Introduction
Intrahepatic cholangiocarcinoma (iCCA) is the second
most prevalent type of primary liver cancer, comprising ~15% of
cases of primary liver cancers (1).
Recently, iCCA have been classified into small- and large-duct
types. Cholangiolocellular carcinoma (CoCC) is an extremely rare
tumor that accounts for <1% of all primary liver cancers
(2). CoCC has been categorized as a
small-duct type iCCA, renamed cholangiolocarcinoma (CLC), based on
the 2019 World Health Organization classification (3). In clinical practice, large-duct type
iCCA frequently recur despite curative surgical resection and are
resistant to chemotherapy or other drugs owing to their high
invasiveness (4,5). Therefore, large-duct type iCCA have a
worse prognosis than small-duct type iCCA (6). In contrast, CLC has a better prognosis
after curative resection than iCCA, because it is less invasive
(7).
iCCAs arise from every part of the intrahepatic
biliary system, including the peribiliary glands (8). In contrast, CLCs are hypothesized to
originate from hepatic stem cells or hepatic progenitor cells
(HPCs) occupying the canals of Hering or cholangioles and cells of
the peripheral branches of the intrahepatic bile ducts (9,10).
Liver damage and chronic stimulation have been implicated in the
activation of HPCs and carcinogenesis (9).
Histologically, large-duct type iCCA are
characterized by cuboidal or columnar cells with mucus production
constituting an irregular ductal or tubular arrangement (11). In contrast, CLC is characterized by
small cuboidal cells without mucus, forming angular small ductular,
antler-like or branched arrangement patterns with abundant
hyalinized fibrous stroma (11).
However, in clinical practice, tumors with mixed CLC and iCCA, with
varying degrees of differentiation are often encountered. Hence, it
is essential to elucidate the significance of tumor heterogeneity
related to CLC components.
Several histological variants of iCCAs have been
previously reported. Recently, iCCA tumor heterogeneity has been
the focus of attention because it is among the factors contributing
to iCCA resistance to therapy (12–14).
However, it is unclear how tumors with mixed CLC and iCCA differ
clinically from iCCAs that do not contain any CLC components. A
previous study defined pure CLC as a tumor comprising >80% of
CLC components within a single tumor (15,16).
We hypothesized that tumors containing various proportions of iCCA
and CLC in a single tumor would have different recurrence or
survival rates after resection compared with pure iCCA (not
containing any CLC components).
In previous years, the pathological diagnosis of CLC
has been made not only by hematoxylin and eosin (H&E) staining
but also by evaluating molecular biology findings by immunostaining
(15). However, most previous
reports on the results of clinicopathological features and surgical
outcomes for CLC included patients with CLC diagnosed with only
H&E staining and few reports included patients with CLC
evaluated based on molecular biology findings (2,17,18).
The present study was performed to identify the CLC
component within an iCCA tumor diagnosed by assessing not only
morphological features but also molecular biological features by
immunostaining and reviewing the clinicopathological features,
surgical outcomes and prognosis. Moreover, the impact on prognosis
of tumor heterogeneity due to CLC components was evaluated.
Materials and methods
Study design
The present study was a retrospective observational
study conducted at a single institution. This study was approved by
the Kanazawa University Ethics Committee (approval no. 3221-2) and
was performed in accordance with the ethical standards stated in
the Declaration of Helsinki. Informed consent for this study was
obtained from all participants, from Kanazawa University Hospital's
website, as an opt-out option. All data, including immunostaining,
were obtained from medical records.
Pathological diagnosis of CLC
CLC was pathologically defined as the proliferation
of tumor cells with insufficient mucus composed of small tubular
glands, antler-like or anastomotic patterns with abundant fibrous
stroma by H&E staining, as previously described (11,19).
H&E staining was performed according to our institutional
protocol. In brief, after deparaffinization, 4 µm sections were
stained with hematoxylin solution for 5 min, and rinsed under
running water for 15 min. Then the sections were stained with eosin
solution for 2 min and followed by dehydration with graded alcohol
and clearing in xylene. In addition to the morphological findings
identified following H&E staining, CLC cells were further
identified using immunohistochemistry and mucicarmine staining.
Positive epithelial membrane antigen (EMA) staining in the
glandular lumen, positive neural cell adhesion molecule 1 (NCAM1)
staining and the absence of mucin production were observed in CLC
(15,20). In contrast, positive EMA staining in
the cytoplasm, negative NCAM1 expression, and mucin production are
usually observed in iCCA (15,20).
For the immunohistochemical analysis of EMA and NCAM1, resected
samples were immediately fixed in 10% neutral buffered formalin at
room temperature for 24 h, embedded in paraffin and sliced into 4
µm sections. For antigen retrieval, sections were treated with 0.05
M citric acid buffer (pH 6) at 95°C for 20 min in a microwave oven.
After blocking endogenous peroxidase activity by 3% hydrogen
peroxide solution at room temperature for 10 min, the sections were
incubated with primary antibodies against EMA clone E29 (cat. no.
IR629; Dako; Agilent Technologies, Inc.) and NCAM1 (cat. no.
418191; Nichirei Biosciences, Inc.) at 4°C overnight. Envision +
solution (Dako; Agilent Technologies, Inc.) was then applied for 30
min at room temperature and the reaction products were visualized
using 3, 3′-diaminobenizidine tetrahydrochloride (MilliporeSigma)
and H2O2. After that the sections were
counterstained with hematoxylin for 1 min at room temperature.
Sections were observed under a BX51 optical microscope (Olympus
Corporation).
The CLC component was distinguished from the iCCA
component based on the morphological characteristics identified
using H&E staining and immunostaining.
