Introduction
Sarcomas are rare malignancies of mesenchymal cell
origin that display a heterogenous mix of clinical and pathologic
characteristics (1). They can be
classified into two main types: Soft tissue sarcoma (STS) and bone
sarcoma. Primary bone tumors and STS account for ~1% of adult
malignant tumors (1), and the most
common primary malignant bone tumors are osteosarcoma,
chondrosarcoma and Ewing sarcoma, while STS have complex
pathological types, the most common being leiomyosarcoma and
liposarcoma. Surgery with or without neoadjuvant chemoradiation is
recommended for patients with resectable lesions. However, ~50% of
patients with sarcoma develop metastases after surgery and the
postoperative recurrence rate is reported to be 10–20% (2). For these metastatic or recurrent
patients, anthracycline-based regimens are first-line therapies,
such as doxorubicin and epirubicin, which have limited efficacy
(1). While patients with NTRK gene
fusion-positive status can be treated with larotrectinib and
entrectinib (1), these cases
constitute <5% of patients with non-gastrointestinal stromal
tumor soft-tissue sarcoma (3). As
of now, there are no approved immune therapies for treating most
subtypes of sarcoma except for alveolar soft part sarcoma, due to
the severe immune suppressive tumor microenvironment (4).
Oncolytic virus is either wild-type or genetically
modified virus selectively replicating in tumor cells and thereby
lysing the cells and leading to tumor antigen release (5,6). In
addition, they can stimulate anti-cancer immunity by infecting
tumor cells and expressing exogenous immune-stimulating factors
carried by the virus (5,6). Therefore, oncolytic virotherapy can be
considered for anti-cancer immune therapy. VG161 is a genetically
modified herpes simplex virus type 1 (HSV-1) oncolytic virus. The
neurovirulence of wild-type HSV-1 is removed by deletion of ICP34.5
(two copies) in the wild-type backbone. It carries IL-12,
IL-15/IL-15 with its receptor α unit (IL-15RA) and programmed cell
death 1 ligand 1 (PD-L1) blocking peptide, which synergistically
stimulate innate and adaptive anti-tumor immunity (7,8). In
tumor-bearing immune-competent mice, VG161 has demonstrated
systemic anti-cancer activity and shown to induce anti-cancer
immune memory in a rechallenge mouse model (7,8). VG161
has now entered the stage of clinical development; current data
indicate its safety and potential clinical efficacy in certain
cancers. In the present study, two cases of sarcoma are reported
who were enrolled in a phase I dose escalation trial for patients
with solid tumor who had failed to respond to all standard
therapies and treated with VG161 monotherapy.
Materials and methods
Virus DNA detection and
quantification
VG161 virus DNA was quantified as described
previously (7). In brief, DNA was
isolated using the DNeasy Blood and Tissue Kit (Qiagen Sciences,
Inc.) and VG161 virus copies were measured by quantitative PCR
using primers and a probe specific to the codon optimized IL-15RA1
payload of VG161. The primers used were as follows: Forward,
5′-CTCTCCAAGCTCCAACAATACA-3′ and reverse,
5′-GAGGACTCGTGGCTAGAGAT-3′; and probe,
5′-CAGCAACCACAGCAGCAATCGTG-3′ (Integrated DNA Technologies).
Cytokine level detection
Cytokine levels were measured using the Meso Scale
Discovery (MSD) platform [V-PLEX Plus Proinflammatory Panel 1 Human
Kit (MSD) and the V-PLEX Plus Human IL-15 Kit (MSD)] based on the
manufacturers' instructions. In brief, plates precoated with
capture antibodies on predefined spots were incubated with serum
samples prediluted two-fold in assay diluent for 2 h. Detection
antibodies conjugated with electro-chemiluminescent labels (MSD
GOLD™ SULPHO TAG) were applied to the analytes to complete the
sandwich immunoassay. An MSD electrochemiluminescence detection
instrument was used for reading the V-Plex plate and V-PLEX
(Multiplex) data acquisition and analysis were performed using MSD
Discovery Workbench® 4.0 software (MSD).
Flow cytometric detection
A flow cytometry assay was performed on peripheral
blood mononuclear cells (PBMCs) (9)
isolated from patients by Ficoll Paque (Milipore-Sigma) using an
Attune NxT Flow Cytometer (Thermo Fisher Scientific, Inc). Anti-CD3
FITC (cat. no. 11-0038-41), CD56 PE (cat. no. 12-0567-41;
Invitrogen; Thermo Fisher Scientific, Inc.) and anti-CD8 BV605
(cat. no. 564115; BD Biosciences) antibodies were used to quantify
natural killer (NK) cells (CD3−CD56+) and
CD8+ cells (CD3+CD8+) in
peripheral blood. All antibodies were diluted 1:40 before use.
RNA-sequencing (seq) detection and
analysis
Total RNA from the biopsy samples was isolated using
the RNeasy Plus Isolation Kit (Qiagen Sciences, Inc.), and was used
to generate PCR-free cDNA Nanopore sequencing libraries (cat. no.
