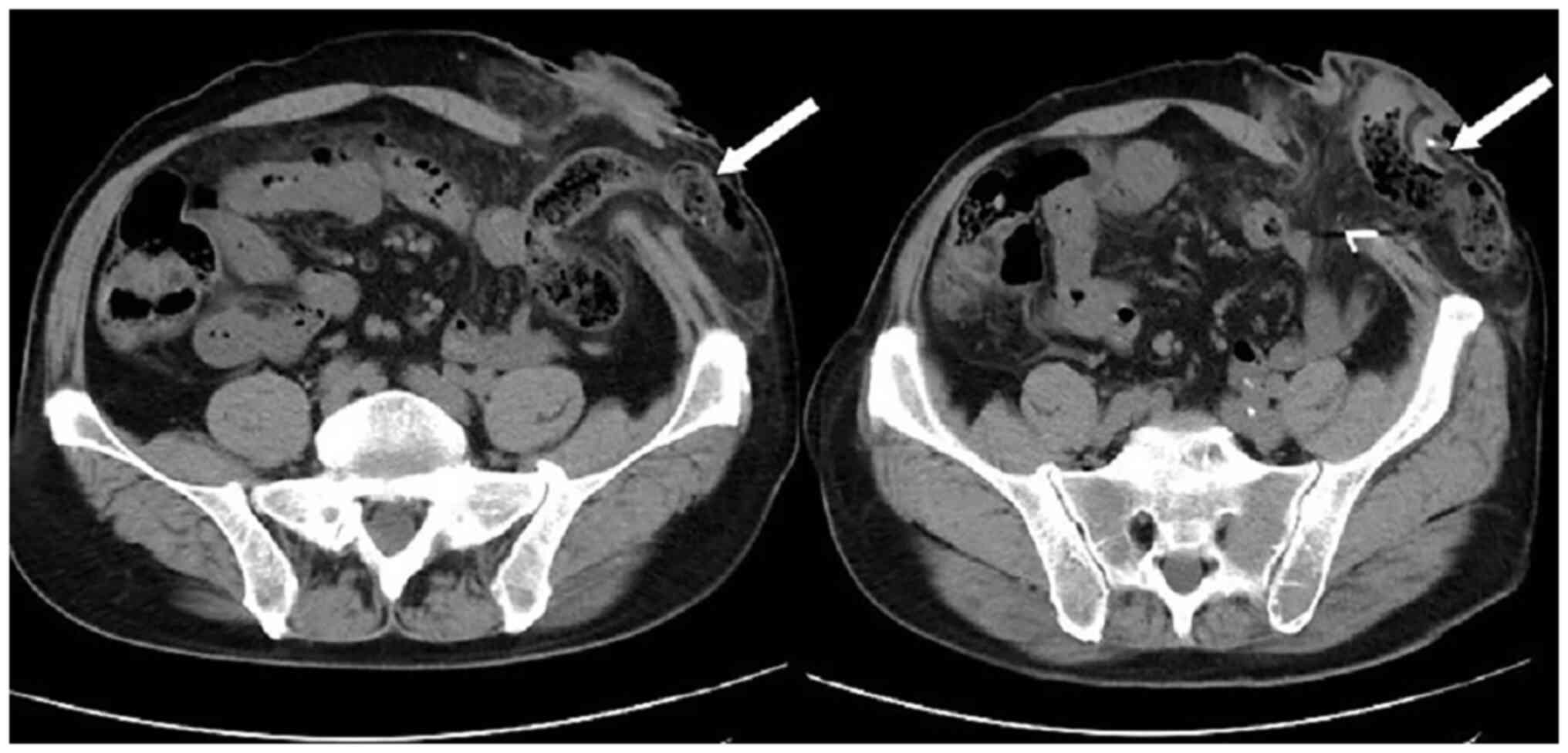
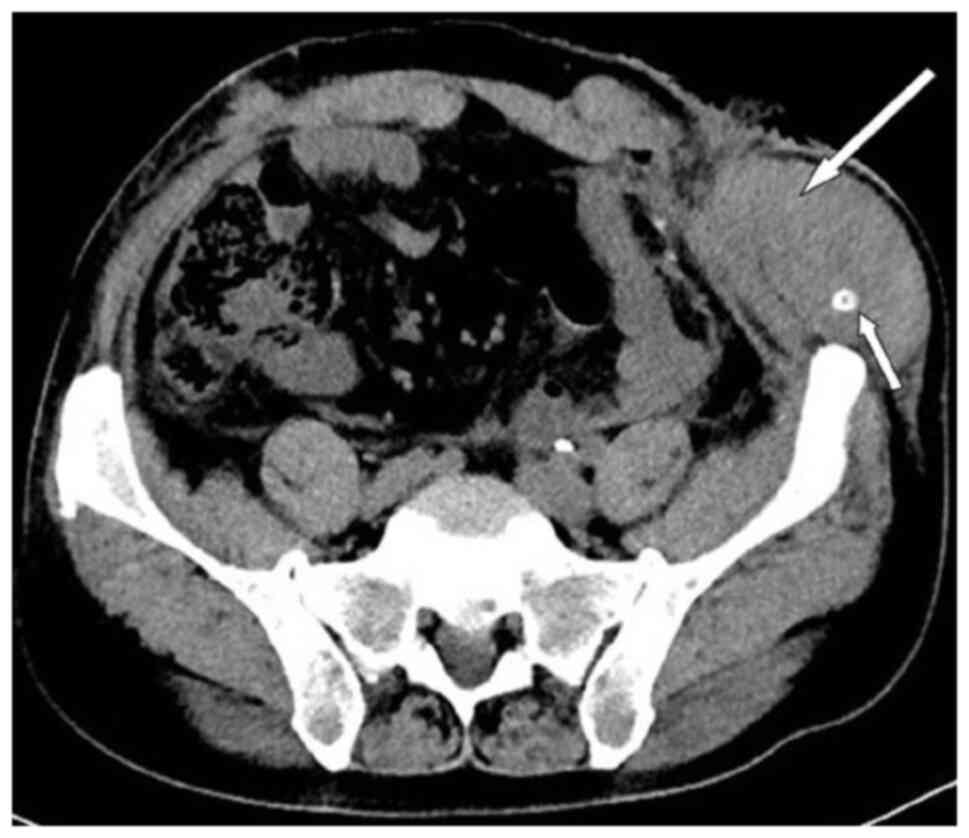
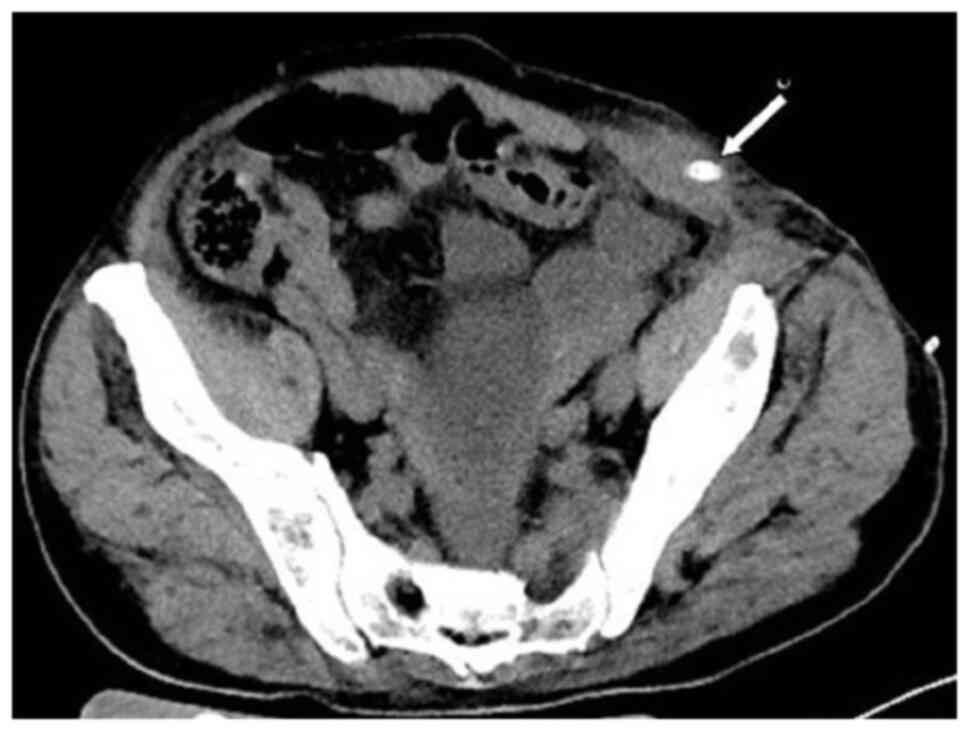
patient was lying flat. Abdominal CT (Fig. 4) revealed that the intestinal tube
had protruded into the subcutaneous fat layer of the abdomen. Based
on the patient's medical history, physical examination (a lump
around the colostomy stoma site) and the result of abdominal CT
(Fig. 4), a parastomal hernia was
suspected. Surgery was the recommended treatment. Routine
preoperative blood tests, coagulation function, and liver and
kidney functions showed no abnormalities. Parastomal hernia repair
surgery was performed using the keyhole technique in November 2020.
A relatively soft drainage tube was placed subcutaneously in the
surgical area. The patient recovered well after the surgery and was
discharged on the third postoperative day without removing the
drainage tube. During discharge, the surgical area was not
compressed. However, five days after discharge, the patient
experienced swelling, pain and bleeding at the surgical site.
Abdominal CT showed a hematoma in the surgical area (Fig. 5). After taking hemostatic treatment