Selection of patients with CLC
Between April 2006 and June 2022, 774 patients
underwent liver resection for primary liver cancer in Kanazawa
University Hospital. This period was determined by the availability
of the clinical data to be analyzed in the present study. Of the
774 patients, only 65 (8.4%) were diagnosed with iCCA after
surgery. Of these 65 iCCA patients, only 14 (21.5%) patients
containing CLC component in a single tumor met the selection
criteria and were defined as patients with CLC. Inclusion criteria
were: i) That curative resection was intended and ii) that the CLC
component should be present in >5% of a single tumor. Exclusion
criteria were: i) No synchronous cancer in other organs, ii) no
preoperative chemotherapy, and iii) no hepatocellular carcinoma
component in a tumor. Pathological diagnosis and evaluation were
independently performed by four board-certified pathologists.
Classification of pure type and
partial type CLC
Based on the pathological proportion of the CLC
component within a single iCCA tumor, CLC were classified into pure
and partial types. A tumor in which the CLC component occupied
>95% of the whole tumor was defined as a pure type and the rest
(CLC component ≤95%) were defined as a partial type. For the
classification, a board-certified expert pathologist blindly
performed morphological evaluation of all H&E slides and
immunostaining to determine the percentage of CLC component.
Representative cases with the pathological findings of the CLC
component in pure-type CLC (Fig.
1A) and the iCCA component in partial-type CLC (Fig. 1B) are presented.
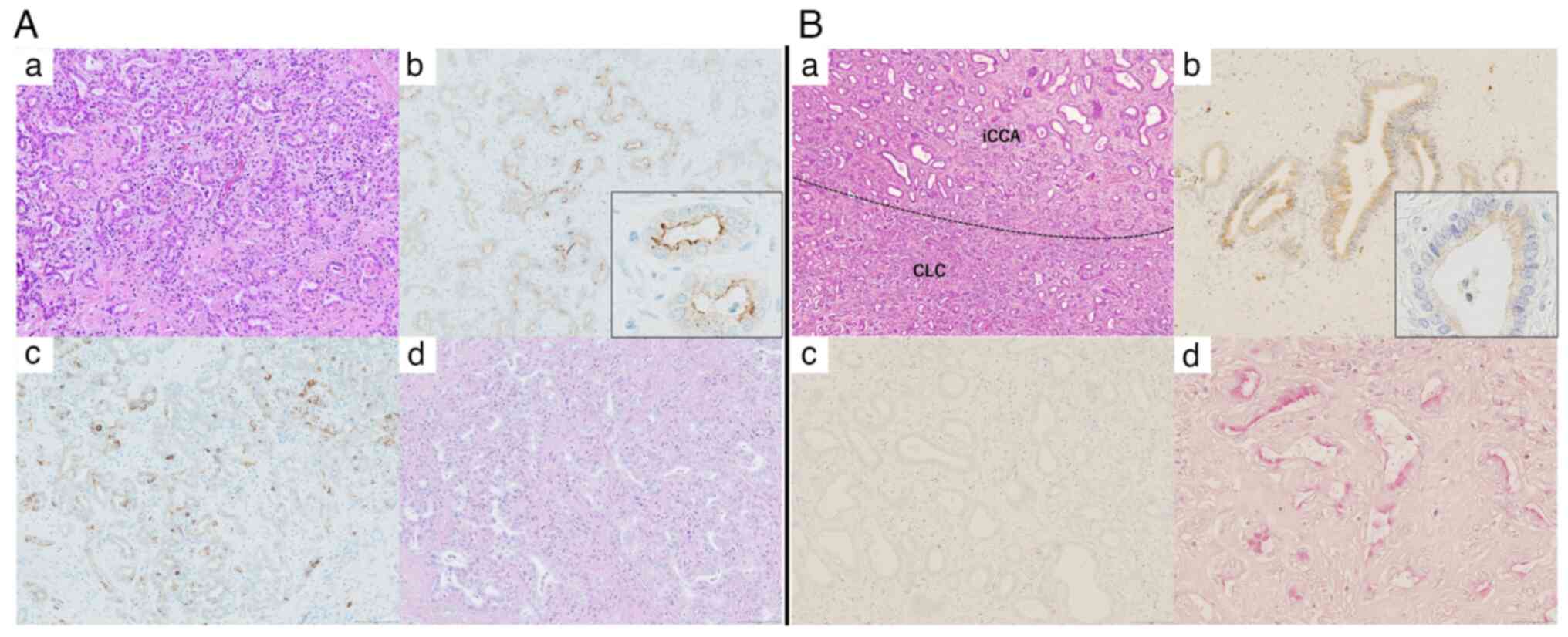 | Figure 1.Histopathological findings of CLC.
Histopathological findings indicating the CLC component (pure-type
CLC). (A-a) Small tubular gland-forming cells proliferate in
antler-like and anastomosing patterns with abundant fibrous stroma
(hematoxylin and eosin staining; magnification, ×100). (A-b)
Immunohistochemical result for EMA is positive in the apical
membrane of the tumor glands (magnification, ×100). The inset shows
image of grandular duct (magnification, ×400). (A-c)
Immunohistochemical result for NCAM1 is positive in the tumor cells
(magnification, ×100). (A-d) Mucin production is not observed in
tumor cells stained with mucicarmine (magnification, ×100).
Histopathological findings indicating the iCCA component
(partial-type CLC). (B-a) The iCCA and CLC components within a
single tumor. Proliferation of atypical large tubular glands with
fibrous stroma is identified in the iCCA component (hematoxylin and
eosin staining; magnification, ×100). (B-b) Immunohistochemical
result for EMA is positive in the cytoplasm of tumor cells
(magnification, ×100). The inset shows image of grandular duct
(magnification, ×400). (B-c) Immunohistochemical result for NCAM1
is negative in tumor cells (magnification, ×100). (B-d) Mucin
production is observed in tumor cells on mucicarmine staining
(magnification, ×100). CLC, cholangiolocarcinoma; NCAM1, neural
cell adhesion molecule 1; EMA, epithelial membrane antigen; iCCA,
intrahepatic cholangiocarcinoma. |
Clinicopathological evaluation of CLC
patients
Each patient was pathologically staged according to
the 8th edition of the Union for International Cancer Control
(UICC) Staging System (21) after
surgery. The gross morphology of the tumor was classified according
to the general rules for the clinical and pathological study of
primary liver cancer (22). The
location of the tumor in the liver was classified as hilar or
peripheral, depending on the presence or absence of contact with
the hepatic hilum (between the right side of the umbilical portion
of the left portal vein and the left side of the origin of the
right posterior portal vein) based on preoperative computed
tomography (CT) and pathological findings of the resected specimen,
as previously described (23). The
extent and number of lymph nodes dissected were in accordance with
the Japanese Society of Biliary Surgery classification (24). Postoperative complications were
classified according to the Clavien-Dindo classification (25).