SQK-DCS109; Oxford Nanopore) following the manufacturer's protocol
and sequenced in PromethION R9 flow cells (Oxford Nanopore). Data
were analyzed using in-house bash scripts, including automated bash
scripts running Guppy basecaller v5 (https://genomebiology.biomedcentral.com/articles/10.1186/s13059-019-1727-y),
FastQC v0.11.9 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/,
minimapper2 (https://academic.oup.com/bioinformatics/article/34/18/3094/4994778,
Samtools v1.13 (https://academic.oup.com/gigascience/article/10/2/giab008/6137722?login=false)
and featureCounts v2.0.3 (https://academic.oup.com/bioinformatics/article/30/7/923/232889,
and packages in R v4.3.2 (DEseq2, ComplexHeatmap, Tidyverse,
ggplot2, ggpubr and clusterprofiler). RNA-seq analysis was used to
calculate the immune-cell composition using single-cell reference
profiles. The calculated immune cell values were relative to all
the immune cells identified in a particular sample, such that the
sum of the total value is equal to 1.
Results
Trial information
The two reported patients were part of a phase I
clinical trial conducted and registered in Australia
(ACTRN12620000244909). It was a first-in-human, open label, dose
escalation study to evaluate the safety, tolerability,
pharmacokinetics (PK) and biologic effect of VG161 in subjects with
advanced malignant solid tumors who are refractory to conventional
therapies, which was composed of two parts (Part A: Single dose
with three different dose levels of 5.0×107,
1.0×108 and 2.0×108 PFU/dose; Part B:
Multiple doses with three different dose levels of
5.0×107, 1.0×108 and 2.0×108
PFU/dose). In Part B, VG161 (Virogin) was administered as
intratumoral injections by five daily injections on Days one
through eight of each cycle with 28 days per cycle until
intolerability or tumor progression. Eligible patients were aged
≥18 years with advanced malignant solid tumor refractory/relapsed
after and/or intolerant to standard therapies or for which no
standard therapy exists or is available. Patients had at least one
injectable cutaneous or subcutaneous or hepatic lesions. Safety was
assessed according to the Common Terminology Criteria for Adverse
Events version 5.0 (10). Antitumor
activity was assessed using the Response Evaluation Criteria in
Solid Tumours (RECIST) version 1.1 (11) and the modified RECIST version 1.1
