Introduction
DLBCL is a mature B-cell malignancy that is
frequently found among adults with non-Hodgkin's lymphoma (NHL)
(1). In China, DLBCL accounts for
40.1% of all NHL cases, and it has attracted increasing attention
due to its high morbidity and mortality rates. At present, the
pathogenesis of DLBCL is unclear, although several studies have
indicated a close association with inflammation-triggered immune
dysfunction, a condition mediated by cytokines (2,3). The
rituximab, cyclophosphamide, Adriamycin, vincristine and prednisone
(R-CHOP) regimen is the current first-line treatment for DLBCL, and
it produces satisfactory remission rates in most patients. However,
30–40% of patients either fail to respond to R-CHOP or relapse
within the first months of treatment (4,5). These
differences in response and prognosis indicate the importance of
establishing an accurate method for patient stratification. The
role of immune escape in disease development is not adequately
reflected by the International Prognostic Index (IPI) score, which
is now the gold standard for the prognostic assessment of patients
with DLBCL. Moreover, the accuracy of the IPI tends to be low for
the standard treatment, even when the treatment includes rituximab
(6). Thus, it is essential to
identify novel biomarkers to improve the accuracy of the IPI
scoring system. It has been found that the levels of certain
cytokines are strongly correlated with the onset, severity and
prognosis of DLBCL (7–9), although there is limited information
on this topic in patients with DLBCL in China. Thus, in view of the
differences observed between domestic and foreign environments,
patient pathogenic factors and high DLBCL heterogeneity, the
present study examined the cytokine profiles of Chinese patients
with DLBCL using flow cytometry. These findings will provide new
ideas for the prognostic analysis and clinical treatment of DLBCL
worldwide.
Materials and methods
General information
The study enrolled 60 patients with pathologically
confirmed B-cell NHL (B-NHL) (38 men and 22 women; age range, 36–91
years; median age, 65 years) who were treated at Zhejiang
Provincial People's Hospital (Hangzhou, China) between January 2017
and January 2020. The diagnostic criteria were in accordance with
the reported literature (10).
Among the selected participants were 11 with chronic lymphocytic
leukemia/small lymphocytic lymphoma (CLL/SLL), 49 with primary
DLBCL and 21 patients with DLBCL in remission after treatment.
Patients with dry syndrome or other diseases involving the immune
system that affect cytokine levels, severe infections, a second
malignant haematological disease, severe target organ damage with a
survival rate of <1 year, other neoplasms, diabetes or other
metabolic diseases were excluded. The control cohort consisted of
67 healthy volunteers. The four groups were comparable in terms of
sex and age. The final follow-up was conducted in January 2021, the
minimum follow-up period was 12 months and the follow-up was
terminated at the death of the patient. Written informed consent
was obtained from all patients for participation in this study.
Sub-cohort of patients with DLBCL
General clinical information was collected for all
patients. This included LDH and CRP levels, bone marrow aspiration
and biopsy results, and ultrasonography and imaging results, such
as radiographs, computed tomography (CT) or positron emission
tomography-CT scans. During the analysis, CRP was found to be a
more sensitive indicator, and the correlation between the elevation
of this indicator and other cytokines showed a phase correlation,
so it was divided into four groups with different degrees of
elevation: Group 0, 0–10 mg/l CRP; group 1, 10–50 mg/l CRP; group
2, 50–100 mg/l CRP; and group 3, >100 mg/l CRP. Patients also
received an IPI score based on five factors, including age,
behavioral status, Ann Arbor stage (11), LDH expression level and number of
invaded sites of extranodal lesions. The IPI score can be used to
classify patients into four different risk groups: A score of 0/1
for low risk; 2 for low to medium risk; 3 for medium to high risk;
and 4/5 for high risk.
The 49 patients with DLBCL were treated with the
R-CHOP regimen over a 21-day treatment period. The R-CHOP
chemotherapy regimen is as follows: i) Rituximab, the dosage of
which is usually determined according to 365 mg/m2 body
surface area, and is administered intravenously on day 1, during
which time the patient should be observed for possible side effects
such as allergic reactions, and it is best to monitor the patient
using automatic ECG monitoring. ii) Cyclophosphamide, the dosage of
which is calculated according to 750 mg/m2 body surface
area, and is administered intravenously on day 2. iii) Vincristine,
the dosage of which is calculated according to 1.4 mg/m2
body surface area, and is administered intravenously on day 2. iv)
Adriamycin, the dosage of which is calculated according to 50
mg/m2 body surface area, and is also administered
intravenously on day 2. v) Prednisone, which is usually
administered orally at 100 mg once a day, from day 2 to day 6, for
a chemotherapy cycle of 21 days, where the dose is given for the
first 6 days, and the time after that is the inter-chemotherapy
period, when side effects are observed and managed. The above body
surface area is generally calculated according to a formula based
on the patient's height and weight. All patients received three
courses, after which an efficacy evaluation was conducted according
to the NCCN Clinical Practice Guidelines in Oncology for B-Cell
Lymphomas (12). Patients with
complete and partial remission were included in the cohort of
effectively treated patients (n=21), while those showing disease
progression and no remission formed the ineffectively treated
cohort (n=28).
Overall, long-term follow-up data were available for
39 patients with DLBCL, while the remaining patients were lost to
follow-up. In addition, 16 patients died during the first year
after treatment, while 23 patients survived for 1 year or more.
These patients were included in the deceased and survived cohorts,
respectively (Fig. S1). For
survival curves, the reference ranges of cytokines were consistent
with those reported by the Department of Pathology of Zhejiang
Provincial People's Hospital. The IL-6 cut-off value was 5.00
pg/ml, the IL-10 cut-off value was 5.00 pg/ml and the IL-17 cut-off
value was 3.00 pg/ml.
Cytokine assays
Cytokine levels were measured in the sera of all
participants before treatment using a Beckman Navios flow cytometer
(cat. no. B47905; Beckman Coulter, Inc.) and a Th1/Th2/Th17
Cytometric Bead Array cytokine kit (cat. no. 560484; BD
Biosciences), according to the manufacturer's protocols. Fasting
venous blood (5 ml) was collected from the arm of all subjects. The
blood samples were allowed to stand for 2 h at room temperature and
then centrifuged at 1,000 × g for 5 min at 4°C. The sera were
stored at 4°C. The measurement of IL-2, IL-4, IL-6, IL-10, IL-17,
TNF-α and IFN-γ levels was completed within 24 h. The software used
for data analysis was FCAP Array™ version 3.0.1 from BD
Biosciences.
Statistical analysis
All data were analyzed using SPSS 20.0 (IBM Corp.).
Cytokine levels between two groups were compared utilizing the
Mann-Whitney U test. Differences in cytokine levels among multiple
groups were assessed through the Kruskal-Wallis test. The post hoc
analysis used was the Bonferroni test. Two-tailed Spearman
correlations were used to assess associations between cytokine
levels and CRP levels and IPI scores. P<0.05 were considered to
indicate a statistically significant difference.