measures (hemostatic drugs, compression hemostasis), fresh blood
still slowly flowed out from the drainage tube. Dynamic
reexamination of coagulation function showed that it gradually
deteriorated, and the activated partial thromboplastin time was
gradually delayed to 60 sec, which was 20 sec longer than normal
(reference range, 20–40 sec). The patient had stubborn anemia, and
after multiple blood transfusions, no significant increase was
identified in hemoglobin, which remained between 45–68 g/l (normal
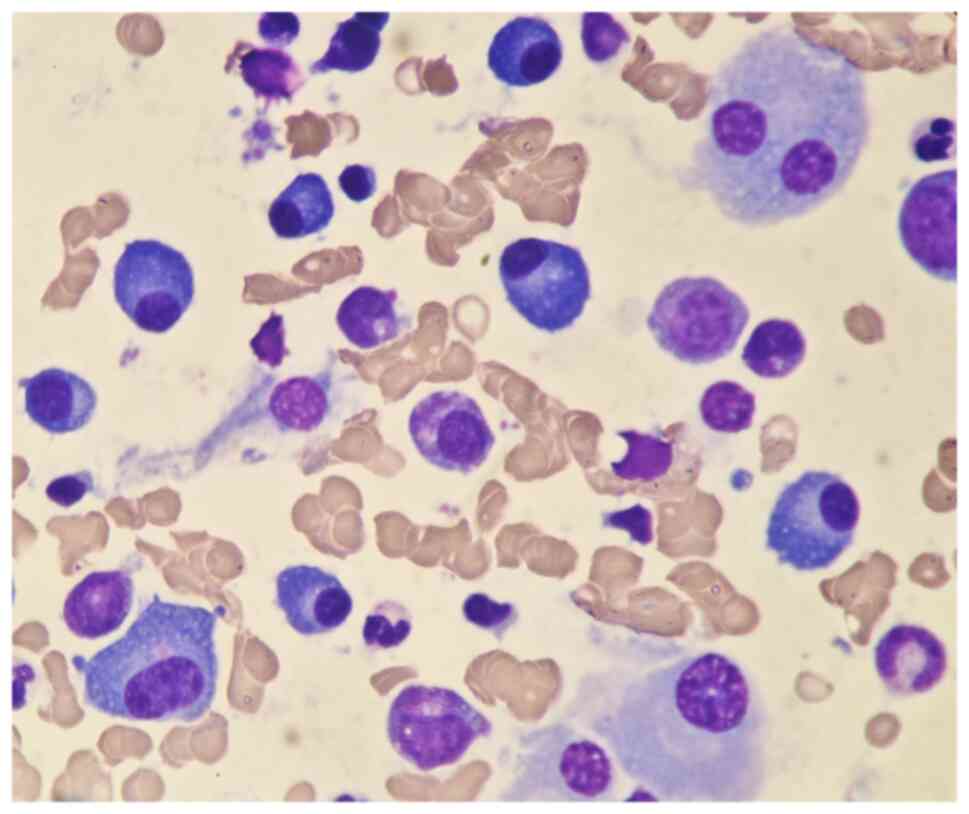
range, 130–175 g/l). A bone marrow biopsy was also performed to
investigate the cause of persistent bleeding (Fig. 6). The bone marrow smear was stained
using the Wright staining method and 200 cells were counted under a
microscope. The results showed abnormal proliferation of plasma
cell lines in bone marrow smears, accounting for 35% of total
cells, with an immature plasma cell composition accounting for
21.0% of total cells (normal range, 0–0.8%). This result is
consistent with the diagnosis of multiple myeloma (10,11).