for immune-based therapeutics (termed iRECIST) criteria (12). For PK and viral shedding analysis,
viral DNA was measured in needle biopsy samples of injected tumor,
blood, urine, and swab of oral mucosa and the injection site.
Changes in cytokines and lymphocyte subsets in the blood were also
observed as pharmacodynamic parameters. Anti-drug antibody was
tested using HSV-1 IgG ELISA commercial kits (cat. no. H1029G;
Calbiotech). Biological activity was tested by RNA sequencing on
needle biopsy samples collected pre- and post-injection.
Case report
The two patients reported in the present study
received intratumoral injection of VG161 at 5.0×107
PFU/dose by five daily injections on Days one through eight per
cycle. The first case was a 68-year-old white male diagnosed with
right scapular conventional chondrosarcoma with lung metastasis at
an external hospital in November 2019, and received partial
scapulectomy for symptom control in November 2019. Post-operative
anti-cancer therapies included doxorubicin and local radiation, and
the patient progressed after completing doxorubicin treatment and
radiation. The patient had shoulder pain, which may have been
related to the tumor and was treated with paracetamol, ibuprofen
and Targin (naloxone hydrochloride; oxycodone hydrochloride). The
concomitant diseases included neuropathy, gastroesophageal reflux,
hypertension and Meniere's disease, which were treated with
esomeprazole, pregabalin and amlodipine.
Since October 2021, the subject (diagnosed as TxN0M1
Stage IV based on imaging examinations at screening) (13) had received intratumoral injection of
VG161 for a total of 13 cycles in the Southern Oncology Clinical
Research Unit (Adelaide, Australia) and the treatment was
discontinued due to disease progression in October 2022. The
maximum changes of the sum of the longest diameters of the target
lesions were −17.96% (evaluated at Cycle 5, Day 28) from baseline
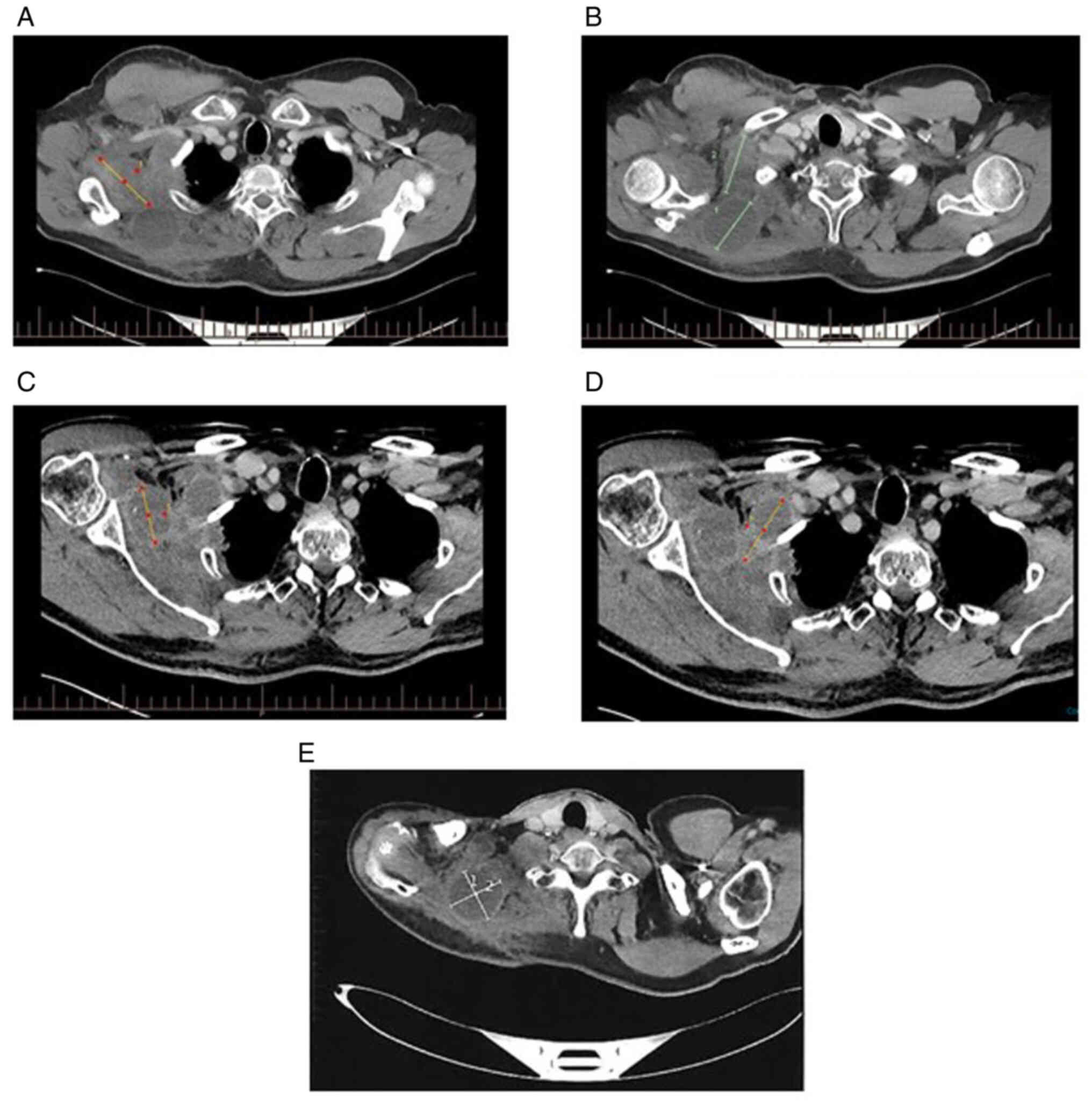
and the best response was stable disease (SD). The CT scans are
presented in Fig. 1. The
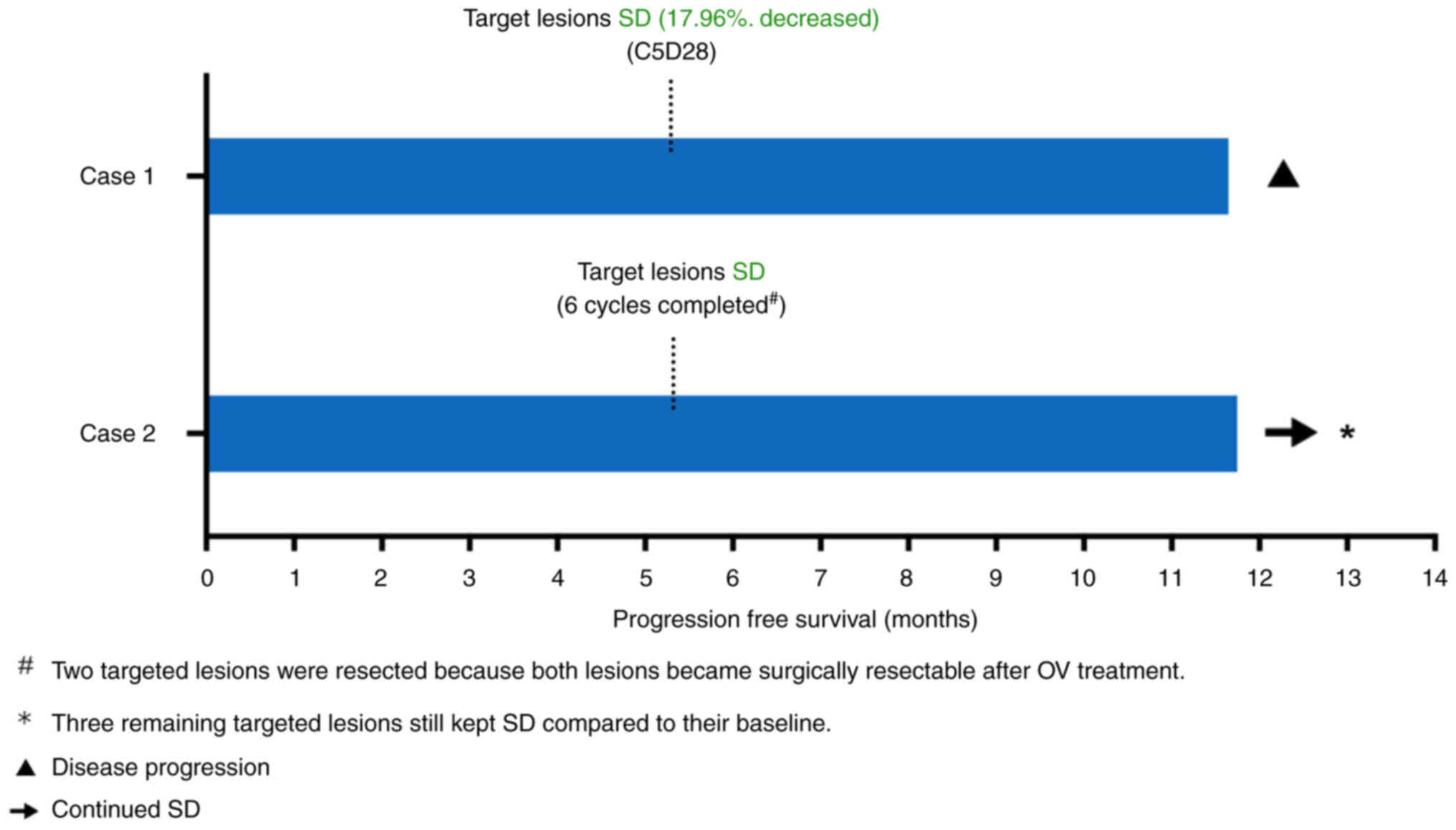
progression-free survival (PFS) was 11.8 months and overall
survival (OS) was not defined, as the patient was still alive at
the data cut-off date in late June 2023 (follow-up for >20.5
months). The data are shown in Fig.