Generation of the prediction
model
The support vector machine (SVM) algorithm was used
to construct a model for prognosis prediction for patients with
DLBCL (13). This was generated
using the results of the cytokine analysis, as determined by flow
cytometry. The data on cytokine levels were then separated into two
categories, namely, 80% for training and 20% for validation. Using
the e1071 package with the random number set to 123, optimization
of the penalty coefficient C was conducted via tune.svm (14). The optimization range was between
0.005–1, the optimization step was 0.005, and γ was set to 1. The
optimal SVM model was derived with a C-classification, radial SVM
kernel and an optimal C of 0.895.
Results
Expression profiles of seven cytokines
in patients with primary DLBCL and primary CLL/SLL, and normal
control volunteers
The IL-6 (P<0.001) and IL-10 (P<0.01) levels
were significantly raised in patients with primary DLBCL compared
with those in the controls (Table
I). Meanwhile, although the TNF-α, IFN-γ and IL-17 levels were
increased slightly in primary DLBCL patients, they did not reach
significance. No changes were seen in the levels of IL-2 and IL-4
between the two cohorts (P>0.05; Table I; Fig.
S2) nor were there significant differences in cytokine levels
between patients with primary CLL/SLL and healthy controls
(P>0.05; Table I), suggesting
that altered serum cytokine levels are specific to the early
diagnosis of DLBCL. Representative flow cytometry plots of two
patients are presented in Fig.
S3A, where it can be seen that both patients had relatively
elevated IL-6 and IL-10 in their test results.
 | Table I.Expression of seven cytokines in the
primary DLBCL, primary CLL/SLL and healthy control groups. |
Table I.
Expression of seven cytokines in the
primary DLBCL, primary CLL/SLL and healthy control groups.
| Groups | IL-2, ng/l | IL-4, ng/l | IL-6, ng/l | IL-10, ng/l | TNF-α, ng/l | IFN-γ, ng/l | IL-17, ng/l |
|---|
| Healthy | 1.13 | 1.49 | 2.45 | 2.35 | 2.10 | 2.35 | 2.30 |
| control | (0.90, 1.94) | (1.22, 2.09) | (1.10, 2.98) | (1.34, 3.15) | (1.03, 2.09) | (1.35, 3.01) | (1.30, 2.77) |
| Initial | 1.07 | 1.31 | 1.69 | 2.48 | 1.03 | 1.63 | 1.42 |
| CLL/ SLL group | (0.85, 1.25) | (0.78, 1.88) | (1.00, 4.02) | (1.18, 4.40) | (0.72, 2.15) | (1.15, 2.13) | (0.72, 1.53) |
| Initial | 1.16 | 1.45 | 35.82 | 25.04 | 2.15 | 2.65 | 1.91 |
| DLBCL group | (0.66, 2.37) | (0.92, 2.27) | (10.49, 104.0) | (6.85, 150.43) | (1.76, 4.08) | (1.61, 4.64) | (0.63, 2.66) |
| Z-value | 0.377a | 0.897b | 0.556a | 0.897b | 8.177a | 0.330b | 7.258a | 0.136b | 0.017a | 1.393b | 2.211a | 1.945b | 0.942a | 2.061b |
| P-value | 0.706a | 0.369b | 0.578a | 0.370b |
<0.001a | 0.741b | 0.000a | 0.892b | 0.987a | 0.164b | 0.027a | 0.052b | 0.346a | 0.159b |
Cytokine expression in patients with
DLBCL in sustained remission after treatment
Apart from a rise in the serum IL-6 levels
(P<0.05), no significant differences were observed between serum
cytokine levels in the 21 patients with DLBCL who achieved
sustained remission following standard treatment and healthy
controls during the same period (P>0.05; Table II). This suggested a close
association between cytokine production and disease progression in
patients with DLBCL.
 | Table II.Expression of seven cytokines in
patients with DLBCL in sustained remission. |
Table II.
Expression of seven cytokines in
patients with DLBCL in sustained remission.
| Groups | IL-2, ng/l | IL-4, ng/l | IL-6, ng/l | IL-10, ng/l | TNF-α, ng/l | IFN-γ, ng/l | IL-17, ng/l |
|---|
| Control | 1.13 | 1.49 | 2.45 | 2.35 | 2.10 | 2.35 | 2.30 |
|
| (0.90, 1.94) | (1.22, 2.09) | (1.10, 2.98) | (1.34, 3.15) | (1.03, 2.09) | (1.35, 3.01) | (1.30, 2.77) |
| DLBCL in
sustained | 1.19 | 1.54 | 3.29 | 3.44 | 2.15 | 2.47 | 2.07 |
| remission | (0.88, 2.19) | (1.06, 2.23) | (2.07, 20.32) | (2.20, 10.26) | (0.91, 2.96) | (1.47, 3.53) | (0.83, 2.77) |
| Z-value | 0.242 | 0.247 | 2.505 | 1.267 | 0.750 | 0.024 | 0.437 |
| P-value | 0.809 | 0.805 | 0.012 | 0.205 | 0.453 | 0.981 | 0.662 |
Association between serum cytokine
levels and clinicopathological features in patients with DLBCL at
first presentation
Elevated LDH expression at the first presentation
was found to be closely associated with prevalence. Patients with
increased levels of serum LDH had considerably higher IL-10 values
than those with normal LDH (Z=2.368 and Z=3.143; P=0.018 and
P=0.002, respectively), although the levels of the remaining
cytokines showed no significant differences (P>0.05; Fig. 1). It was also observed that raised
CRP was associated with markedly higher IL-6 and IL-10 levels,
compared with patients with normal CRP, with significance shown for
10–50 and 50–100 mg/l for IL-6, and for 10–50 mg/l for IL-10
(P<0.05; Fig. 2A-E). Moreover,
the degree of increase in IL-6 was positively correlated with the
serum CRP (P<0.001, r=0.662; Fig.
2F). By contrast, the levels of IFN-γ only increased in
patients with CRP >100 mg/l, respectively (P<0.05; Table SI). There were 12 patients with
missing CRP data; therefore, CRP data for 37 patients are counted
here.