Follow-up of patients with CLC
All the patients were followed up after surgery as
an outpatient in our institution for >5 years (unless they died
or dropped out). In principle, contrast-enhanced CT and blood
examination including tumor markers were performed every 3–6 months
for evaluation of recurrence. The survival period was defined as
the date of surgery to death or last contact. If recurrence was
detected, systemic chemotherapy, local treatment if suitable or
best supportive care was considered depending on the condition of
the patient.
Statistical analysis
Overall survival (OS), recurrence-free survival
(RFS), and disease-specific survival (DSS) rates were calculated
using the Kaplan-Meier method, and the log-rank test was used for
comparison. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
Graph Pad Prism (9.4.1; Dotmatics).
Results
Patient characteristics
The characteristics of all patients are presented in
Table I. The median age of these
patients was 70.5 (range, 62–80) years. There were 10 males (71.4%)
and four females (28.6%). Nine patients (64.3%) had a chronic liver
injury (alcohol, n=1; chronic hepatitis due to hepatitis B virus,
n=2; chronic hepatitis due to hepatitis C virus, n=4; liver
cirrhosis due to hepatitis C virus, n=1; and nonalcoholic
steatohepatitis, n=1). The liver function of all patients was well
preserved based on the Child-Pugh classification A. Six patients
(42.9%) had elevated carbohydrate antigen 19-9 (CA19-9) levels
(>37.0 ng/ml), and two (14.3%) had elevated alpha-fetoprotein
(AFP) levels (>10.0 ng/ml). Only one patient (7.1%) had elevated
carcinoembryonic antigen (CEA) protein levels (>5.0 ng/ml) and
one patient (7.1%) had elevated levels of protein induced by the
absence of vitamin K or antagonist-II (PIVKA-II; ≥40.0 mAU/ml),
respectively. CLC was diagnosed preoperatively in eight of 14
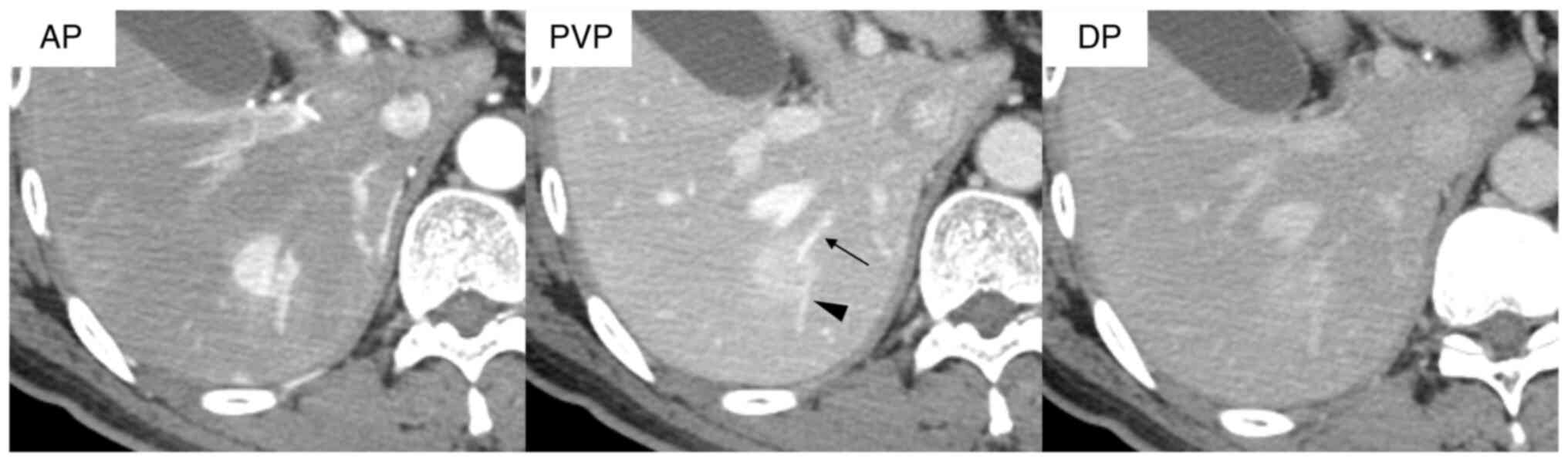
patients (57.1%) based on characteristic imaging features,
including hypervascularity and enhancement during the arterial
phase (rim arterial phase hyperenhancement; AP), prolonged
enhancement in the delayed phase, and the presence of intratumoral
pre-existing vessels, such as the portal and hepatic veins, on
dynamic contrast-enhanced CT. A representative of these finding is
presented for one patient with CLC (Fig. 2). According to the classification by
tumor location from the preoperative CT findings, there were 11
peripheral-type (78.6%) and three hilar-type CLCs (21.4%).