2.
For Case 1, treatment-related adverse events (TRAEs)
included pyrexia (grade 2, occurred during Cycle 4, resolved after
hospitalization), fatigue (grade 1, occurred during Cycle 4,
resolved after ibuprofen treatment), nausea (grade 1, occurred
during Cycle 2, resolved after ondansentron treatment), neck and
face pain (grade 2, occurred during Cycle 2, resolved after
amitriptyline treatment), injection site reaction (injection site
pain, discharge and swelling, grade 1, occurred after Cycle 1,
resolved after oxycodone treatment), pruritus (grade 1, occurred
during Cycle 9, resolved spontaneously) and lymphocyte count
decreased (grade 3, occurred during Cycle 4, resolved
spontaneously). One severe adverse event (SAE), pyrexia (grade 2,
occurred during Cycle 4, resolved after hospitalization), was
reported, possibly related to treatment. All of the above AEs had
resolved. No TRAEs led to death or dose reduction or treatment
discontinuation.
The second case was a 52-year-old white female
diagnosed with STS, hemangiopericytoma, at an external hospital in
August 2011 and received radical popliteal resection. Post multiple
sequential anti-cancer therapies included radiotherapy, KN046 (a
recombinant antibody targeting PD-L1 and cytotoxic T-lymphocyte
antigen 4, doxorubicin and T3011 (HSV-1 oncolytic virus) and the
disease was progressive after these treatments. The patient had
pain in the thigh, cervical spine, shoulder and lower back
(intermittent pain), which may have been related to the tumor. The
concomitant diseases included neuropathy, hypothyroidism and
trochanteric bursitis, and the patient was treated with thyroxine
for hypothyroidism.
At screening, there were five target lesions
observed in this subject. The five target lesions were located at
the right axilla, right supraclavicular, right posterior thigh,
right diaphragm and anterior mediastinal node, respectively. Since
August 2022, the subject (diagnosed as TxN0M1 Stage IV based on
imaging examinations at screening) (13) had received intratumoral injection of
VG161 for a total of six cycles at the Southern Oncology Clinical
Research Unit (Adelaide, Australia) and maintained SD until
treatment discontinuation followed by surgical resection of the two
injected targeted lesions located respectively at the right axilla
and right supraclavicular lesions in January 2023. The above two
targeted lesions were resected because both lesions became
surgically resectable after oncolytic virus treatment. During the
follow-up visit after surgery, except for the two resected targeted
lesions, the three remaining targeted lesions still maintained SD
compared to their baseline. Therefore, the best overall response of
this subject was SD and the PFS was >11.9 months and OS was not
defined (>11.9 months), as the patient still kept SD at the data
cut-off date in late July 2023 (11.9 months from first dosing). The
data are shown in Fig. 2.
For Case 2, no TRAEs and SAEs were reported. The
treatment-emergent AEs (TEAEs) included cough, nausea, headache and
vomiting. These TEAEs were all grade 1 or 2 and had resolved. None
of the TRAEs led to death or dose reduction or treatment
discontinuation.
PK and viral shedding
Viral DNA was tested in injected lesion biopsy
samples after VG161 treatment, which demonstrated VG161 entered and
replicated in the injected tumor lesion. No viral DNA was detected
in the serum and urine samples, and swab of oral mucosa. While
viral DNA was detected at the injection site swab after injection
for case 1, it turned to be negative 24 h after the last dose. No
viral DNA was found in any of the injection site swabs from case 2.
The above results showed that multiple intratumoral injections of
VG161 have a low risk of viral spreading or shedding.