Fig. 6 shows the characteristics of
abnormal plasma cells: This type of cell was significantly
different in size, with the cell body and nucleus appearing
circular, elliptical, ovoid or irregular in shape. The nucleus was
misaligned, the chromatin of the nucleus appeared as a granular or
loose network and certain cells showed obvious nucleoli. The
cytoplasm was rich, stained opaque dark blue and flame-like, with
obvious light staining bands around the nucleus. Nodular
protrusions and vacuoles were easily observed, while no particles
were seen. The morphological features were consistent with those of
MM (10). Further testing revealed
elevated serum immunoglobulin A (IgA) levels of 76.7 g/l (normal
range, 0.82–4.53 g/l) and significantly increased serum β2
microglobulin (β2-microglobulin) levels of 16,205 ng/ml (normal
range, 604–2,286 ng/ml). Based on the results of the bone marrow
puncture, the bone destruction, anemia and bleeding were attributed
to MM. After consultation with a hematologist, the patient was
diagnosed with MM (IgA-λ type, Durie-Salmon Stage III). The
Durie-Salmon staging system is a classic staging system for MM. The
staging criteria for Stage III are as follows: One or more of the
following abnormalities must be present: Hemoglobin <8.5 g/dl;
serum calcium >12 mg/dl; very high myeloma protein production;
IgG peak >7 g/dl; IgA peak >5 g/dl; Bence Jones protein
>12 g/24 h; and >3 lytic lesions on bone survey (11). The patient was transferred to the
