Introduction
The majority of newly diagnosed cancers are breast
cancers and breast cancer is also a leading cause of cancer death
in women globally (1). In spite of
the early diagnosis, radiation and chemotherapy, breast cancer is
the second leading cause of cancer death in the United States
(2). One of the major reasons for
such a high morbidity and mortality of breast cancer is the
invasive behavior of breast cancer cells leading to cancer
metastasis. Certain natural/dietary compounds, presented in
vegetables and fruits, can affect various signaling pathways and
molecular targets leading to their possible use in the combination
therapy (3). The use of dietary
supplements is highest among breast cancer survivors (4) suggesting that these supplements can
prevent the exacerbation of comorbid conditions associated with
breast cancer (5). Proper
evaluation of toxicity and biological effects of polybotanical
dietary supplements in cancer in general and in breast cancer in
particular is of great importance.
BreastDefend™ (BD) is a polybotanical dietary
supplement which inhibits growth and invasive behavior of highly
metastatic human breast cancer cells in vitro(6). BD contains mycelium from Asian
medicinal mushrooms (Coriolus versicolor, Ganoderma lucidum, and
Phellinus linteus), which separately suppressed growth and
inhibited invasiveness of breast cancer cells by different
mechanisms (6–10). In addition, some of the natural
agents in BD also demonstrated anticancer activities against breast
cancer cells. For example, extracts or purified compounds from
Scutellaria barbata, Astragalus membranaceus and Curcuma
longa suppressed growth, induced oxidative stress and
apoptosis, inhibited breast cancer cell invasiveness and prevented
breast cancer metastases in mice (11–14).
Quercetin, a flavonoid found in fruits, vegetables, leaves and
grains inhibited proliferation of invasive breast cancer cells and
its combination with other polyphenols further suppressed tumor
growth and site-specific metastasis (15,16).
Finally, 3,3′-diindolylmethane (DIM), the biologically active
compound derived from the digestion of indole-3-carbinol, found in
cruciferous vegetables such as broccoli, Brussels sprouts, cabbage
and kale suppressed growth, migration and invasion of metastatic
breast cancer cells (17,18).
In the present study, we evaluated toxicity and
anti-cancer activities of BD in an animal model of breast-to-lung
cancer metastasis with triple-negative highly invasive humane
breast cancer cells MDA-MB-231 implanted in the mammary pad of nude
mice. Here, we show that BD is not toxic and its oral application
significantly suppresses time-dependent increase in tumor sizes and
inhibits breast-to-lung cancer metastasis. In addition, BD inhibits
expression of pro-metastastic genes PLAU and CXCR4,
in breast cancer xenografts. Our results confirm that BD is not
toxic and inhibits growth and metastasis of invasive human breast
cancer cells in vivo.
Materials and methods
Cell culture and reagents
Highly invasive human breast cancer cells
(MDA-MB-231) were obtained from ATCC (Manassas, VA). MDA-MB-231
cells were maintained in DMEM medium supplemented with penicillin
(50 U/ml), streptomycin (50 U/ml), and 10% fetal bovine serum
(FBS). Media and supplements came from Gibco BRL (Grand Island,
NY). FBS was obtained from Hyclone (Logan, UT). BreastDefend (BD)
was supplied by EcoNugenics®, Inc. (Santa Rosa, CA). BD
contains the following active weight components: herb and active
nutritional blend 57.56%, [Quercetin (98% bioflavonoids), Turmeric
rhizome extract (Curcuma longa) complex with enhanced
bioavailability (BCM-95®), Scutellaria barbata
herb extract, and Astragalus membranaceus root extract],
mushroom mycelium blend 30.3% (Coriolus versicolor, Ganoderma
lucidum, Phellinus linteus), and 3,3′-diindolylmethane 12.12%.
BD is manufactured consistent with the FDA Good Manufacturing
Practices (GMP) regulation for dietary supplements as defined in 21
CFR§111, for batch to batch consistency and quality controls. BD
stock solution was prepared by dissolving BD in dimethylsulfoxide
(DMSO) at a concentration 25 mg/ml and stored at 40°C.
Toxicology studies
Toxicity of BD was evaluated in the 6-week old
female nude mice (Harlan, Indianapolis, IN, USA). The mice were
acclimatized for 1 week, and BD was applied by intragastrical
gavage 5 times/week for additional 4 weeks at the following doses:
0, 100, 200 and 400 mg/kg of body weight, n=10 per group. The body
weight was evaluated three times per week. At the end of the
experiment animals were euthanized by CO2 inhalation.
Blood was collected and a gross pathology examination performed.
Liver, spleen, kidney, lung and heart were harvested, fixed in 10%
neutral buffered formalin at 4°C for 24 h followed tissue
processing overnight, and then embedded in paraffin.
Five-micrometer sections were stained with hematoxilyn and eosin
(H&E). The levels of alanine aminotransferase (ALT), aspartate
aminotransferase (AST), alkaline phosphatase (ALP), albumin and
total protein were determined at the Department of Pathology and
Laboratory Medicine, Indiana University (Indianapolis, IN,
USA).