Translational findings
Levels of cytokines, including IL-12, IL-15, IL-6,
IFN-γ and TNF-α were tested in the blood before and after VG161
treatment. The results showed increases in these cytokines,
particularly INF-γ, after VG161 treatment, indicating activation of
immune function. The data are presented in Table I. Changes in peripheral
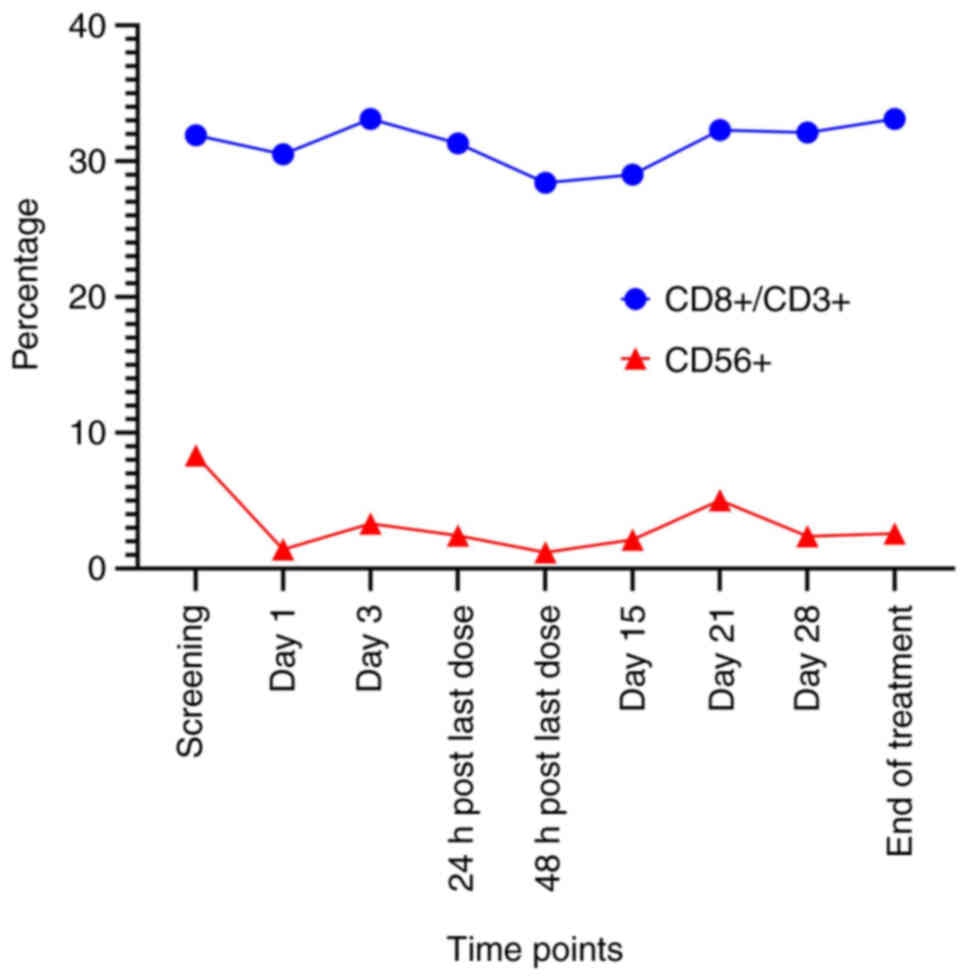
CD8+ and NK cells were determined by flow cytometry of
PBMC samples of Case 2, and no significant changes were observed
between the time-points Screening and End of treatment in Fig. 3. The data are shown in Figs. 3 and S1. The NK cells decreased slightly after
the first dose of VG161 (Day 1) compared to the baseline
(Screening), which may prompt the transfer of peripheral NK cells
into the injected lesions. For RNA-seq analysis, bulk RNA was
extracted from the biopsy samples obtained from one injected lesion
at the right supraclavicular lesion of Case 2 pre- and post-VG161
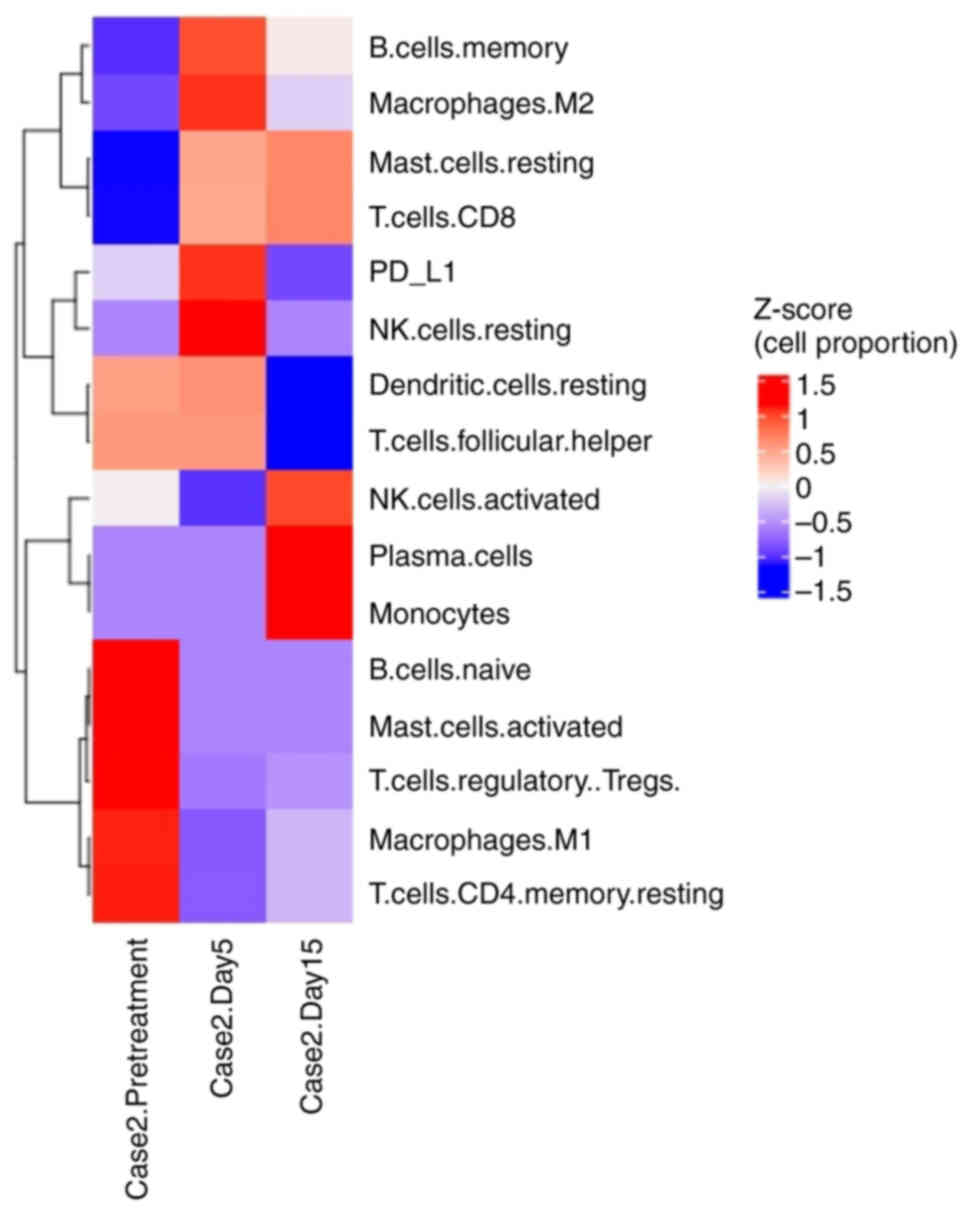
treatment. The heatmap constructed was using the Z-score, which
represents the deviation of individual sample values from the row
mean for a given cell type. Changes in the tumor microenvironment
before and after the VG161 treatment are displayed in Fig. 4. Although the levels of
CD8+ cells and NK cells in PBMCs only changed marginally
over the treatment course (Figs. 3
and S1), the tumor samples showed
more significant changes in CD8+ and NK cells, and
T-regulatory cells were decreased after VG161 treatment (Fig. 4). Due to a low quantity and quality
of sample for Case 1, there were insufficient data to analyze, and
it was not possible to present the lymphocyte cell and RNA-seq
results for Case 1. Furthermore, through the RNA-seq analysis, the
presence of a fusion gene (‘RP11-680G10.1:GSE1’ on chromosome 16
‘+’ strand) was found in Case 2, which has been previously reported
to be associated with sarcomas (14). Due to low RNA quantity and quality,
there were insufficient sequencing data from Case 1, and it was not
possible to detect any gene fusion in Case 1.
 | Table I.Changes of cytokines (pg/ml) in
peripheral blood in cycle 1 after VG161 treatment. |
Table I.
Changes of cytokines (pg/ml) in
peripheral blood in cycle 1 after VG161 treatment.
| Cytokines | Baseline Case 1/Case
2 | Day 3 Case 1/Case
2 | Day 8 Case 1/Case
2 | Day 15 Case 1/Case
2 | Day 28 Case 1/Case
2 | Max fold change Case
1/Case 2 |
|---|