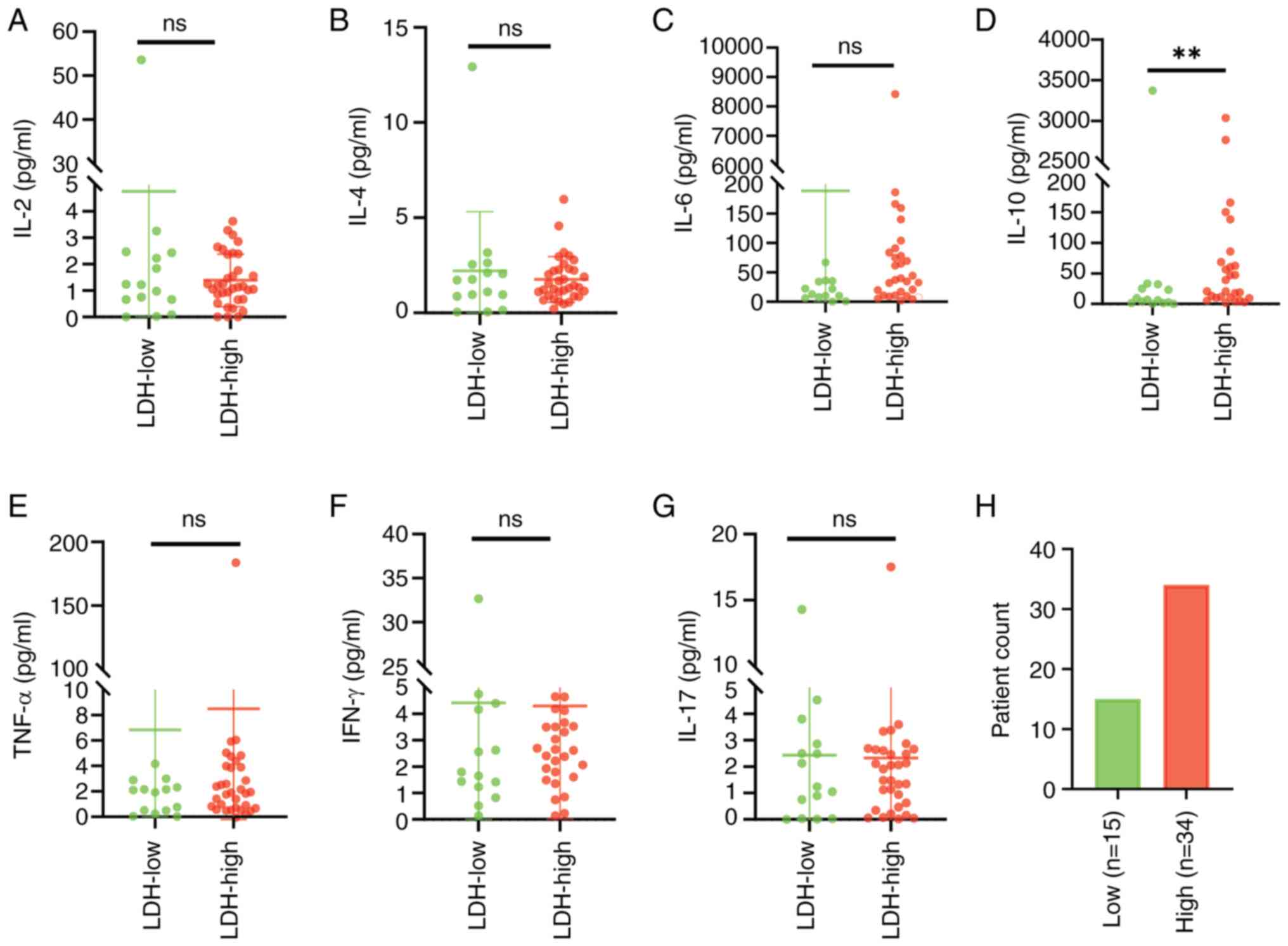 | Figure 1.Differences in serum cytokine and LDH
levels in patients with diffuse large B-cell lymphoma. Patients
were separated into low and high LDH-expression cohorts, according
to their levels of LDH. (A-G) Differences in the levels of (A)
IL-2, (B) IL-4, (C) IL-6, (D) IL-10, (E) TNF-α, (F) IFN-γ and (G)
IL-17 in the low- and high-LDH cohorts. (H) The low-LDH cohort
included 15 patients and the high-LDH cohort included 34 patients.
**P<0.01. LDH, lactate dehydrogenase; IL, interleukin; TNF-α,
tumor necrosis factor-α; IFN-γ, interferon-γ. |
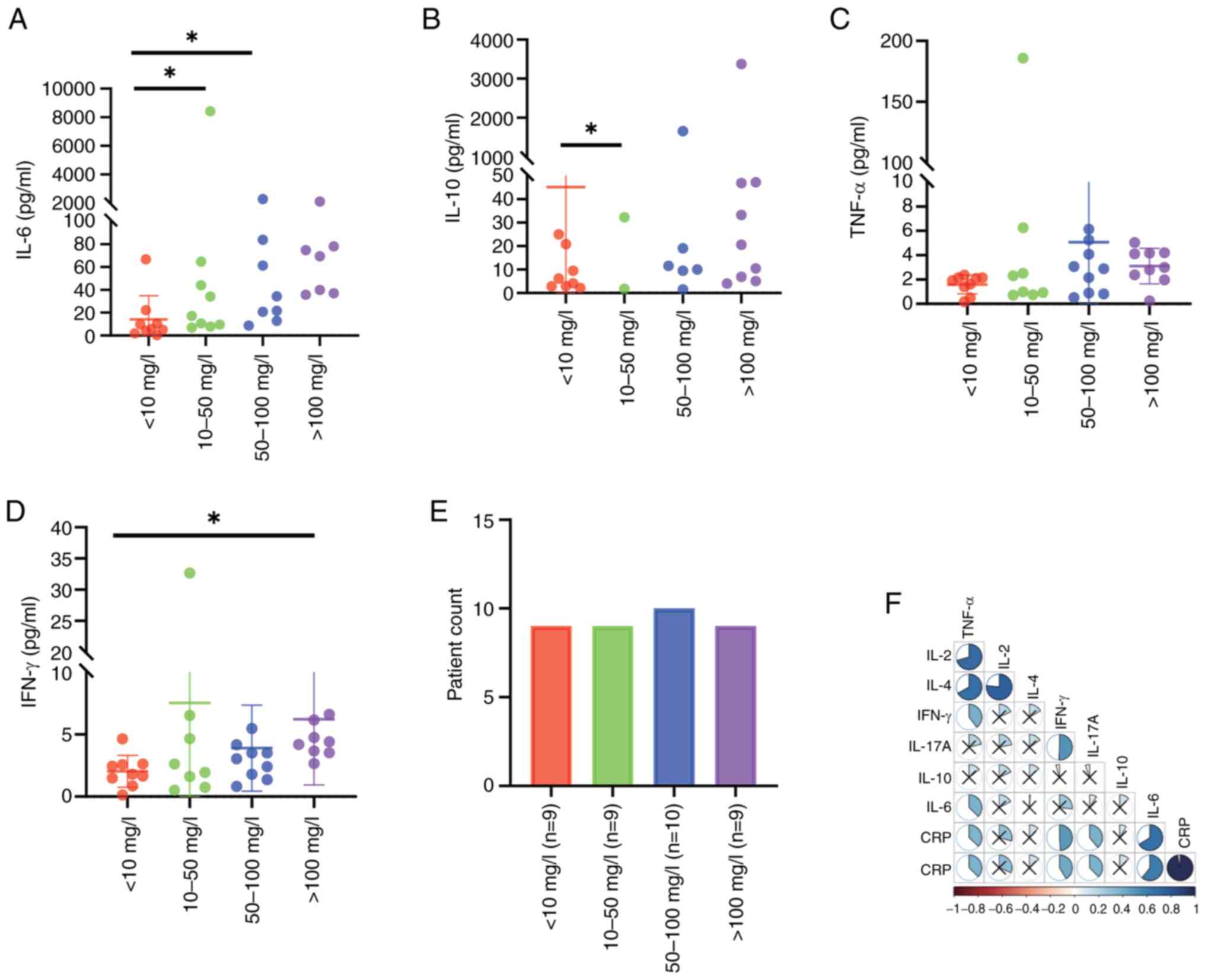 | Figure 2.Differences and correlations between
cytokine levels in patients with diffuse large B-cell lymphoma
exhibiting varying CRP levels. Patient CRP levels were divided into
four categories, namely, 0–10, 10–50, 50–100 and >100 mg/l.