hematology department and was treated with the PCD regimen
(bortezomib, cyclophosphamide, dexamethasone), chemotherapy and
blood transfusion. The specific dosage of medication is calculated
based on the patient's body surface area. One chemotherapy cycle is
4 weeks and this patient received 6 cycles of chemotherapy.
Afterwards, the patient received maintenance treatment with
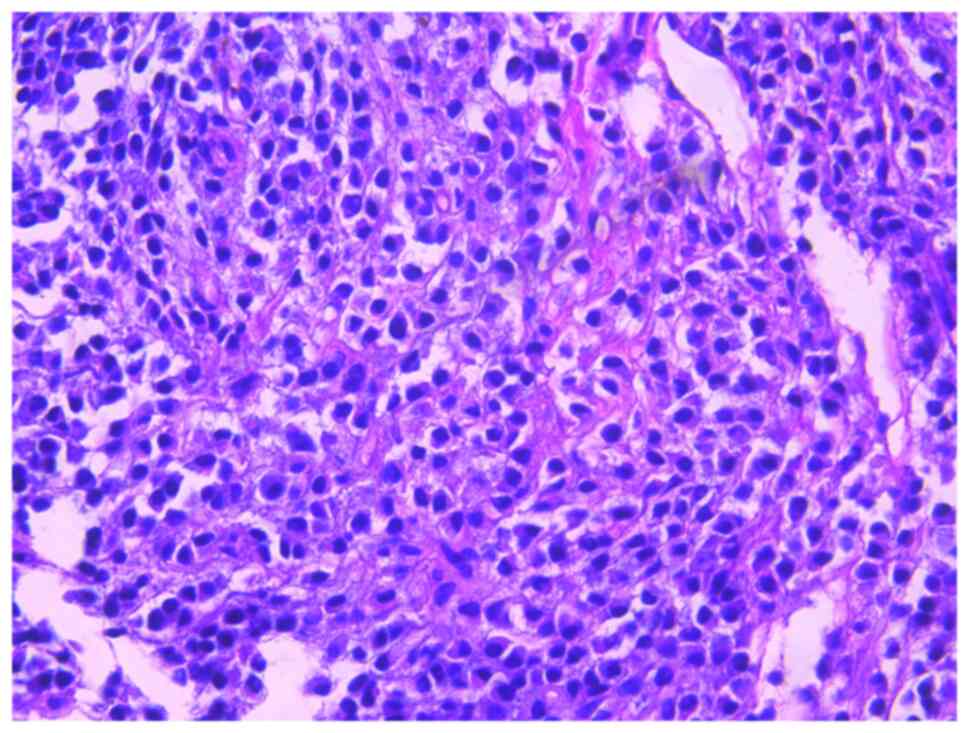
bortezomib monotherapy. The seventh rib lesion invaded the
surrounding soft tissue, and the interventional department
performed an empty needle puncture biopsy on it. The pathological
and immunohistochemical results of the puncture tissue are
consistent with multiple myeloma (10). The lesion was determined to be
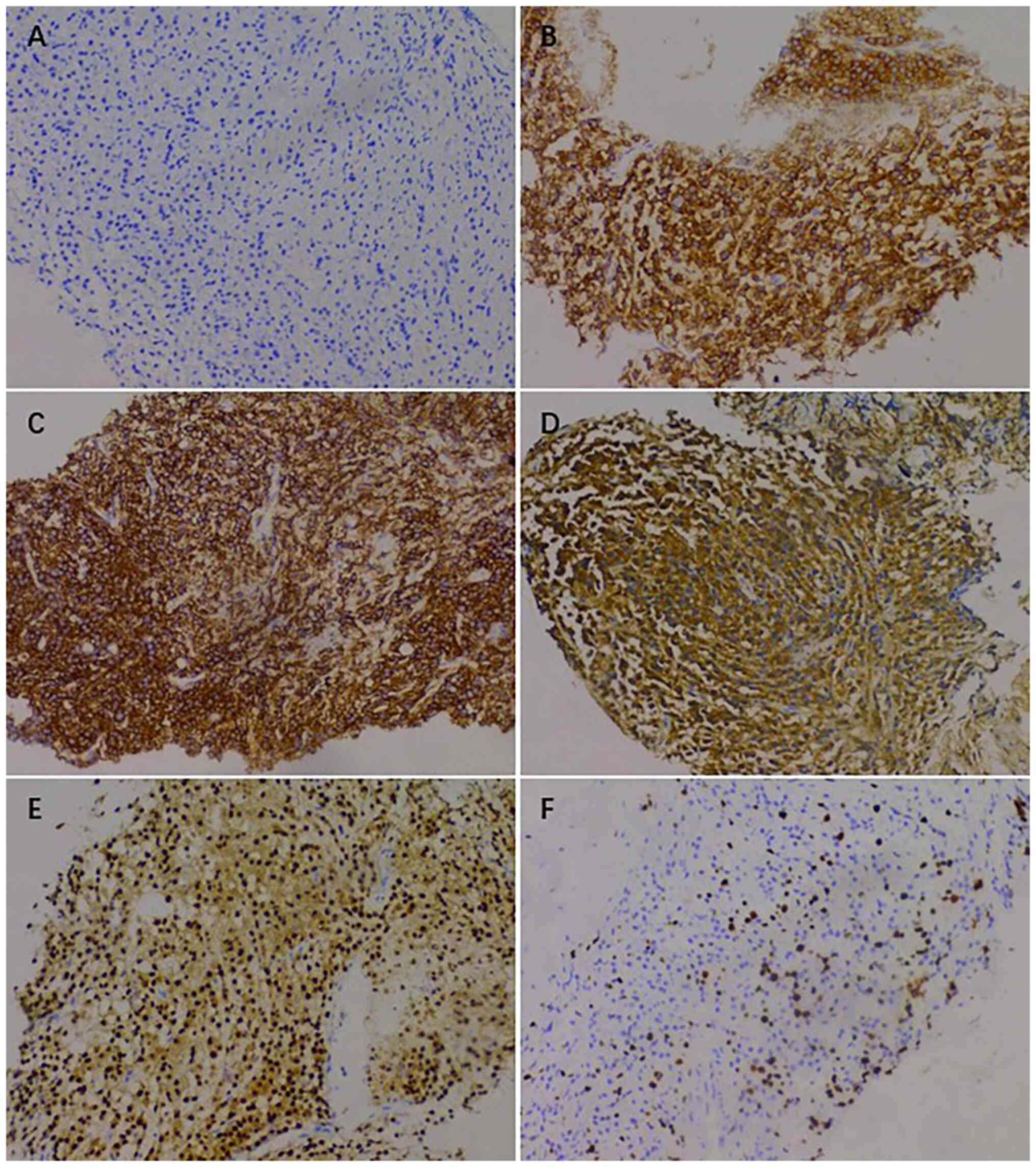
plasmacytoma, suggesting involvement of MM (Fig. 7), and the immunohistochemical
results were as follows: CD138 (+), CD38 (+), cytokeratin (CK) (−),
Ki67 (15%, +), Lambda (+) and MM oncogene 1 (+) (Fig. 8). CK negativity indicated the
absence of malignant cells of epithelial origin. The patient's
condition gradually improved, with increasing hemoglobin levels,
recovering coagulation function, absorption of the hematoma around
the stoma (Fig. 9) and alleviation
of bone pain. For the past 2 years, the patient has been regularly
treated in the hematology department and the progression of the MM
has been slow (Figs. 10 and
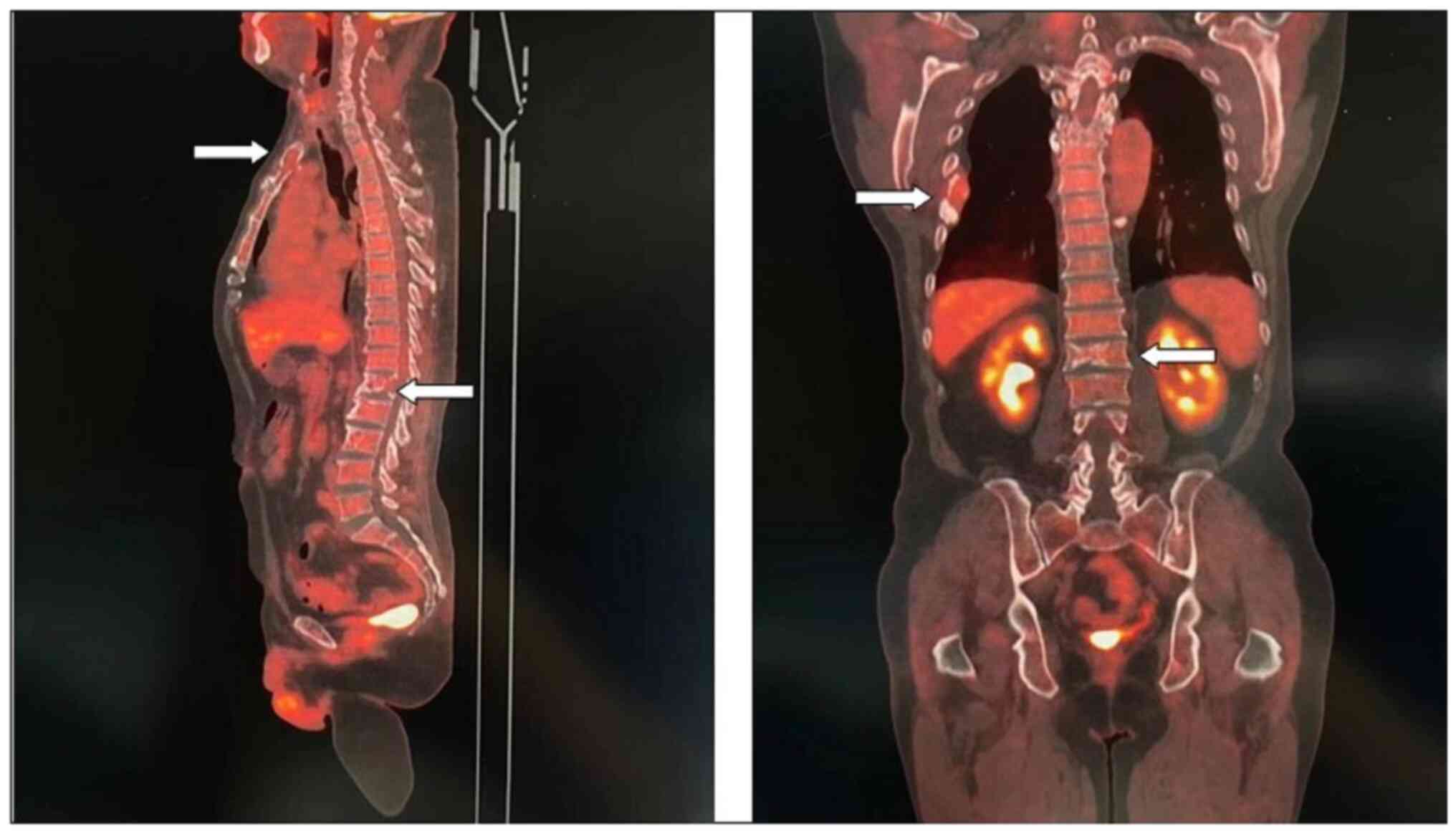