| IL-15 | 3.30/5.20 | 4.13/4.93 | 2.80/3.78 | 2.56/6.62 | 4.48/4.17 | 1.36/1.27 |
| IL-6 | 2.89/1.13 | 3.46/1.56 | 2.22/7.91 | 1.78/1.39 | 2.51/1.00 | 1.20/7.00 |
| INF-γ | 9.68/8.03 | 46.24/15.66 | 9.70/6.80 | 4.61/167.71 | 9.53/4.96 | 4.78/20.89 |
| TNF-α | 0.84/0.54 | 0.89/0.71 | 0.89/0.63 | 0.92/1.13 | 0.86/0.57 | 1.10/2.09 |
Discussion
Sarcomas can be classified into two main types: STS
and bone sarcoma. The systemic treatment of unresectable sarcoma
remains a clinical challenge, as the efficacy of approved standard
therapies is limited. Chondrosarcoma, one subtype of sarcoma,
accounts for 9.2% of all primary malignant bone tumors with an
incidence of ~1/200,000. Conventional chondrosarcoma accounts for
~85% of all chondrosarcoma, including primary and secondary
chondrosarcoma. Chemotherapy is commonly ineffective in
chondrosarcoma, particularly for conventional chondrosarcoma, and
there is currently no standard systemic treatment for conventional
chondrosarcoma (15). In recent
clinical trials, patients with inoperable advanced chondrosarcoma
who received apatinib treatment achieved a median PFS of 4.7 months
(16). In the current study, a
patient with chondrosarcoma in whom chemotherapy failed but who
achieved a prolonged PFS of 11.8 months was presented. Similarly,
STS also poses challenges with standard therapy, as there is
limited efficacy with an objective response rate (ORR) of 49%, a
median PFS of 4.2 months and a median OS of 16.8 months (1). A phase II study recently reported a
PFS of 4.1 months among unresectable and/or metastatic soft tissue
sarcoma (17). By contrast, the
present study demonstrated that VG161 monotherapy achieved a
prolonged PFS (>11.9 months) of a case of STS that previously
progressed after chemotherapy. Although the OS is undefined so far,
improved OS data are expected as the follow-up time for one of
these two patients exceeds 14.3 months, which is the median OS
reported in the literature (17).
With this encouraging result, a clinical trial of VG161 monotherapy
focusing on sarcoma is being planned to be launched.
So far, only limited subtypes of sarcoma have
demonstrated a response to immune therapies. Atezolizumab, as the
first systemic therapy, was approved for alveolar soft part
sarcoma, which reported an overall response rate of 24% and a
durable response rate at 6 and 12 months of 67 and 42%,
respectively (18). The clinical
trial has reported that responses to immune therapies occurred in
numerous subtypes of sarcoma (19).