Human breast tumor xenograft
experiments
MDA-MB-231 cells (1×106) in 0.2 ml DMEM
were implanted subcutaneously in the mammary fat pad of the 6-week
old female nude mice (Harlan). After 1–2 weeks of implantation with
tumor cells, when tumors reached ~20–30 mm3, the animals
were randomized into control and treatment groups (15 animals per
group). The animals received intragastrical gavage 5 times/week
with water (control) or 100 mg BD/kg of body weight (treatment) for
additional 33 days. The tumor size was measured using calipers, and
the tumor volume was estimated by the formula: tumor volume
(mm3) = (W × L) 2 × 1/2, where L is the length and W is
the width of the tumor. At the end of the experiment (day 33), the
tumors were snap frozen and stored separately in liquid nitrogen.
The lung were harvested, fixed in 10% neutral buffered formalin at
4°C for 24 h followed tissue processing overnight, and then
embedded in paraffin. Five-micrometer sections were stained with
hematoxylin and eosin (H&E) and number of metastasis counted
under the light microscope.
The protocol for animal experiments was approved by
the Animal Research Committee at the Methodist Hospital according
to the NIH guidelines for the Care and Use of Laboratory
Animals.
Quantitative RT-PCR
The quantitative real-time polymerase chain reaction
(qRT-PCR) was performed using the ABI PRISM 7900HT Fast Real-Time
PCR system (Applied Biosystems) according to the manufacturer’s
instructions. Total RNA was isolated from tumors with RNAeasy
(Qiagen, Valencia, CA). The RNA samples were reverse transcribed
into cDNA (RT-PCR) using random hexamer primers and TaqMan reverse
transcription kit (Applied Biosystems). The cDNA (100 ng per
sample) was subjected to qPCR analysis in quadruplicate using
forward and reverse primers, TaqMan Universal Master Mix, and probe
(10 μl per reaction) in fast optical 96-well plates. The data were
analyzed using the ABI PRISM 7900 relative quantification (DDCt)
study software (Applied Biosystems). In this study we have used
primers for PLAU, CXCR4, EZR, HRAS, S100A4, CDKN1A and
HTATIP2 genes with β-actin gene as internal control
(Applied Biosystems). The gene expressions levels are normalized to
β-actin and are presented as arbitrary fold changes compared
between control and treated groups.
Statistical analysis
Toxicology analyses of plasma were summarized using
median (min, max) and compared across groups using Kruskal-Wallis
tests and Mann-Whitney U tests with significance level adjusted
using the Bonferroni correction. The changes in body weight and
tumor volume over time were tested using a random effects mixed
model. Metastasis incidence was summarized using percentage of
animals with metastases and compared between control and BD
treatment groups using Fisher’s exact test. Metastasis multiplicity