Preoperative patient characteristics were presented in Table II.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case | Age, years | Sex | Underlying liver
disease | CEA, ng/ml
(≤5.0) | CA19-9, ng/ml
(≤37.0) | AFP, ng/ml
(≤10.0) | PIVKA-II, mAU/ml
(<40.0) | Tumor location | Type of liver
resection | Number of lymph nodes
removed |
|---|
| 1 | 80 | F | HCV | 2.5 | 19 | 5 | 17 | peripheral | Partial hepatectomy
(S5/6) | - |
| 2 | 62 | M | NL | 3.4 | 76 | 5 | 22 | hilar | Left hepatectomy | + (8,12) |
| 3 | 73 | M | HCV | 4.5 | 45 | 202 | 24 | peripheral | Left lateral
sectionectomy | - |
| 4 | 75 | M | NL | 4.2 | 41 | 5 | 18 | peripheral | Left
hepatectomy | + (12) |
| 5 | 62 | M | NL | 4.2 | 24 | 5 | 31 | peripheral | Partial hepatectomy
(S7/8) | - |
| 6 | 79 | F | HCV | 2 | 59 | 3 | 753 | hilar | Left extended
hepatectomy with bile duct resection | + (12) |
| 7 | 70 | F | LC(C) | 0.9 | 44 | 4 | 11 | peripheral | Partial hepatectomy
(S5/6) | - |
| 8 | 69 | M | NL | 4.8 | 4 | 2 | 17 | peripheral | Right posterior
sectionectomy | - |
| 9 | 63 | M | HBV | 1.9 | 23 | 89 | 27 | peripheral | S7
segmentectomy | - |
| 10 | 78 | F | NASH | 2 | 7 | 4 | 14 | Peripheral | Laparoscopic
partial hepatectomy (S8) | - |
| 11 | 78 | M | NL | 1.8 | 24 | 4 | 16 | hilar | Left extended
hepatectomy with bile duct resection | +
(3,7,8,12,13a) |
| 12 | 71 | M | HBV | 2.1 | 25 | 7 | 16 | peripheral | Laparoscopic S3
segmentectomy | - |
| 13 | 66 | M | HCV | 1.5 | 17 | 4 | 16 | peripheral | S7
segmentectomy | - |
| 14 | 70 | M | Alcoholic | 5.3 | 208 | 6 | 21 | peripheral | Laparoscopic
partial hepatectomy (S8) | - |
 | Table II.Preoperative patient
characteristics. |
Table II.
Preoperative patient
characteristics.
| Variable | Value |
|---|
| CLC, no. | 14 |
| Sex male, no.
(%) | 10 (71.4) |
| Age, median
(range), year | 70.5 (62–80) |
| Underlying liver
disease, no. (%) |
|
|
Present | 9 (64.3) |
|
Alcohol | 1 (11.1) |
|
HBV | 2 (22.2) |
|
HCV | 5 (55.6) |
|
NASH | 1 (11.1) |
| CEA |
|
| Median
(range), ng/ml | 2.3 (0.9–5.3) |
| >5.0
ng/ml, no. (%) | 1 (7.1) |
| CA19-9 |
|
| median
(range), ng/ml | 24.5
(4.0–208.0) |
|
>37.0 ng/ml, no. (%) | 6 (42.9) |
| AFP |
|
| median
(range), ng/ml | 5.0
(2.0–202.0) |
|
>10.0 ng/ml, no. (%) | 2 (14.3) |
| PIVKA-II |
|
| median
(range), ng/ml | 17.5
(11.0–753.0) |
| ≥40.0
mAU/ml, no. (%) | 1 (7.1%) |
| Preoperative
diagnosis of CLC, no. (%) | 8 (57.1) |
| Number of tumor,
no. (%) |
|
|
single | 13 (92.8) |
|
multiple | 1 (7.1) |
Laparoscopic hepatectomy was performed in three
patients (21.4%), all of whom had peripheral-type CLC based on the
preoperative image findings. Anatomical liver resection was
performed in nine patients (left extended hepatectomy, n=2; left
hepatectomy, n=2; right posterior sectionectomy, n=1; left lateral
sectionectomy, n=1; and segmentectomy, n=3), and partial
hepatectomy was performed in five patients. Lymph node dissection
was performed in four patients (28.6%), which consisted of all
three patients with hilar-type CLC and one with peripheral-type
CLC. Two patients (14.3%) with hilar-type CLC underwent
extrahepatic bile duct resection. The median operative time and
blood loss were 367 min and 310 ml, respectively. The
intraoperative transfusion rate was 21.4%. Early postoperative
complications with Clavien-Dindo classification IIIa occurred in
three patients (21.4%; bile leakage, n=2; pleural fluid, n=1). The
median length of hospital stay was 21 days. No postoperative deaths