Among the 38 patients that received nivolumab monotherapy, the
confirmed ORR was 5% [92% CI (1–15%)]. Responses occurred in
undifferentiated pleomorphic sarcoma (UPS) and sarcoma not
otherwise specified (NOS). For the 38 patients that received
nivolumab in combination with ipilimumab therapy, the confirmed ORR
was 16%, [92% CI (7–29%)]. Responses occurred in UPS,
leiomyosarcoma, myxofibrosarcoma and angiosarcoma. Nivolumab alone
demonstrated limited efficacy in an unselected sarcoma population;
however, nivolumab combined with ipilimumab demonstrated
preliminary efficacy in certain sarcoma subtypes. To date, no
immune therapy has been approved for the two subtypes of sarcoma
reported in the current study.
Unlike conventional chemotherapy, the ORR usually
does not represent the prolonged PFS and OS with anti-cancer immune
therapy. The ORR of ipilimumab in combination with nivolumab for
patients with metastatic sarcoma was reported to be only 16%, but
significantly prolonged PFS and OS were achieved, which appears to
be more important for efficacy assessment (19). Instead of directly killing cancer
cells, immune therapy can cause tumor dormancy through activation
of anti-cancer immunity, resulting in prolonged PFS and OS. None of
the two cases reported in the present study achieved partial
response, but the best overall response was SD. Together with the
prolonged PFS and OS, it reflected the activation of anti-cancer
immunity by VG161 treatment, which was also demonstrated by the
laboratory findings, as discussed in the following section.
Certain progress has been made in therapies of
oncolytic viruses in sarcoma. A phase II trial of Talimogene
laherparepvec plus pembrolizumab in patients with locally advanced
or metastatic sarcoma who had failed at least one standard systemic
therapy reported the efficacy data. The primary endpoint was the
ORR at 24 weeks, which was 35% (20). A phase II clinical trial is
investigating the combination of JX-594, a thymidine kinase
gene-inactivated oncolytic virus expressing the
granulocyte-macrophage colony-stimulating factor, combined with
metronomic cyclophosphamide (arm 1) compared with metronomic
cyclophosphamide (arm 2) in patients with advanced STS. None of the
patients in arm 1 were progression-free at six months, while one
out of four was progression-free at six months in arm 2 (21). Of note, the present study found a
clinical meaningful PFS benefit of the treatment with
immune-stimulating HSV-1 oncolytic virus VG161 in patients with
sarcoma, with high tolerance and a good safety profile.
The clinical benefit observed in the two patients of
the present study may be explained by the upregulation of IFN-γ
induced by VG161. The IFN pathway has an important role in the
human immune response. Following the virus entering the human body,
the innate and adaptive immune responses are being triggered to
defend against the virus. One of the critical pathways against the
viral invasion is the IFN pathway (22); however, it also stimulates
anti-tumor activity. Therefore, IFNs are used in the treatment of
numerous types of cancer (23). In
the present trial, the level of IFN-γ was apparently increased
after VG161 treatment in both cases where prolonged PFS/OS was
observed.
Along with IFN-γ, and when biopsy samples from Case
2 were examined, an increase in the number of immune cells,
including activated and resting NK cells, CD8+ cells and
memory B cells were also detected post-treatment. On the other
hand, the numbers of T-regulatory cells were decreased in the
post-treatment samples. Although systemic changes in
CD8+ and NK cells were not significant, it may be an
important indication for the safety of VG161 treatment.
Furthermore, these results, along with the tumor biopsy immune cell
composition, may indicate local changes in the tumor
microenvironment, as well as infiltration of immune cells, which
may be more significant than systemic changes in the peripheral
immune cell composition.
Taken together, the current findings point out an
increased activation of the immune system, locally and
systemically. Another interesting finding was the upregulation of
PD-L1 in post-treatment biopsy samples. This finding strongly
suggests that combination therapy of VG161 with checkpoint
inhibitors (CPI) may be warranted in these patients. Furthermore,
clinical trials of combination therapy of VG161 with CPI are
ongoing in China and the US (NCT05162118, NCT05223816, NCT06008925
and NCT06124001).
Finally, another incidental finding through the
RNA-seq analysis was the presence of a fusion gene in Case 2
(‘RP11-680G10.1:GSE1’ on the chromosome 16 ‘+’ strand) that has
been previously reported to be associated with sarcomas (14), while the association between the
reported antitumor activity and the gene fusion remains to be
elucidated.
In conclusion, patients with advanced chondrosarcoma
and STS treated with VG161 intratumoral injection had a prolonged
PFS and potentially OS benefit compared to previously reported data
in the literature (1,16,17).
This promising outcome may be attributed to the ability of VG161 to
activate anti-cancer immunity and transform an immune-suppressive
tumor microenvironment into an immune-active one. The encouraging
results observed in these two patients provide strong support for
conducting further investigations into the efficacy of VG161 in
sarcoma through well-designed clinical trials in the future.
The potential of VG161 to induce a durable response
and activate the immune system against sarcomas may open up new
avenues for effective treatment options, offering hope for improved
outcomes for patients facing this challenging disease. Further
research through clinical trials will be essential to validate and
fully understand the benefits and mechanisms of VG161 therapy in
treating sarcomas.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Virogin provided funding for this research.
Availability of data and materials
The data generated in the present study may be found
in the National Center for Biotechnology Information (NCBI)
Sequence Read Archive under BioProject no. PRJNA1066249 or the
following URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1066249.
Authors' contributions
Conceptualization: YQ, AQ, RZ, WJ, QT and GK.
Methodology: YQ, AQ, QT and GK. Writing-original draft preparation:
YQ and AQ. Writing-review and editing: YQ, AQ, JD, WJ and YM.
Translational findings-analysis: JD, WJ, MS and YM. All authors
have read and agreed to the published version of the manuscript. YQ
and AQ confirm the authenticity of the raw clinical data. JD and YM
confirm the authenticity of the raw non-clinical data.