(A-D) Differences in the levels of (A) IL-6, (B) IL-10, (C) TNF-α
and (D) IFN-γ, according to grouping. (E) Specific grouping
according to CRP level, showing 11 patients in the 0–10 mg/l group,
10 patients in the 10–50 mg/l group, 9 patients in the 50–100 mg/l
group and 7 patients in the >100 mg/l group. (F) Correlations of
cytokine and CRP levels in individual patients. *P<0.05. IL,
interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ;
CRP, C-reactive protein. |
Associations between serum cytokine
profiles and IPI scores in patients with DLBCL at first
presentation
Fig. 3A-G shows the
distribution of IPI scores in the 48 patients with DLBCL; 1 patient
had an IPI score of 1 and its data were therefore not included.
There were significant differences in serum IL-6 and IL-10 levels
between sub-cohorts of patients with different IPI scores compared
with those with an IPI score of 2 (IL-6: H=11.214, P=0.011; IL-10:
H=15.203, P=0.002). Levels of both IL-6 and IL-10 were increased in
patients with IPI scores of 4 (P<0.05; Table SII), and there was a positive
correlation between the IPI score and serum IL-6 and IL-10 levels
(IL-6: P=0.007, r=0.380; IL-10: P=0.002, r=0.438; Fig. 3I). Moreover, although differences in
TNF-α between the two cohorts did not reach statistical
significance (H=3,474, P=0.324), levels were higher in patients
with IPI scores of ≥3, relative to those with IPI scores of 2
(Fig. 3E).
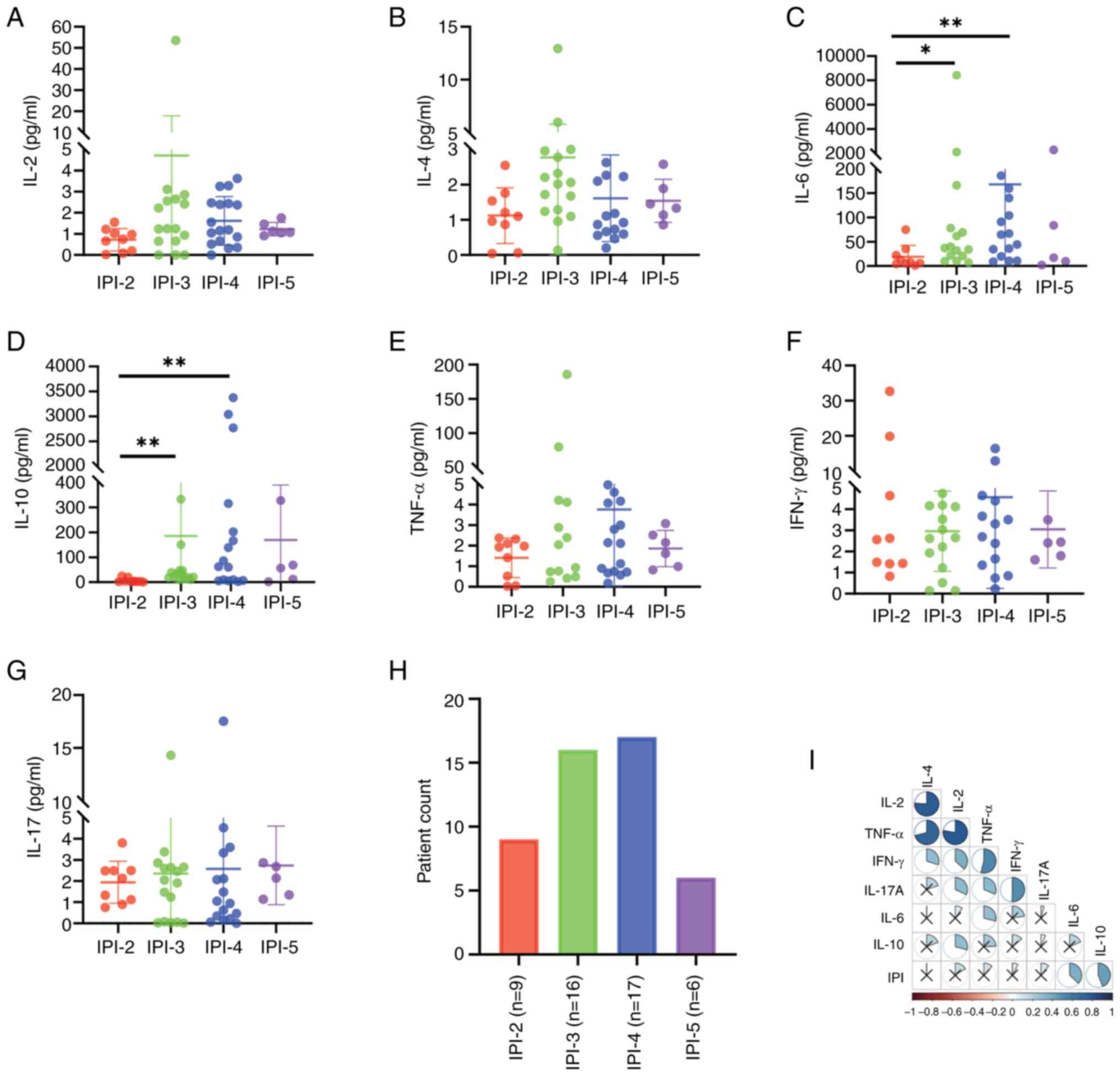 | Figure 3.Cytokine levels and correlations in
patients with DLBCL with varying IPI scores. (A) IL-2, (B) IL-4,
(C) IL-6, (D) IL-10, (E) TNF-α, (F) IFN-γ and (G) IL-17 cytokine
levels of patients with DLBCL in relation to IPI scores. (H)
Numbers of patients in the different IPI groups. (I) Correlations
between individual cytokines and IPI scores. *P<0.05 and
**P<0.01. IPI, International Prognostic Index; DLBCL, diffuse
large B-cell lymphoma; IL, interleukin; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ. |
Predictive model of short-term
treatment response in patients with DLBCL using the SVM analysis of