11). Fig. 10 is a PET/CT image of the patient
diagnosed with MM one year later. The arrows in Fig. 10 indicate the metabolic status of
the lesionsin the right seventh posterior rib, upper sternum, and
first lumbar spine. The increased metabolism of these three main
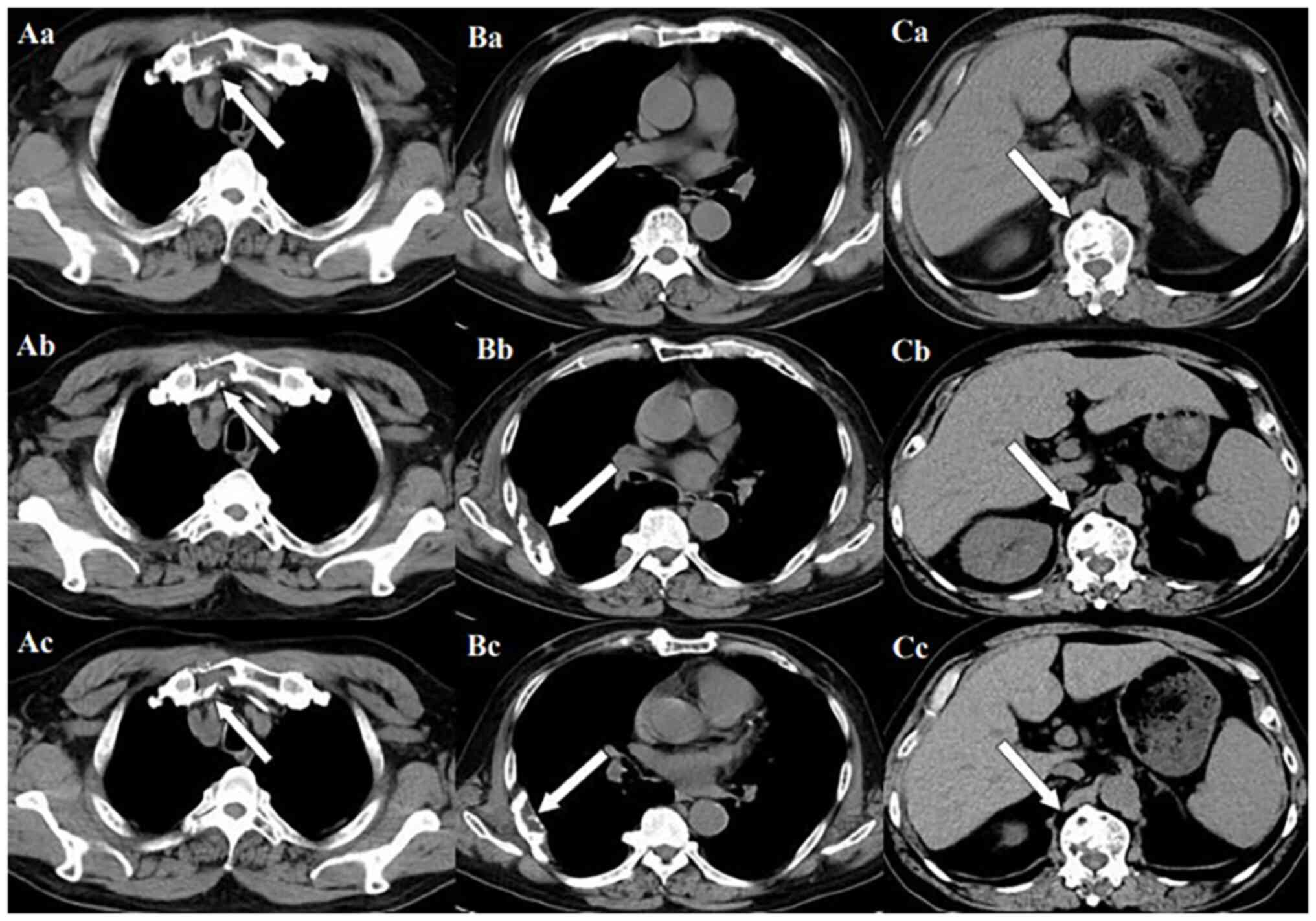
lesions is consistent with the manifestation of MM. In Fig. 11, row A represents the situation of
lesion in the upper sternum at different time-points; row B shows
the situation of lesion in the upper sternum at different
time-points; and row C shows the situation of lesion in the upper
sternum at different time-points. The arrows in Fig. 11Aa, Ba and Ca refer to the lesions
of the upper sternum, the seventh rib on the right side and the
first lumbar vertebra when MM was diagnosed. Fig. 11Ab-Cb shows the respective lesions
1 year after the diagnosis of MM and Fig. 11Ac-Cc shows them at 2 years after
the diagnosis of MM. After comparison, the progression of these
three lesions was not obvious. After treatment, the soft tissue
mass around the lesion of the right seventh rib gradually became
smaller. The patient has not experienced any worsening bone pain
symptoms since being diagnosed with MM. The patient has been
regularly visiting the hematology outpatient department. During the
follow-up period, the patient's blood routine, coagulation function
and serum immunoglobulin are being tested every two months, and
chest CT and spinal CT examinations conducted every 6 months.