occurred within 90 days.
Pathological findings and
prognosis
The pathological findings and prognosis are
presented in Table III. The gross
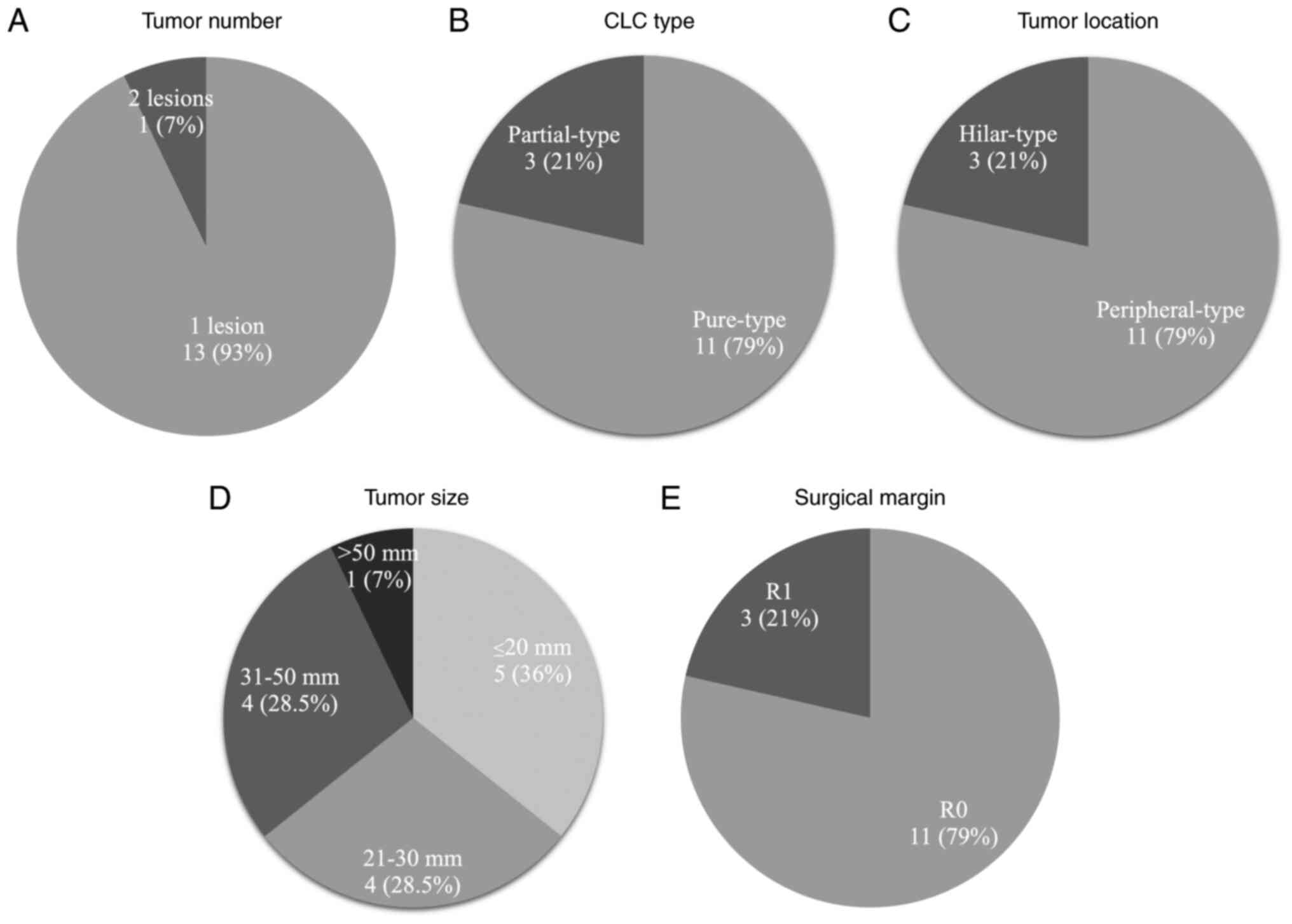
type of all the resected CLCs based on macroscopic findings was the
mass-forming (MF) type. One patient (7.1%) had two lesions in the
resected specimen (Fig. 3A).
According to the classification of tumor heterogeneity by the CLC
component proportion, there were 11 patients with pure (78.6%) and
three with partial (21.4%) type CLCs (Fig. 3B). According to the pathological
classification of tumor location, there were 11 peripheral-type
(78.6%) and three hilar-type (21.4%) CLCs (Fig. 3C).
 | Table III.Pathological findings and
prognosis. |
Table III.
Pathological findings and
prognosis.
| Case no. | Tumor no. | CLC type (CLC
component) | Tumor location | Size (mm) | Lymph node
metastasis | Vascular
invasion | T (UICC) | Stage (UICC) | Surgical
margin | Recurrence
site | Treatment for
recurrence | Follow-up period
(months) | Death (cause of
death) |
|---|
| 1 | 1 | Pure | peripheral | 35 | n.a. | + | 2 | II | R0 | - | - | 114 | no |
| 2 | 1 | Pure | hilar | 52 | - | + | 2 | II | R0 | - | - | 103 | no |
| 3 | 1 | Partial (50%) | peripheral | 20 | n.a. | + | 2 | II | R0 | - | - | 113 | no |
| 4 | 1 | Pure | peripheral | 25 | - | + | 2 | II | R0 | - | - | 57 | no |
| 5 | 1 | Partial (15%) | peripheral | 35 | n.a. | + | 2 | II | R0 | - | - | 57 | yes (other
disease) |
| 6 | 1 | Pure | hilar | 26 | + | + | 3 | IIIB | R1 | lymph node,
peritoneum | chemotherapy | 31 | yes (CLC) |
| 7 | 1 | Pure | peripheral | 32 | n.a. | + | 2 | II | R0 | - | - | 95 | no |
| 8 | 1 | Pure | peripheral | 23 | n.a. | - | 1a | IA | R0 | - | - | 93 | no |
| 9 | 1 | Pure | peripheral | 19 | n.a. | + | 2 | II | R0 | - | - | 59 | yes
(pneumonia) |
| 10 | 1 | Pure | peripheral | 15 | n.a. | + | 2 | II | R0 | - | - | 60 | no |
| 11 | 2 | Pure | hilar | 50 | - | + | 2 | II | R1 | local | BSC | 64 | yes (CLC) |
| 12 | 1 | Pure | peripheral | 17 | n.a. | + | 2 | II | R0 | - | - | 38 | no |
| 13 | 1 | Pure | peripheral | 16 | n.a. | - | 1a | IA | R0 | - | - | 19 | no |
| 14 | 1 | Partial (25%) | peripheral | 28 | n.a. | + | 2 | II | R1 | - | - | 12 | no |
The median maximum diameter of the primary tumors
was 25.5 mm (Fig. 3D). Lymph node
metastasis was detected in one patient (7.1%) and vascular invasion
was detected in 12 patients (85.7%). Based on the eighth edition of
the UICC, two, 11 and one patient had stage IA, II and IIIB CLC,
respectively.
R0 resection was achieved in 11 patients (78.6%) and
R1 resection in 3 patients (21.4%, Fig.
3E). Two of the three patients (66.6%) who underwent R1
resection had hilar-type CLC. These patients underwent left
extended hepatectomy and extrahepatic bile duct resection; one was
surgical margin-positive at the bile duct transection and dissected
surface (case 6) and the other was surgical margin-positive at the
transection surface (case 11). Another case of R1 resection was a
peripheral-type, and the patient underwent laparoscopic partial
hepatectomy with tumor exposure on part of the transection surface
(case 14).
In the median follow-up period of 59.5 (range,
12–114) months, recurrence of CLC occurred in two patients with
positive surgical margins (cases 6 and 11). In one patient (case
6), distant lymph node metastases (para-aortic lymph nodes) and
peritoneal dissemination were observed 14 months postoperatively.