Ethics approval and consent to
participate
The protocol was approved by the Institutional
Review Board/Independent Ethics Committee of the Southern Oncology
Clinical Research Unit, St Vincent's Public Hospital and Royal
Brisbane and Women's Hospital (ethical approval nos.
2019-11-950-AA-A-1, 2019-11-950-A-9 and HREC/2021/QRBW/78009,
respectively). The above three hospitals have participated in this
Phase I study. Informed consent was obtained from all subjects
participated in the study, including the statement of participation
in an experimental study/receiving experimental treatment.
Patient consent for publication
Written informed consent was obtained from the
patients to publish their data and images presented in this
paper.
Competing interests
YQ, AQ, RZ, JD, WWGJ, MS, YM and QT are either
current or former employees of Virogin Biotech, a company focusing
on developing oncolytic virotherapy. This company funded this study
and provided the oncolytic virus VG161 used in the study.
References
|
1
|
NCCN, . NCCN Clinical Practice Guidelines
in Oncology (NCCN Guidelines®) Soft Tissue Sarcoma
version 2.2023. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1464August
3–2023
|
|
2
|
Damerell V, Pepper MS and Prince S:
Molecular mechanisms underpinning sarcomas and implications for
current and future therapy. Signal Transduct Target Ther.
6:2462021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cocco E, Scaltriti M and Drilon A: NTRK
fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin
Oncol. 15:731–747. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albarrán V, Villamayor ML, Pozas J,
Chamorro J, Rosero DI, San Román M, Guerrero P, Pérez de Aguado P,
Calvo JC, García de Quevedo C, et al: Current landscape of
immunotherapy for advanced sarcoma. Cancers. 15:22872023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macedo N, Miller DM, Haq R and Kaufman HL:
Clinical landscape of oncolytic virus research in 2020. J
Immunother Cancer. 8:e0014862020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su Y, Su C and Qin L: Current landscape
and perspective of oncolytic viruses and their combination
therapies. Transl Oncol. 25:1015302022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chouljenko DV, Ding J, Lee IF, Murad YM,
Bu X, Liu G, Delwar Z, Sun Y, Yu S, Samudio I, et al: Induction of
durable antitumor response by a novel oncolytic herpesvirus
expressing multiple immunomodulatory transgenes. Biomedicines.
8:4842020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding J, Murad YM, Sun Y, Lee IF, Samudio
I, Liu X, Jia WW and Zhao R: Pre-existing HSV-1 immunity enhances
anticancer efficacy of a novel immune-stimulating oncolytic virus.
Viruses. 14:23272022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hokland P and Heron I: Analysis of the
lymphocyte distribution during Isopaque-Ficoll isolation of
mononuclear cells from human peripheral blood. J Immunol Methods.
32:31–39. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Institues of Health National
Cancer Institute, . Common Terminology Criteria for Adverse Events
(CTCAE). Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdfNovember
27–2017
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao T, Liu JJ, Zhao LJ, Zhou JY, Wang JQ,
Wang Y, Wang ZQ, Wei LH, Wang JL and Li XP: Identification of new
fusion genes and their clinical significance in endometrial cancer.
Chin Med J (Engl). 132:1314–1321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monga V, Mani H, Hirbe A and Milhem M:
Non-conventional treatments for conventional chondrosarcoma.
Cancers. 12:1–12. 2020. View Article : Google Scholar
|
|
16
|
Xie L, Xu J, Sun X, Liu K, Li X, He F, Liu
X, Gu J, Lv Z, Yang R, et al: Apatinib for treatment of inoperable
metastatic or locally advanced chondrosarcoma: What we can learn
about the biological behavior of chondrosarcoma from a two-center
study. Cancer Manag Res. 12:3513–3525. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ayodele O and Abdul Razak AR:
Immunotherapy in soft-tissue sarcoma. Curr Oncol. 27 (Suppl
1):S17–S23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bergsma EJ, Elgawly M, Mancuso D, Orr R,
Vuskovich T and Seligson ND: Atezolizumab as the first systemic
therapy approved for alveolar soft part sarcoma. Ann Pharmacother.
19:106002802311874212023.
|
|
19
|
D'Angelo SP, Mahoney MR, Van Tine BA,
Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap
WD, Schwartz GK and Streicher H: Nivolumab with or without
ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two
open-label, non-comparative, randomised, phase 2 trials. Lancet
Oncol. 19:416–426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly CM, Antonescu CR, Bowler T, Munhoz
R, Chi P, Dickson MA, Gounder MM, Keohan ML, Movva S, Dholakia R,
et al: Objective response rate among patients with locally advanced
or metastatic sarcoma treated with talimogene laherparepvec in
combination with pembrolizumab: A phase 2 clinical trial. JAMA
Oncol. 6:402–408. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toulmonde M, Cousin S, Kind M, Guegan JP,
Bessede A, Le Loarer F, Perret R, Cantarel C, Bellera C and
Italiano A: Randomized phase 2 trial of intravenous oncolytic virus
JX-594 combined with low-dose cyclophosphamide in patients with
advanced soft-tissue sarcoma. J Hematol OncolJ Hematol Oncol.
15:1492022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barber GN: Host defense, viruses and
apoptosis. Cell Death Differ. 8:113–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldstein D and Laszlo J: The role of
interferon in cancer therapy: A current perspective. CA Cancer J
Clin. 38:258–277. 1988. View Article : Google Scholar : PubMed/NCBI
|