cytokines
The 49 patients with primary DLBCL included 21 that
responded to treatment and were effectively treated, and 28 that
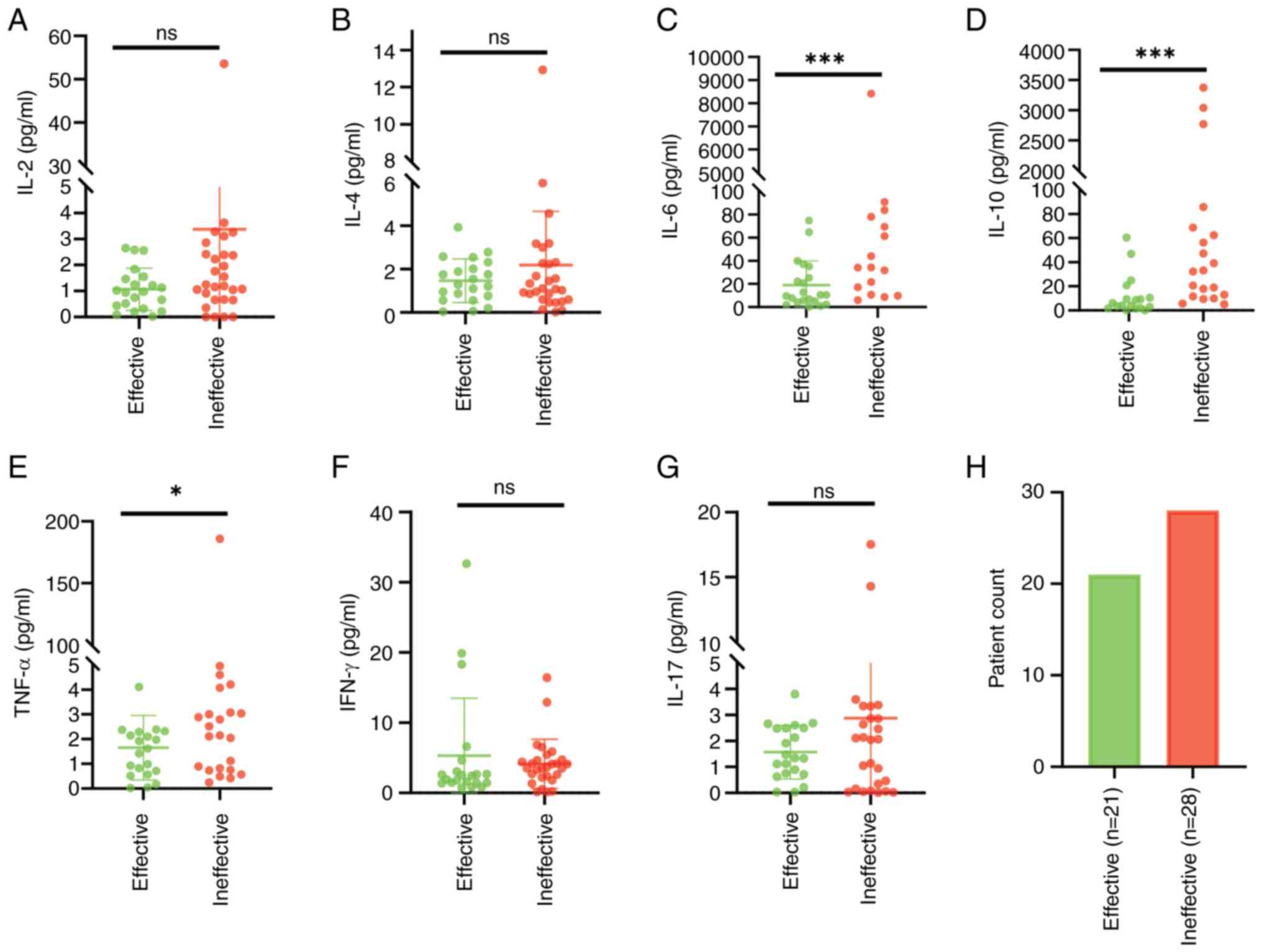
did not and were ineffectively treated. Analysis showed that
compared with the effectively treated patients, those that were
ineffectively treated had significantly higher levels of IL-6 and
IL-10 (both P<0.01), as well as TNF-α (P<0.05) (Table III). However, IFN-γ did not differ
significantly between the two groups, despite an observed increase
in ineffectively treated patients (Fig.
4).
 | Table III.Serum cytokines in patients with
diffuse large B-cell lymphoma in the effective and ineffective
groups of short-term treatment. |
Table III.
Serum cytokines in patients with
diffuse large B-cell lymphoma in the effective and ineffective
groups of short-term treatment.
| Groups | IL-2, ng/l | IL-4, ng/l | IL-6, ng/l | IL-10, ng/l | TNF-α, ng/l | IFN-γ, ng/l | IL-17, ng/l |
|---|
| Ineffective
group | 1.22 | 1.39 | 87.43 | 59.31 | 2.84 | 3.6 | 2.09 |
|
| (0.67, 2.40) | (0.90, 2.44) | (33.53,
232.49) | (18.79,
230.51) | (0.88, 4.68) | (2.36, 4.68) | (0.31, 3.34) |
| Effective
group | 0.98 | 1.29 | 10.47 | 8.73 | 1.62 | 2,45 | 1.35 |
|
| (0,76, 2,02) | (0.76, 2.02) | (5.09, 25.32) | (2.94, 25.04) | (0.72, 2.29) | (1.44, 3.04) | (0.75, 3.34) |
| Z-value | 1.384 | 0.778 | 4.303 | 3.374 | 2.293 | 1.283 | 0.667 |
| P-value | 0.166 | 0.437 | <0.001 | 0.001 | 0.022 | 0.200 | 0.505 |
The data on cytokine levels were then separated into
two categories, namely, 80% for training and 20% for validation.
Using the e1071 package with the random number set to 123,
optimization of the penalty coefficient C was conducted via
tune.svm. The optimization range was between 0.005–1, the
optimization step was 0.005, and gamma was set to 1. The optimal
SVM model was derived with a C-classification, radial SVM kernel
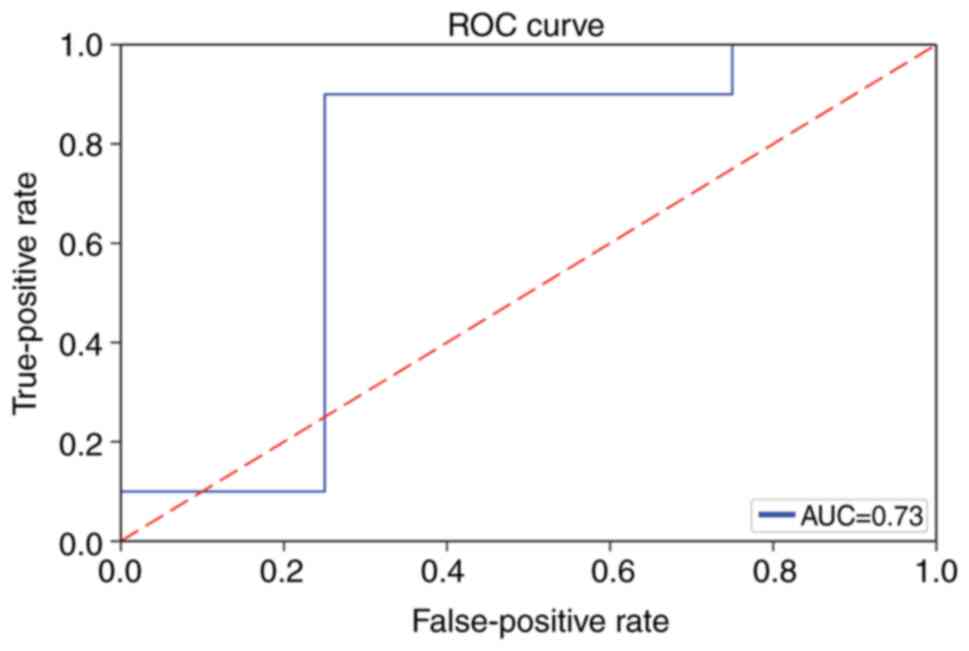
and an optimal C of 0.895. Using this optimal SVM model, the
prediction test group accuracy was 78.57% (Fig. S3B), and the area under the ROC
curve was 0.73 (Fig. 5).