Although chemotherapies with tegafur-gimeracil-oteracil potassium,
gemcitabine and paclitaxel were administered sequentially, the
disease progressed, and the patient died 31 months postoperatively.
In one patient (case 11), a local recurrence was detected on the
transection surface of the liver 63 months postoperatively. The
patient did not wish treatment for recurrence and died 64 months
postoperatively. Recurrence of lymph node metastasis was observed
in only one patient with hilar-type CLC. There were no CLC
recurrences in patients who underwent R0 resection. The cumulative
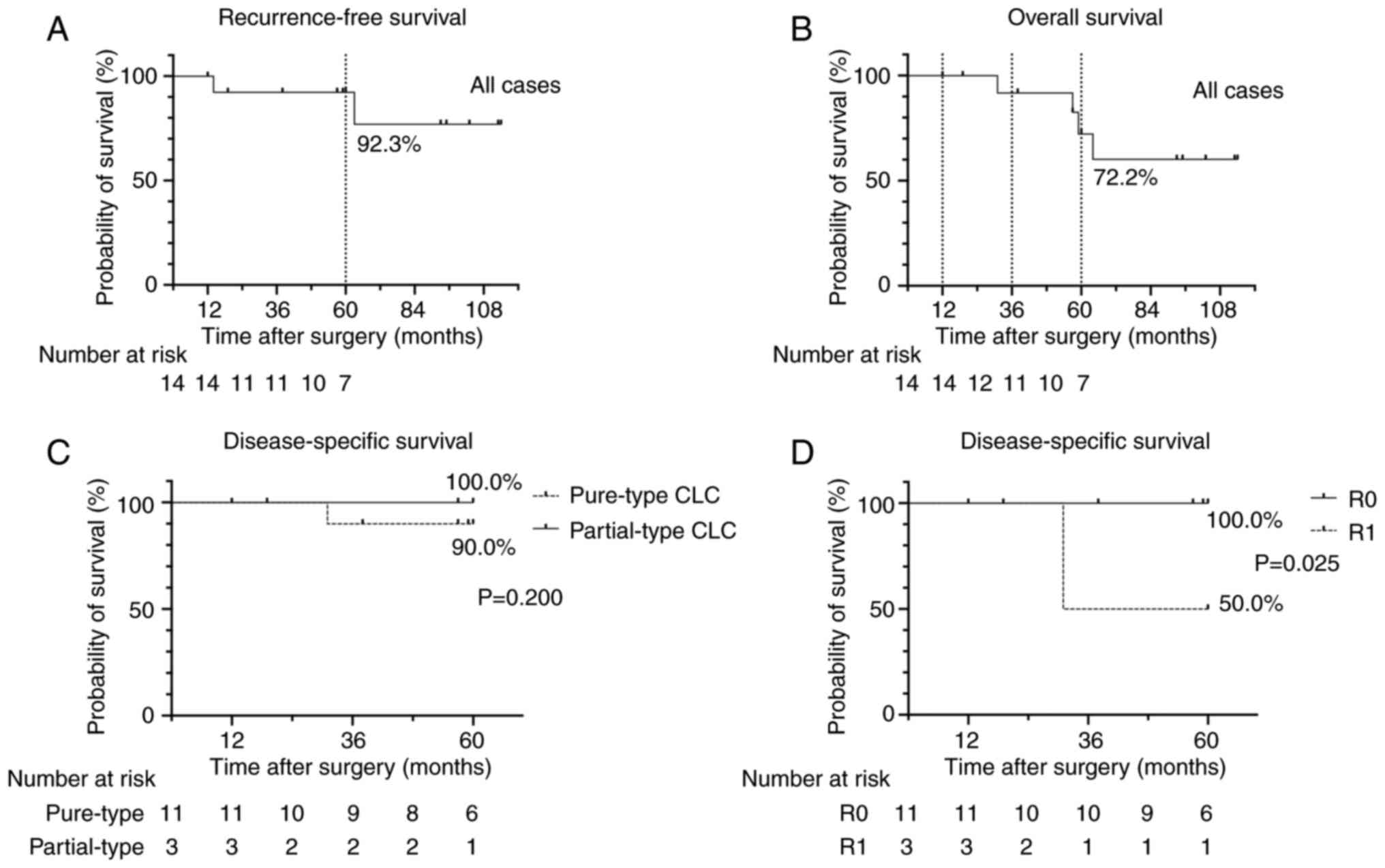
5-year RFS rate in the 14 patients after surgery was 92.3%
(Fig. 4A). The cumulative 1, 3 and
5-year OS rates in the 14 patients were 100, 91.7 and 72.2%,
respectively (Fig. 4B). Comparison
of the cumulative 5-year DSS rate between patients with pure-type
and partial-type CLC showed no significant differences (Fig. 4C). The cumulative 5-year DSS rate
was significantly higher in the R0 resection group (100.0%) than
that in the R1 resection group (Fig.
4D).
Discussion
CLC is an extremely rare primary liver tumor that
accounts for ~0.6% of all primary liver tumors (2), and to the best of our knowledge, there
are few reports on the clinicopathological features of patients
with CLC. In the present study, 14 patients with CLC who underwent
resection at a single institution were included. Additionally, all
CLC tumors were diagnosed using molecular biology markers with
immunostaining in addition to morphological evaluation of H&E
staining.
Regarding the characteristics of underlying liver
disease, a previous study reported that >50% of patients with
CLC had chronic liver injury (26).
The frequency of chronic liver injury in patients with CLC has also
been reported to be higher than that in patients with iCCA
(27). In the present study, nine
patients (64.3%) had an underlying chronic liver injury, most of
whom had chronic viral hepatitis. These results support the
hypothesis that chronic inflammation is associated with the
development of CLC. With regards the characteristics of CLC in
blood tests, it has been reported that the number of patients with
abnormal CA19-9 levels is lower among those with CLC than among
those with iCCA (7). In the present
study, only six of the patients (42.9%) had elevated CA19-9;
however, this was the most frequently elevated of the tumor
markers.