During follow-up, there has been occasional mild anemia but no
coagulation abnormalities.
Discussion
Based on the case data, the patient of the present
study developed bone pain and was eventually diagnosed with MM
within 1 year (from October 2019 to November 2020). The patient's
condition did not worsen significantly and he received timely,
specialized treatment. We used the search term ‘multiple myeloma
and colorectal cancer’ in the PubMed database and two relevant case
reports were found. The first report documented a patient who was
diagnosed with MM shortly after undergoing CRC surgery (12). The authors proposed that both
primary tumors existed simultaneously. The second case report
described a patient with MM who, during the course of treatment,
was found to have colon adenocarcinoma due to abdominal pain and
melena (13). Owing to the unique
nature of this case, it was chosen to report it and analyze the
reason behind the initial misdiagnosis.
First, on analyzing the characteristics of the two
diseases, MM without specific manifestations was found to be
similar to bone metastasis. MM is a disease characterized by clonal
proliferation of malignant plasma cells in the bone marrow
microenvironment. It accounts for ~1% of tumors and 13% of
hematological cancers (5,14). Clinical manifestations of MM include
bone pain, anemia, bleeding and hypercalcemia. These lesions can
affect the spine, ribs, sternum, pelvis and other body parts
(6–8). Bone metastasis often leads to bone
diseases, commonly referred to as skeletal-related events. Common
clinical manifestations include bone pain, pathological fractures
and hypercalcemia (15). The bone
is a common site of metastasis of CRC, besides the liver and lungs,
accounting for ~3–7% of postoperative bone metastasis in CRC
(16). Rectal cancer is a risk
factor for bone metastasis and previous studies found that bone
metastasis of rectal cancer is more common than that of colon
cancer (16,17). The most common clinical
manifestations of bone metastasis of CRC are pain at the site of
tumor invasion and pathological fractures, and it may be
accompanied by symptoms of nerve compression (18–20).
In addition to the clinical manifestations, the
imaging features of MM and CRC bone metastases are similar, with
both showing osteolytic destruction. CT, MRI and ECT are important
methods for diagnosing bone metastasis of CRC, as they can detect
bone metastases (21). In the
present case, MRI showed abnormal enhancement of multiple vertebral
bodies, appendages and bilateral iliac bones in the thoracolumbar
and sacral vertebrae. Multiple ECT examinations indicated an
abnormal increase in bone metabolism. PET/CT has better specificity
and sensitivity for detecting metastatic tumors than ECT and can
evaluate the overall metastasis to help in the staging of tumors
(22,23). In the present case, PET/CT showed
bone destruction in the sternum, multiple vertebrae, and right
sixth and seventh ribs, with slightly increased glucose metabolism.
No lesions, other than those in the bone, were observed. Bone
metastases, primary bone tumors and MM are all associated with bone
destruction. However, no other suspicious lesions were found during
PET/CT imaging in this patient. Relying solely on imaging results
and clinical symptoms may not provide definitive evidence to
differentiate between these two conditions, so it may be necessary
to perform invasive methods such as bone marrow aspiration and
biopsy of pathological tissues to establish an accurate diagnosis.
This can help avoid misdiagnosis and ensure appropriate management
and treatment for the patient. The bone marrow smear showed
abnormal proliferation of plasma cells, morphologically consistent
with MM. The biopsy of the lesion indicated plasmacytoma,
suggesting the involvement of MM.