Association between overall survival
and serum cytokine levels in patients with DLBCL
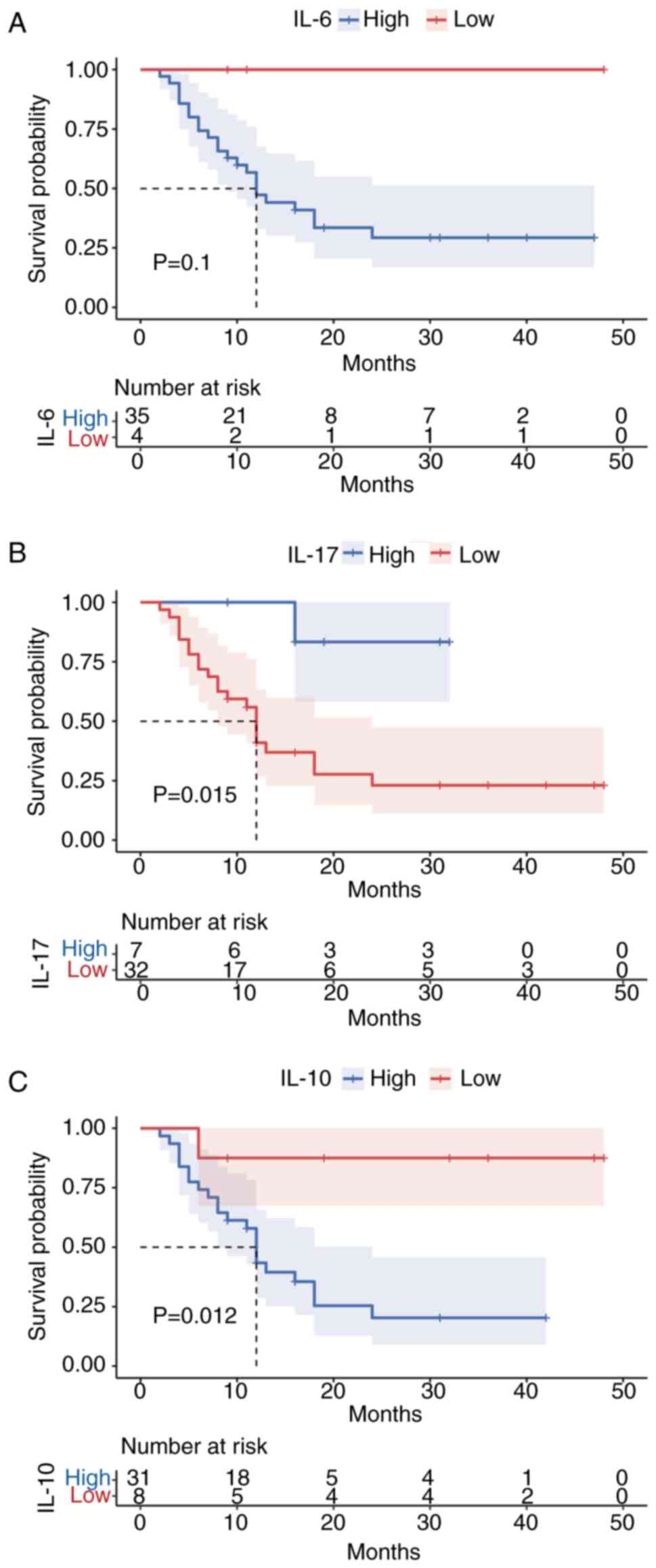
Of the 39 patients with DLBCL who had long-term
follow-up data, 16 died (deceased cohort) and 23 survived (survived
cohort) during the 12 months of follow-up. The deceased patients
showed higher serum IL-6 and IL-10 levels, while the patients who
survived had higher IL-17 levels (P<0.05); no significant
changes were seen in the remaining cytokines (P>0.05) (Table IV). Notably, based on the survival
plots, only IL-6 was not significantly associated with survival
prognosis in patients with DLBCL (P>0.05) (Fig. 6).
 | Table IV.Differences in serum cytokines
between patients with diffuse large B-cell lymphoma in the clinical
deceased and survival groups. |
Table IV.
Differences in serum cytokines
between patients with diffuse large B-cell lymphoma in the clinical
deceased and survival groups.
| Groups | IL-2, ng/l | IL-4, ng/l | IL-6, ng/l | IL-10, ng/l | TNF-α, ng/l | IFN-γ, ng/l | IL-17, ng/l |
|---|
| Deceased group | 1.19 | 1.33 | 78.05 | 60.51 | 2.8 | 3.51 | 1.47 |
|
| (0.75, 2.29) | (0.73, 2.27) | (20.73,
186.11) | (10.05,
327.34) | (0.82, 4.17) | (1.80, 4.65) | (0.72, 2.65) |
| Survival group | 0.96 | 1.48 | 23.81 | 5.88 | 2.01 | 2.07 | 2.75 |
|
| (0.12, 1.47) | (0.93, 2.57) | (23.81, 66.18) | (5.88, 27.52) | (0.63, 2.31) | (1.39, 2.64) | (1.37, 3.53) |
| Z-value | 1.699 | 0.485 | 2.056 | 2.827 | 1.371 | 1.571 | 2.142 |
| P-value | 0.089 | 0.627 | 0.04 | 0.005 | 0.17 | 0.116 | 0.037 |
Discussion
As a particularly aggressive form of NHL, DLBCL has
higher morbidity and mortality rates than other NHL subtypes
(15). Thus, it is imperative to
identify novel indicators that can assist in the early diagnosis
and prognostic assessment of DLBCL patients. Recently, the
measurement of cytokine levels and the expression of their
associated receptors has become an essential component of basic and
clinical immunology research, as serum cytokine levels have been
shown to be important in the early diagnosis of disease and the
prediction of prognosis, and even in assessing the efficacies of
antitumor drugs and formulating individualized treatment plans
(16). In the present study, the
serum levels of cytokines were measured in patients with DLBCL
using flow cytometry to provide novel ideas for the early
diagnosis, clinical treatment and prognostic analysis of DLBCL.
In recent decades, in the process of exploring new
methods for the diagnosis and treatment of B-NHL, particularly
DLBCL, the number of studies on IL-6, IL-10, TNF-α and IFN-γ has
gradually increased. IL-6 is a potent cytokine that promotes the
growth and differentiation of B lymphocytes, and is an essential
component of the lymphoma microenvironment; it also induces
angiogenesis in tumors, disrupts adhesion between tumor cells, and
strongly counteracts antitumor actions in the body, thereby
promoting the growth and proliferation of tumor cells, while
inhibiting apoptosis, initiating a vicious cycle (17,18).
Although IL-10 is known to possess antitumor effects mediated by
CD8+ T-cell responses, in the presence of
CD19+ tumor cells, IL-10 can potentially serve as a
growth factor for tumorigenic B lymphocytes, and promote the immune
escape of tumor cells (19,20). IL-10 also inhibits apoptosis through
the upregulation of Bcl-2 expression, thus promoting tumorigenesis
(21). TNF-α is one of the first
cytokines released during inflammation, and it is critical for the
initiation of the cytokine cascade. Elevated expression of TNF-α
and its associated receptors [soluble TNF-receptor 1 (sTNF-R1) and
sTNF-R2] has been linked with reduced overall survival in numerous
tumors, including breast and stomach cancer (22–25).