The characteristic radiological findings of CLC
include hypervascularity in the AP, peritumoral enhancement (either
ring-like or wedge-shaped in the AP), small intratumoral portal
tracts, rare peripheral bile duct dilatation and prolonged staining
in the equilibrium phase (28).
However, the imaging findings of CLC are similar to those of iCCA
or HCC, owing to CLC tumor heterogeneity (29). Therefore, many patients with CLC
tend to be misdiagnosed with iCCA or HCC preoperatively. In a study
performed by Ariizumi et al (7), 34% of patients with CLC were
preoperatively diagnosed with iCCA and 55% with HCC. In the present
study, eight patients (57.1%) were suspected to have CLC
preoperatively based on these characteristic imaging findings.
Although preoperative diagnosis based on imaging findings alone is
challenging, it is crucial to consider CLC in the differential
diagnosis from a comprehensive perspective.
Although an appropriate therapeutic strategy for CLC
has not yet been established, curative resection is reported to be
the more effective treatment compared with chemotherapy or hepatic
arterial infusion (7). The
prognosis of CLC is reported to be better after curative resection
than that of iCCA (7). The present
study also demonstrated that the prognosis of all CLC patients was
favorable, with a 5-year OS rate of 72.2%. However, recurrences of
CLC were observed in two patients. Both individuals exhibited
hilar-type, positive vascular invasion, and positive surgical
margins (R1 resection) pathologically. In contrast, no recurrence
was observed in the patients who underwent pathological R0
resection. Moreover, the 5-year DSS rate was 100.0% in these
patients. These results indicate that R0 resection is an important
therapeutic strategy for CLC, due to the complete removal of
cancerous tissue. In the present study, all patients with
hilar-type CLC underwent major hepatectomy with lymph node
dissection. In contrast, most patients with peripheral-type CLC do
not undergo lymph node dissection in the present study. Only one of
the patients with CLC developed lymph node metastatic recurrence,
and this was the only patient positive for lymph node metastasis at
the time of surgery. These results suggest that the rate of lymph
node positivity in CLC is low. Moreover, even in cases without
lymph node dissection, lymph node metastasis can be considered
negative at the time of surgery since no lymph node recurrence was
found during the observation period.
Pathological examination indicates that CLC is often
composed of a CLC component and an iCCA component in variable
proportions (30), which
complicates CLC diagnosis. Komuta et al. (16) defined CLC as a condition when the
CLC component accounted for >90% of the whole tumor, but the
definition of ‘a significant proportion of CLC components’ has not
been determined previously. In the present study, pure-type CLC
(CLC component >95%) and partial-type CLC (CLC component ≤95%)
were defined as indicated. As a result, in partial-type CLC
patients, each tumor containing 15, 25 or 50% CLC components, was
categorized into groups. No recurrence was observed in any patient.
Furthermore, there was no statistically significant difference in
the DSS between patients with pure- and partial-type CLC. Few
previous reports have compared prognoses focusing on CLC
heterogeneity. The present study showed that partial-type CLC,
comprising a small portion of the CLC component (15–50%), was
similar to pure-type CLC in prognosis. This was because even in
partial-type CLC a better prognosis was observed when R0 resection
was achieved.
The present study has certain limitations. First,
the number of patients with CLC included in the present study was
small because of the rarity of CLC and the single institution
nature of the study. Multivariate analyses could not be performed
for prognostic comparisons because of the small sample size.
Secondly, as this was a retrospective observational study, lymph
node dissections were not performed in all patients; therefore, the
assessment of lymph node metastasis could not be performed
reliably. In addition, selection bias or information bias may exist
due to this study design. Third, due to the long study time period,
it is possible that medical developments during that period may
have affected perioperative outcomes and prognosis.
In conclusion, the clinicopathological features and
prognoses of 14 patients with CLC who underwent resection and were
diagnosed based on molecular biological and immunohistochemical
findings, are reported. Patients with CLC were categorized into
pure and partial type CLC based on tumor heterogeneity, and the
results suggest that regardless of the proportion of CLC
components, patients with resected CLC may have a favorable
prognosis. Curative resection is crucial to achieve a better
prognosis in patients with CLC. Further case accumulation and
assessment in a larger study are necessary to validate the findings
of the present study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HS, SN and SY contributed to study design, drafting
the manuscript, acquisition and analysis of all clinical data and
confirm the authenticity of all the raw data. RG, TT, RT, MO, KKa,
ST and IM contributed to collecting data, conception and design of
the study, and drafting and revising the manuscript. KKo
contributed to evaluation of imaging data. KH contributed to
evaluation of pathological findings. All authors read and approved
the final manuscript.
Ethics approval and consent to
participation
This study was conducted in accordance with the
ethical standards stated in the Declaration of Helsinki and with
approval from the Kanazawa University Ethics Committee (approval
no. 3221-2). Informed consent for this study was obtained from all
participants from Kanazawa University Hospital's website as an
opt-out option.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Altekruse SF, Devesa SS, Dickie LA,
McGlynn KA and Kleiner DE: Histological classification of liver and
intrahepatic bile duct cancers in SEER registries. J Registry
Manag. 38:201–205. 2011.PubMed/NCBI
|
|
2
|
Shiota K, Taguchi J, Nakashima O,
Nakashima M and Kojiro M: Clinicopathologic study on
cholangiolocellular carcinoma. Oncol Rep. 8:263–268.
2001.PubMed/NCBI
|
|
3
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA:
The 2019 WHO classification of tumours of the digestive system.