Analysis of the possibility of simple bone
metastasis after radical resection of rectal cancer is crucial for
accurate diagnosis. The present patient underwent regular follow-up
for 6 years after radical treatment and all follow-up indicators
were normal. However, multiple skeletal abnormalities were
discovered 7 years after treatment. The first consideration is the
possibility of distant metastasis in patients with rectal cancer
who have been well followed up for several years without local
recurrence. Bone metastasis is relatively rare in CRC and accounts
for only 1% of all bone metastases. Cases of bone metastases
without evidence of visceral (lung or liver) metastases are even
rarer (24). Kanthan et al
(25) reviewed patients with rectal
cancer who had been treated for 25 years. Among 137 patients with
bone metastases, only 1% had bone metastases without local
recurrence or visceral (liver and lung) metastases (25). Bone is not the main site of
metastasis in rectal cancer and CRC is not a common source of bone
metastases (16,26). A retrospective study of 516 patients
with CRC found that the incidence rate of metachronous bone
metastasis was 6.0% and the median time of occurrence was 15 months
(range, 1–89 months). Bone metastasis occurred more often after
rectal cancer surgery than after colon cancer surgery. Tumor
location (P=0.039) and lymph node involvement (P=0.003) were
independent risk factors for metachronous bone metastasis (4). The median interval between initial
treatment of CRC and metachronous diagnosis of bone metastasis is
20.0 months (interquartile range, 9.0–46.5 months) (27). There are even case reports of local
recurrence and bone metastasis in the 10th year after colon cancer
surgery (28). Another study showed
that, compared with synchronous bone metastases from CRC,
metachronous bone metastases are more likely to have multiple bone
metastases (63.0 vs. 7.9%; P<0.001) and originate from rectal
cancer (60.9 vs. 41.3%; P=0.033) (29). In summary, patients with rectal
cancer and good follow-up may experience metachronous bone
metastasis several years after surgery and multiple bone metastases
may also occur.
Based on the above analysis, the patient of the
current study mainly presented with bone pain and destruction,
which are highly similar to the symptoms of MM without specific
manifestations. Although the incidence of metachronous bone
metastasis after radical resection of rectal cancer is relatively
low, a patient's diagnosis of postoperative bone metastasis at
multiple hospitals may be reasonable. The diagnosis of MM in this
patient was reached incidentally. When the patient underwent
surgical treatment for a hernia near the colostomy stoma at our
hospital 3 years earlier, no abnormalities were observed in the
preoperative blood routine parameters, coagulation function, liver
function, kidney function or urine routine parameters.
Postoperative hematoma around the stoma, persistent anemia and
abnormal coagulation function were observed. To clarify the cause
of the abnormal coagulation function, a bone marrow puncture was
performed, which led to the diagnosis of MM. If the patient had not
undergone this surgery, the diagnosis may have remained elusive,
and he may have continued to be misdiagnosed with postoperative
bone metastasis from rectal cancer, potentially leading to a delay
in addressing the patient's condition.
Delay in diagnosis can potentially lead to tumor
progression, bringing a greater physical burden to the patient,
such as worsening pain and organ dysfunction. If the disease
progresses rapidly, this may result in changes and limitations to
the treatment options, as well as potentially reducing the success
rate of treatment and the patients' survival rate. Patients who
experience delayed diagnosis may face prolonged uncertainty and
anxiety, which can have a negative impact on their mental
well-being.
During the 10-year follow-up period after surgery
for rectal cancer, regular monitoring was conducted, including
regular evaluations of digestive system tumor markers (CEA, CA199),
abdominal CT scans and colonoscopies, all of which did not reveal
any abnormalities. The prognosis for rectal cancer in this patient
was relatively favorable, with a low probability of recurrence.
Before 2000, the median overall survival of MM was close to 30
months, but now the median overall survival can exceed 10 years
(30). This patient had been
receiving standardized treatment since the diagnosis of MM, and to
date, 4 years have passed with slow disease progression.
The misdiagnosis of the patient of the present was
unlikely to have occurred due to the characteristics of the case.
However, the phenomenon of misdiagnosis remains widespread. The
attending doctors from multiple hospitals unanimously thought that
the patient had bone metastasis after rectal cancer surgery, and
imaging experts from these hospitals considered the possibility of
bone metastatic tumors when drafting their reports. Therefore, the
diagnosis and treatment of multiple primary tumors should be taken
seriously. Because errors in cancer diagnosis may be the most
harmful type of diagnostic error, they are increasingly being
valued (31). Diagnostic errors,
defined as omissions, delays or misdiagnoses, are common causes of
medical errors in the US (32).
Various factors can lead to these errors, depending on the specific
types of cancer. Raab and Grzybicki (33) found that diagnostic errors are
related to five areas of complex healthcare systems: Doctor-patient
contact in clinical settings, diagnostic testing or program
performance, pathological diagnosis, patient follow-up or
examination results, and patient-related delays. One study found
that ~4% of abnormal imaging results were missed when evaluating
results in a computerized test result notification system; among
them, the vast majority involved the diagnosis of potential new
malignant tumors (34).
In conclusion, challenges remain when distinguishing
between metachronous bone metastases and MM in patients radically
treated for rectal cancer with good long-term follow-up, making
these patients prone to misdiagnosis. The risk of misdiagnosis is
higher in patients with MM who lack clear clinical manifestations.
If no increase in digestive tract tumor markers is identified, but
multiple bone destructions are observed during long-term follow-up
after rectal cancer surgery, clinicians should routinely perform
differential diagnoses of the lesions. The lesions may be primary
bone tumors, bone metastases or MM. Primary bone tumors discovered
in the short term are usually solitary, so in this scenario,
differentiation between bone metastases and MM is crucial.
Diagnosis requires bone marrow smears or pathological examination
of the lesion tissue. Meanwhile, with an increasing number of
reports on multifocal primary tumors, clinicians can accumulate
experience and refer to the literature to enhance vigilance for
suspicious cases and minimize the risk of misdiagnosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present case report may be
requested from the corresponding author.
Authors' contributions
YH and ZC collected image materials from the
hospital's information, pathology and hematology departments. YH,
ZC and KW acquired and interpreted the clinical data, drafted and
revised the manuscript and confirmed the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nielsen DL, Palshof JA, Larsen FO, Jensen
BV and Pfeiffer P: A systematic review of salvage therapy to
patients with metastatic colorectal cancer previously treated with
fluorouracil, oxaliplatin and irinotecan +/- targeted therapy.
Cancer Treat Rev. 40:701–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Augestad KM, Bakaki PM, Rose J, Crawshaw
BP, Lindsetmo RO, Dørum LM, Koroukian SM and Delaney CP: Metastatic
spread pattern after curative colorectal cancer surgery. A
retrospective, longitudinal analysis. Cancer Epidemiol. 39:734–744.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun C, Deng Y, Zhou H and Hu ZQ: Risk
factors for the development of metachronous bone metastasis in
colorectal cancer patients after curative resection. Int J Surg.