However, the role of TNF-α in DLBCL remains unclear. IFN-γ has
classical antitumor effects, but these may change in the tumor
microenvironment. Some studies have demonstrated that IFN-γ
significantly enhances oncogenic activity via B invasive lymphoma
protein 1/ADP ribose convertase 9 in patients with high-risk DLBCL
(26). The role of IL-17, a
characteristic Th17 cell-secreted cytokine in tumors, remains
controversial. It was previously found that in B-NHL, increased
levels of transforming growth factor β inhibited Th17 cell
differentiation while promoting the differentiation of regulatory T
cells, reducing the antitumor activity and thus enabling tumor
immune escape (27).
The levels of IL-6, IL-10 and IFN-γ were
significantly higher in patients with DLBCL compared with those in
healthy controls in the present study. Significantly increased
levels of TNF-α were also observed, confirming the association
between aberrant cytokine expression and DLBCL occurrence. No
significant differences were observed in these cytokines when
comparing between the patients with CLL/SLL and the healthy
controls. Although some prior studies reported that serum IL-6 and
IL-10 levels remained high in patients with CLL/SLL, these results
were primarily associated with certain high-grade patients
(28). The present study found that
serum cytokine levels have limited diagnostic significance for the
inert B-cell lymphoma represented by CLL, while showing specificity
for DLBCL. Additionally, in patients with DLBCL, the levels of most
cytokines such as OL-10 and IL-17, with the exception of IL-6,
often returned to normal on remission of the disease. This
suggested that cytokines are significantly associated with disease
progression, suggesting a potential treatment strategy for patients
with DLBCL. Furthermore, elevated LDH and CRP were associated with
prevalence rate in patients with DLBCL, and it was found that
patients with increased serum CRP or LDH levels also had higher
levels of IL-6 and IL-10, and that the increased production of
these factors was positively associated with serum CRP. This was
consistent with the report by Nacinovic-Duletic et al
(29). Furthermore, there was a
strong correlation between the IPI risk stratification and
circulating IL-6 and IL-10 levels in the patients with DLBCL. IL-10
was significantly higher in patients with low-risk IPI scores,
relative to those with high-risk scores, and the difference was
greater with higher scores. This is in agreement with the findings
reported by Aydin et al (30); however, the assay used in the
present study is simpler and more efficient than the ELISA method
that was used by this study. In terms of treatment efficacy, it was
found that elevated IL-6, IL-10 and TNF-α levels were often
predictive of poor treatment effectiveness, consistent with the
findings reported by Dlouhy et al (7). In addition, several studies have
reported the roles of single cytokines or combinations of a few
cytokines in NHL (31–35) while the present study included a
more complete range of cytokines and a more detailed analysis of
the various correlations with the disease. The present study
further tested cytokines using the SVM algorithm, and the accuracy
of the optimal SVM model for the prediction of short-term treatment
efficacies in patients with DLBCL was 81.63%. These findings
suggest that cytokines are important indicators of the DLBCL
treatment response.
Analysis of long-term follow-up data showed that, in
comparison with DLBCL patients who survived, those who died within
1 year of treatment had elevated levels of IL-6, IL-10 and IL-17.
However, analysis of the survival curves showed that IL-6 levels
did not correlate with overall survival in patients with DLBCL.
This suggests that there are some limits to the use of serum
cytokine levels for predicting the long-term prognosis of patients
with DLBCL.
In conclusion, serum IL-6, IL-10, IL-17, TNF-α and
IFN-γ levels can serve as prognostic indicators for the assessment
of tumor immune status in DLBCL. Moreover, in combination with the
IPI score, they can be important indicators of DLBCL prognosis, and
may also provide a basis for the precise treatment and direction of
novel and efficacious targeted therapies.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Foundation of Science Technology
Department of Zhejiang Province (grant no. LGF22H080012) and the
Zhejiang Provincial Medical Technology Plan Project (grant nos.
2022KY505 and 2020KY052).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SX, LZ and LW collected and analyzed the data, drew
figures and tables, and contributed in writing the manuscript. SW,
LZ and SX performed the statistical analysis. XT procured the
funding for this study. XT, WN, SW participated in the design of
the study, gave administrative or logistical support for this
study, and reviewed drafts of the paper. All the authors agreed
with the conclusions of this study. All authors have read and
approved the final manuscript. SX and WN confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Zhejiang Provincial People's Hospital (approval no. 2021QT150).
Written informed consent was obtained from all patients for
participation in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tout M, Casasnovas O, Meignan M, Lamy T,
Morschhauser F, Salles G, Gyan E, Haioun C, Mercier M, Feugier P,
et al: Rituximab exposure is influenced by baseline metabolic tumor
volume and predicts outcome of DLBCL patients: A lymphoma study
association report. Blood. 129:2616–2623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tárnok A, Hambsch J, Chen R and Varro R:
Cytometric bead array to measure six cytokines in twenty-five
microliters of serum. Clin Chem. 49:1000–1002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malaponte G, Hafsi S, Polesel J,
Castellano G, Spessotto P, Guarneri C, Canevari S, Signorelli SS,
McCubrey JA and Libra M: Tumor microenvironment in diffuse large
B-cell lymphoma: Matrixmetalloproteinases activation is mediated by
osteopontin overexpression. Biochim Biophys Acta. 1863:483–489.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim SH, Woo SY, Kim S, Ko YH, Kim WS and
Kim SJ: Cross-sectional Study of Patients with Diffuse Large B-Cell
Lymphoma: Assessing the effect of host status, tumor burden, and
inflammatory activity on venous thromboembolism. Cancer Res Treat.
48:312–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falduto A, Cimino F, Speciale A, Musolino
C, Gangemi S, Saija A and Allegra A: How gene polymorphisms can
influence clinical response and toxicity following R-CHOP therapy
in patients with diffuse large B cell lymphoma. Blood Rev.
31:235–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Z, Sehn LH, Rademaker AW, Gordon LI,
Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA,
Rodriguez MA, et al: An enhanced International Prognostic Index
(NCCN-IPI) for patients with diffuse large B-cell lymphoma treated
in the rituximab era. Blood. 123:837–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dlouhy I, Filella X, Rovira J, Magnano L,
Rivas-Delgado A, Baumann T, Martínez-Trillos A, Balagué O, Martínez
A, González-Farre B, et al: High serum levels of soluble
interleukin-2 receptor (sIL2-R), interleukin-6 (IL-6) and tumor
necrosis factor alpha (TNF) are associated with adverse clinical
features and predict poor outcome in diffuse large B-cell lymphoma.
Leuk Res. 59:20–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong W, Xu X, Zhu Z, Du Q, Du H, Yang L,
Ling Y, Xiong H and Li Q: Increased expression of IRF8 in tumor
cells inhibits the generation of Th17 cells and predicts
unfavorable survival of diffuse large B cell lymphoma patients.