Histopathology. 76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sirica AE, Gores GJ, Groopman JD, Selaru
FM, Strazzabosco M, Wei Wang X and Zhu AX: Intrahepatic
cholangiocarcinoma: Continuing challenges and translational
advances. Hepatology. 69:1803–1815. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fabris L, Sato K, Alpini G and
Strazzabosco M: The tumor microenvironment in cholangiocarcinoma
progression. Hepatology. 73 (Suppl 1):S75–S85. 2021. View Article : Google Scholar
|
|
6
|
Hayashi A, Misumi K, Shibahara J, Arita J,
Sakamoto Y, Hasegawa K, Kokudo N and Fukayama N: Distinct
clinicopathologic and genetic features of 2 histologic subtypes of
intrahepatic cholangiocarcinoma. Am J Surg Pathol. 40:1021–1030.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ariizumi S, Kotera Y, Katagiri S, Nakano
M, Nakanuma Y, Saito A and Yamamoto M: Long-term survival of
patients with cholangiolocellular carcinoma after curative
hepatectomy. Ann Surg Oncol. 21:451–458. 2014. View Article : Google Scholar
|
|
8
|
Nakanuma Y, Sasaki M, Ikeda H, Sato Y, Zen
Y, Kosaka K and Harada K: Pathology of peripheral intrahepatic
cholangiocarcinoma with reference to tumorigenesis. Hepatol Res.
38:325–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steiner PE and Higginson J:
Cholangiolocellular carcinoma of the liver. Cancer. 12:753–759.
1959. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moeini A, Haber PK and Sia D: Cell of
origin in biliary tract cancers and clinical implications. JHEP
Rep. 3:1002262021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Chen J, Zhang X, Sheng X, Chang
XY, Chen J, Chen MS, Dong H and Duan GJ: Expert consensus on
pathological diagnosis of intrahepatic cholangiocarcinoma (2022
version). J Clin Transl Hepatol. 11:1553–1564. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G and
Andersen JB: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dagogo-Jack I and Shaw AT: Tumour
heterogeneity and resistance to cancer therapies. Nat Rev Clin
Oncol. 15:81–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen Canh H, Takahashi K, Yamamura M, Li
Z, Sato Y, Yoshimura K, Kozaka K, Tanaka M, Nakanuma Y and Harada
K: Diversity in cell differentiation, histology, phenotype and
vasculature of mass-forming intrahepatic cholangiocarcinomas.
Histopathology. 79:731–750. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komuta M, Spee B, Vander Borght S, De Vos
R, Verslype C, Aerts R, Yano H, Suzuki T and Matsuda M:
Clinicopathological study on cholangiolocellular carcinoma
suggesting hepatic progenitor cell origin. Hepatology.
47:1544–1556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuda M, Hara M, Suzuki T, Kono H and
Fujii H: Synchronously resected double primary hepatic
cancers-hepatocellular carcinoma and cholangiolocellular carcinoma.
J Hepatobiliary Pancreat Surg. 13:571–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kihara Y, Takeda Y, Ohmura Y, Katsura Y,
Shinke G, Kinoshita M, Aoyama S, Yanagisawa K, Katsuyama S,
Ikeshima R, et al: Minimally invasive liver resection for
cholangiolocellular carcinoma: A single-institution experience.
Asian J Endosc Surg. 17:2024. View Article : Google Scholar
|
|
19
|
Motosugi U, Ichikawa T, Nakajima H, Sou H,
Sano M, Sano K, Araki T, Iino H, Fujii H and Nakazawa T: Imaging of
small hepatic metastases of colorectal carcinoma: How to use
superparamagnetic iron oxide-enhanced magnetic resonance imaging in
the multidetector-row computed tomography age? J Comput Assist
Tomogr. 33:266–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagata K, Einama T, Kimura A, Murayama M,
Takeo H, Nishikawa M, Hoshikawa M, Noro T and Ogata S: A case of
intrahepatic cholangiocarcinoma that was difficult to diagnose
prior to surgery: A case report. Oncol Lett. 17:823–830.
2019.PubMed/NCBI
|
|
21
|
Lee AJ and Chun YS: Intrahepatic
cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin
Oncol. 7:522018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liver Cancer Study Group of Japan, . The
general rules for the clinical and pathological study of primary
liver cancer. 6th edition. Kanehara; Tokyo: 2019
|
|
23
|
Orimo T, Kamiyama T, Mitsuhashi T, Kamachi
H, Yokoo H, Wakayama K, Shimada S, Nagatsu A and Taketomi A: Impact
of tumor localization on the outcomes of surgery for an
intrahepatic cholangiocarcinoma. J Gastoroenterol. 53:1206–1215.
2018. View Article : Google Scholar
|
|
24
|
Japanese Society of Biliary Surgery, .
General rules for surgical and pathological studies on cancer of
the biliary tract. 6th edition. Kanehara; Tokyo: 2013
|
|
25
|
Dindo D, Demartines N and Clavien P:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akiyama K, Abe T, Oshita A, Shimizu A,
Hanada K, Yonehara S, Kobayashi T, Ohdan H, Noriyuki T and Nakahara
M: Gradually progressive cholangiolocellular carcinoma: A case
report. Surg Case Rep. 7:2632021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sempoux C, Fan C, Singh P, Obeidat K,
Roayaie S, Schwartz M, Fiel MI and Thung SN: Cholangiolocellular
carcinoma: An innocent-looking malignant liver tumor mimicking
ductular reaction. Semin Liver Dis. 31:104–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kozaka K, Matsui O, Kobayashi S, Koda W,
Minami T, Kitao A, Inoue D, Yoneda N and Yoshida K: Dynamic CT
findings of cholangiolocellular carcinoma: Correlation with
angiography-assisted CT and histopathology. Abdom Radiol.
42:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Asayama Y, Tajima T, Okamoto D, Nishie A,
Ishigami K, Ushijima Y, Kakihara D, Aishima S, Taketomi A and Honda
H: Imaging of cholangiolocellular carcinoma of the liver. Eur J
Radiol. 75:120–125. 2010. View Article : Google Scholar
|
|
30
|
Kadono M, Kimura K, Imamura J, Saeki S,
Kurata M, Honda G, Tsuruta K, Horiguchi S and Hayashi S: A case of
a large cholangiolocellular carcinoma. Clin J Gastroenterol.
4:340–346. 2011. View Article : Google Scholar : PubMed/NCBI
|