21:145–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kyle RA and Rajkumar SV: Multiple myeloma.
N Engl J Med. 351:1860–1873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alzrigat M, Párraga AA and
Jernberg-Wiklund H: Epigenetics in multiple myeloma: From
mechanisms to therapy. Semin Cancer Biol. 51:101–115. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durie BG, Harousseau JL, Miguel JS, Bladé
J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J,
Sonneveld P, et al: International uniform response criteria for
multiple myeloma. Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyle RA and Rajkumar SV: Criteria for
diagnosis, staging, risk stratification and response assessment of
multiple myeloma. Leukemia. 23:3–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhai C, Cai Y, Lou F, Liu Z, Xie J, Zhou
X, Wang Z, Fang Y, Pan H and Han W: Multiple primary malignant
tumors-a clinical analysis of 15,321 patients with malignancies at
a single center in China. J Cancer. 9:2795–2801. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
San Miguel JF, Gutiérrez NC, Mateo G and
Orfao A: Conventional diagnostics in multiple myeloma. Eur J
Cancer. 42:1510–1519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katzel JA, Hari P and Vesole DH: Multiple
myeloma: Charging toward a bright future. CA Cancer J Clin.
57:301–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li QL, Ma JA, Li HP, Huang RB, Hu CH, Liu
XL, Gao YW, Feng GH and Wu F: Synchronous colorectal cancer and
multiple myeloma with chest wall involvement: Is this a
coincidence? Curr Probl Cancer. 41:413–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kou CJ, Romain J, Broadwater DR and
Barnett T: Colorectal cancer in a patient with multiple myeloma: A
treatment Dilemma. Cureus. 12:e121122020.PubMed/NCBI
|
|
14
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Moos R, Costa L, Gonzalez-Suarez E,
Terpos E, Niepel D and Body JJ: Management of bone health in solid
tumours: From bisphosphonates to a monoclonal antibody. Cancer
Treat Rev. 76:57–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christensen TD, Jensen SG, Larsen FO and
Nielsen DL: Systematic review: Incidence, risk factors, survival
and treatment of bone metastases from colorectal cancer. J Bone
Oncol. 13:97–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhenghong and Zihua Z: Guoweijian,
Zhangning, Caiyunyun, Yingjiangshan and Xiaomi: Retrospective study
of predictors of bone metastasis in colorectal cancer patients. J
Bone Oncol. 9:25–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sundermeyer ML, Meropol NJ, Rogatko A,
Wang H and Cohen SJ: Changing patterns of bone and brain metastases
in patients with colorectal cancer. Clin Colorectal Cancer.
5:108–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santini D, Tampellini M, Vincenzi B,
Ibrahim T, Ortega C, Virzi V, Silvestris N, Berardi R, Masini C,
Calipari N, et al: Natural history of bone metastasis in colorectal
cancer: Final results of a large Italian bone metastases study. Ann
Oncol. 23:2072–2077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coleman RE, Croucher PI, Padhani AR,
Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R and
Costa L: Bone metastases. Nat Rev Dis Primers. 6:832020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
von Eyben FE and Kairemo K: Meta-analysis
of (11)C-choline and (18)F-choline PET/CT for management of
patients with prostate cancer. Nucl Med Commun. 35:221–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wondergem M, van der Zant FM, van der
Ploeg T and Knol RJ: A literature review of 18F-fluoride PET/CT and
18F-choline or 11C-choline PET/CT for detection of bone metastases
in patients with prostate cancer. Nucl Med Commun. 34:935–945.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Connelly TM, Piggott RP, Waldron RM and
O'Grady P: Unusual osseous metastases from rectal adenocarcinoma: A
case report and review of the literature. J Gastrointest Surg.
19:1177–1186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanthan R, Loewy J and Kanthan SC:
Skeletal metastases in colorectal carcinomas: A Saskatchewan
profile. Dis Colon Rectum. 42:1592–1597. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawamura H, Yamaguchi T, Yano Y, Hozumi T,
Takaki Y, Matsumoto H, Nakano D and Takahashi K: Characteristics
and prognostic factors of bone metastasis in patients with
colorectal cancer. Dis Colon Rectum. 61:673–678. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Assi R, Mukherji D, Haydar A, Saroufim M,
Temraz S and Shamseddine A: Metastatic colorectal cancer presenting
with bone marrow metastasis: A case series and review of
literature. J Gastrointest Oncol. 7:284–297. 2016.PubMed/NCBI
|
|
29
|
Ma CX, Guan X, Wei R, Wang S, Quan JC,
Zhao ZX, Chen HP, Liu Z, Jiang Z and Wang XS: The distinction of
clinicopathological characteristics, treatment strategy and outcome
in colorectal cancer patients with synchronous vs. Metachronous
bone metastasis. Front Oncol. 10:9742020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cowan AJ, Green DJ, Kwok M, Lee S, Coffey
DG, Holmberg LA, Tuazon S, Gopal AK and Libby EN: Diagnosis and
management of multiple myeloma: A review. JAMA. 327:464–477. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh H, Sethi S, Raber M and Petersen LA:
Errors in cancer diagnosis: Current understanding and future
directions. J Clin Oncol. 25:5009–5018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Phillips RL Jr, Bartholomew LA, Dovey SM,
Fryer GE Jr, Miyoshi TJ and Green LA: Learning from malpractice
claims about negligent, adverse events in primary care in the
United States. Qual Saf Health Care. 13:121–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raab SS and Grzybicki DM: Quality in
cancer diagnosis. CA Cancer J Clin. 60:139–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh H, Arora HS, Vij MS, Rao R, Khan MM
and Petersen LA: Communication outcomes of critical imaging results
in a computerized notification system. J Am Med Inform Assoc.
14:459–466. 2007. View Article : Google Scholar : PubMed/NCBI
|