Oncotarget. 8:49757–49772. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashwah H, Bertram K, Stirm K, Stelling A,
Wu CT, Kasser S, Manz MG, Theocharides AP, Tzankov A and Müller A:
The IL-6 signaling complex is a critical driver, negative
prognostic factor, and therapeutic target in diffuse large B-cell
lymphoma. EMBO Mol Med. 11:e105762019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
and Leukaemia Lymphoma Group; Eastern Cooperative Oncology Group, ;
et al: Recommendations for initial evaluation, staging, and
response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano
classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruppert AS, Dixon JG, Salles G, Wall A,
Cunningham D, Poeschel V, Haioun C, Tilly H, Ghesquieres H, Ziepert
M, et al: International prognostic indices in diffuse large B-cell
lymphoma: A comparison of IPI, R-IPI, and NCCN-IPI. Blood.
135:2041–2048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zelenetz AD, Gordon LI, Abramson JS,
Advani RH, Bartlett NL, Caimi PF, Chang JE, Chavez JC, Christian B,
Fayad LE, et al: NCCN Guidelines Insights: B-Cell Lymphomas,
Version 3.2019. J Natl Compr Canc Netw. 17:650–661. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Dong X, Shen W, Ye Z and Xiang R:
Machine learning-based classification of diffuse large B-cell
lymphoma patients by eight gene expression profiles. Cancer Med.
5:837–852. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Zhang J, Sun X, Wang Y and Qian Y:
Mitophagy-mediated molecular subtypes depict the hallmarks of the
tumour metabolism and guide precision chemotherapy in pancreatic
adenocarcinoma. Front Cell Dev Biol. 10:9012072022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martelli M, Ferreri AJ, Agostinelli C, Di
Rocco A, Pfreundschuh M and Pileri SA: Diffuse large B-cell
lymphoma. Crit Rev Oncol Hematol. 87:146–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagai H, Miyaki D, Matsui T, Kanayama M,
Higami K, Momiyama K, Ikehara T, Watanabe M, Sumino Y and Miki K:
Th1/Th2 balance: An important indicator of efficacy for
intra-arterial chemotherapy. Cancer Chemother Pharmacol.
62:959–963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng X, Shi J, Sun W, Ruan X, Guo Y, Zhao
L, Wang J and Li B: Genetic polymorphisms of IL-6 promoter in
cancer susceptibility and prognosis: A meta-analysis. Oncotarget.
9:12351–12364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Narazaki M, Tanaka T and Kishimoto T: The
role and therapeutic targeting of IL-6 in rheumatoid arthritis.
Expert Rev Clin Immunol. 13:535–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiu B, Lin Y, Grote DM, Ziesmer SC,
Gustafson MP, Maas ML, Zhang Z, Dietz AB, Porrata LF, Novak AJ, et
al: IL-10 induces the development of immunosuppressive
CD14(+)HLA-DR(low/-) monocytes in B-cell non-Hodgkin lymphoma.
Blood Cancer J. 5:e3282015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Purdue MP, Lan Q, Kricker A, Grulich AE,
Vajdic CM, Turner J, Whitby D, Chanock S, Rothman N and Armstrong
BK: Polymorphisms in immune function genes and risk of non-Hodgkin
lymphoma: Findings from the New South Wales non-Hodgkin Lymphoma
Study. Carcinogenesis. 28:704–712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park YH, Sohn SK, Kim JG, Lee MH, Song HS,
Kim MK, Jung JS, Lee JJ, Kim HJ and Kim DH: Interaction between
BCL2 and interleukin-10 gene polymorphisms alter outcomes of
diffuse large B-cell lymphoma following rituximab plus CHOP
chemotherapy. Clin Cancer Res. 15:2107–2115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura N, Goto N, Tsurumi H, Takemura M,
Kanemura N, Kasahara S, Hara T, Yasuda I, Shimizu M, Sawada M, et
al: Serum level of soluble tumor necrosis factor receptor 2 is
associated with the outcome of patients with diffuse large B-cell
lymphoma treated with the R-CHOP regimen. Eur J Haematol.
91:322–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakayama S, Yokote T, Hirata Y, Akioka T,
Miyoshi T, Hiraoka N, Iwaki K, Takayama A, Nishiwaki U, Masuda Y,
et al: TNF-α expression in tumor cells as a novel prognostic marker
for diffuse large B-cell lymphoma, not otherwise specified. Am J
Surg Pathol. 38:228–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cruceriu D, Baldasici O, Balacescu O and
Berindan-Neagoe I: The dual role of tumor necrosis factor-alpha
(TNF-α) in breast cancer: Molecular insights and therapeutic
approaches. Cell Oncol (Dordr). 43:1–8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu Y, Wang X, Bai S, Niu L, Zhao G, Yao Y,
Li B and Li H: The effects of TNF-α/TNFR2 in regulatory T cells on
the microenvironment and progression of gastric cancer. Int J
Cancer. 150:1373–1391. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Camicia R, Bachmann SB, Winkler HC, Beer
M, Tinguely M, Haralambieva E and Hassa PO: BAL1/ARTD9 represses
the anti-proliferative and pro-apoptotic IFNү-STAT1-IRF1-p53 axis
in diffuse large B-cell lymphoma. J Cell Sci. 126((Pt 9)):
1969–1980. 2013.PubMed/NCBI
|
|
27
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fayad L, Keating MJ, Reuben JM, O'Brien S,
Lee BN, Lerner S and Kurzrock R: Interleukin-6 and interleukin-10
levels in chronic lymphocytic leukemia: Correlation with phenotypic
characteristics and outcome. Blood. 97:256–263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nacinovic-Duletic A, Stifter S, Dvornik S,
Skunca Z and Jonjic N: Correlation of serum IL-6, IL-8 and IL-10
levels with clinicopathological features and prognosis in patients
with diffuse large B-cell lymphoma. Int J Lab Hematol. 30:230–239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aydin F, Yilmaz M, Ozdemir F, Kavgaci H,
Yavuz MN and Yavuz AA: Correlation of serum IL-2, IL-6 and IL-10
levels with International Prognostic Index in patients with
aggressive non-Hodgkin's lymphoma. Am J Clin Oncol. 25:570–572.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guney N, Soydinc HO, Basaran M, Bavbek S,
Derin D, Camlica H, Yasasever V and Topuz E: Serum levels of
interleukin-6 and interleukin-10 in Turkish patients with
aggressive non-Hodgkin's lymphoma. Asian Pac J Cancer Prev.
10:669–674. 2009.PubMed/NCBI
|
|
32
|
Niitsu N, Okamato M, Nakamine H, Yoshino
T, Tamaru J, Nakamura S, Higashihara M and Hirano M: Simultaneous
elevation of the serum concentrations of vascular endothelial
growth factor and interleukin-6 as independent predictors of
prognosis in aggressive non-Hodgkin's lymphoma. Eur J Haematol.
68:912002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cortes J and Kurzrock R: Interleukin-10 in
non-Hodgkin's lymphoma. Leuk Lymphoma. 26:251–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
D'Mello KP, Zhao L, Kaser EC, Zhu Z, Xiao
H, Wakefield MR, Bai Q and Fang Y: The role of interleukins and the
widely studied TNF-α in non-Hodgkin's lymphoma. Med Oncol.
38:562021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Uskudar Teke H, Gunduz E, Akay OM, Bal C
and Gulbas Z: Are the high serum interleukin-6 and vascular
endothelial growth factor levels useful prognostic markers in
aggressive non-hodgkin lymphoma patients? Turk J Haematol.
32:21–28. 2015. View Article : Google Scholar : PubMed/NCBI
|