and qRT-PCR data were summarized using median (min, max) and
compared between control and BD treatment groups using Wilcoxon
rank sum test.
Results
Toxicity of BD in vivo
Our recent study demonstrated cytostatic effect of
BD on human breast cancer cells MDA-MB-231 (6). Although BD was not toxic for these
cells in vitro, systemic toxicity in animals needs to be
evaluated. To evaluate the toxicity of BD in vivo, female
nude mice were orally gavaged by 0, 100, 200 and 400 mg BD per kg
of body weight for 33 days as described in Materials and methods. A
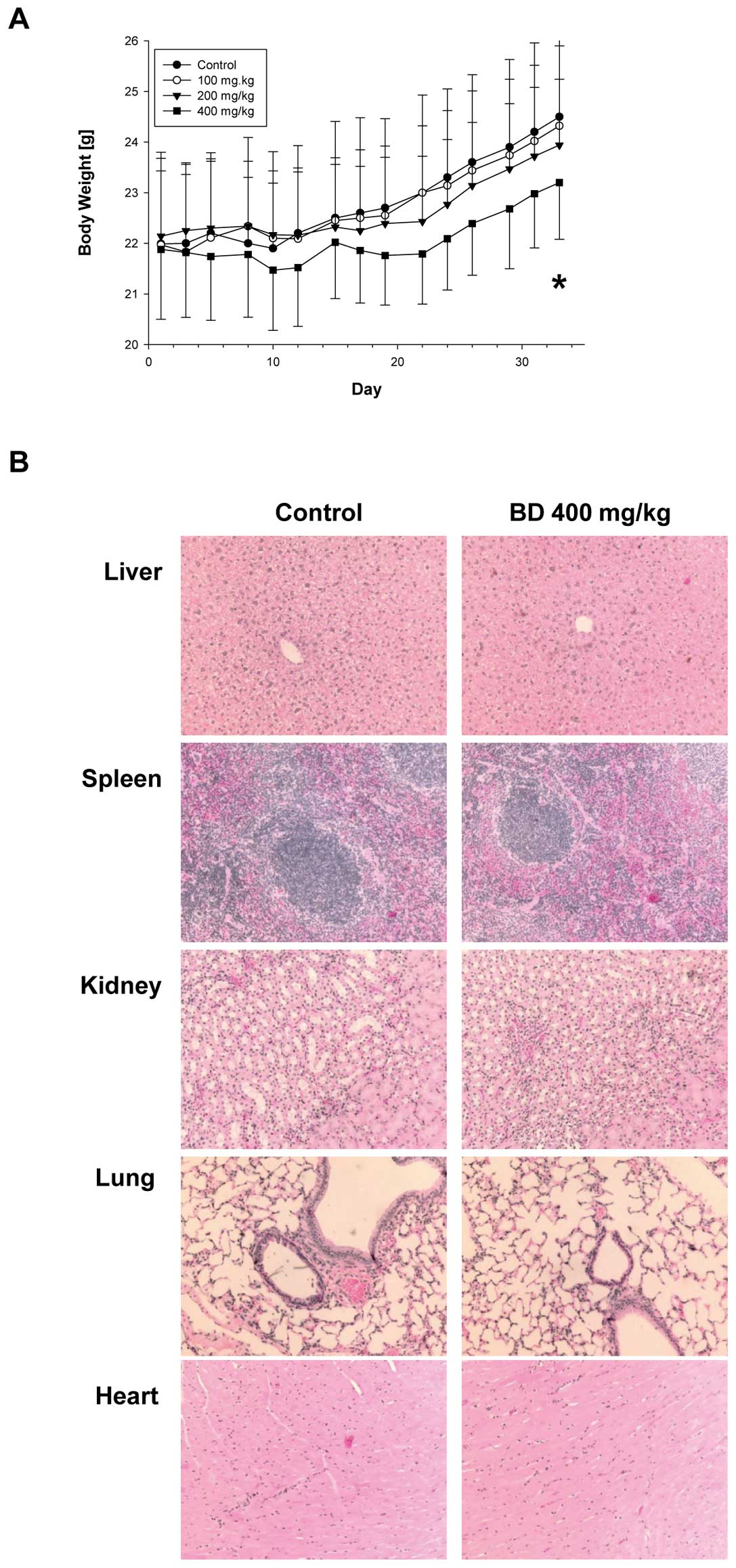
seen in Fig. 1A, all groups
demonstrated increase in body weight but the increase in 400 mg/kg
group was decreased by 5% to control group and this change in body
weight overtime was significant (p<0.001). In addition, gross
necropsy did not show any sign of toxicity and weights of liver,
spleen, kidney, lung and heart were not different between the
treatment and control groups (not shown). H&E staining of
control and highest BD dose treatment group (400 mg/kg) of liver,
spleen, kidney, lung and heart also did not demonstrate any
abnormalities (Fig. 1B). Although
the liver enzyme profiles in plasma (ALT, AST and ALP) were not
changed by BD treatment (0–400 mg/kg), the levels of albumin and
total protein were decreased at the 200 and 400 mg BD per kg groups
(Table I). Therefore, 100 mg BD/kg
dose was decided to be used in our experiments.
 | Table ILevels of liver enzymes, albumin and
total protein in plasma after BD treatment. |
Table I
Levels of liver enzymes, albumin and
total protein in plasma after BD treatment.
| n | Control | n | BreastDefend 100
mg/kg | n | BreastDefend 200
mg/kg | n | BreastDefend 400
mg/kg | P-value |
|---|
| ALT | 9 | 83 (34, 103) | 10 | 64 (51, 215) | 10 | 110 (43, 470) | 4 | 92 (63, 113) | 0.488 |
| AST | 9 | 236 (189, 867) | 9 | 232 (155, 396) | 9 | 414 (149,
1261) | 5 | 442 (197, 940) | 0.483 |
| ALP | 7 | 157 (149, 191) | 7 | 165 (145, 464) | 6 | 113.5 (53,
136)a | 6 | 154 (135, 207) | 0.003 |
| Albumin | 9 | 1.6 (1.3, 1.7) | 9 | 1.7 (1.6, 1.8) | 7 | 1.4 (1.3,
1.4)a | 10 | 1.4 (1.3,
1.5)a | <0.001 |
| Total protein | 6 | 5.3 (4.9, 5.6) | 5 | 5.4 (5.2, 5.6) | 3 | 4.8 (4.6, 5.2) | 4 | 4.8 (4.6, 5.3) | 0.036 |
BD suppresses tumor growth and prevents
breast-to-lung cancer metastasis in an orthotopic model of human
breast cancer
Based on our data demonstrating that 100 mg/kg of BD
is not toxic in vivo, we have employed an animal orthotopic
model of human breast cancer. MDA-MB-231 cells were inoculated into
mammary fat pad of female nude mice. When the forming tumors
reached the size ~20–30 mm3, the mice were divided into
the control group (water) and the treatment group (BD 100 mg/kg of
body weight/3 times/week). There were no changes in the tumor
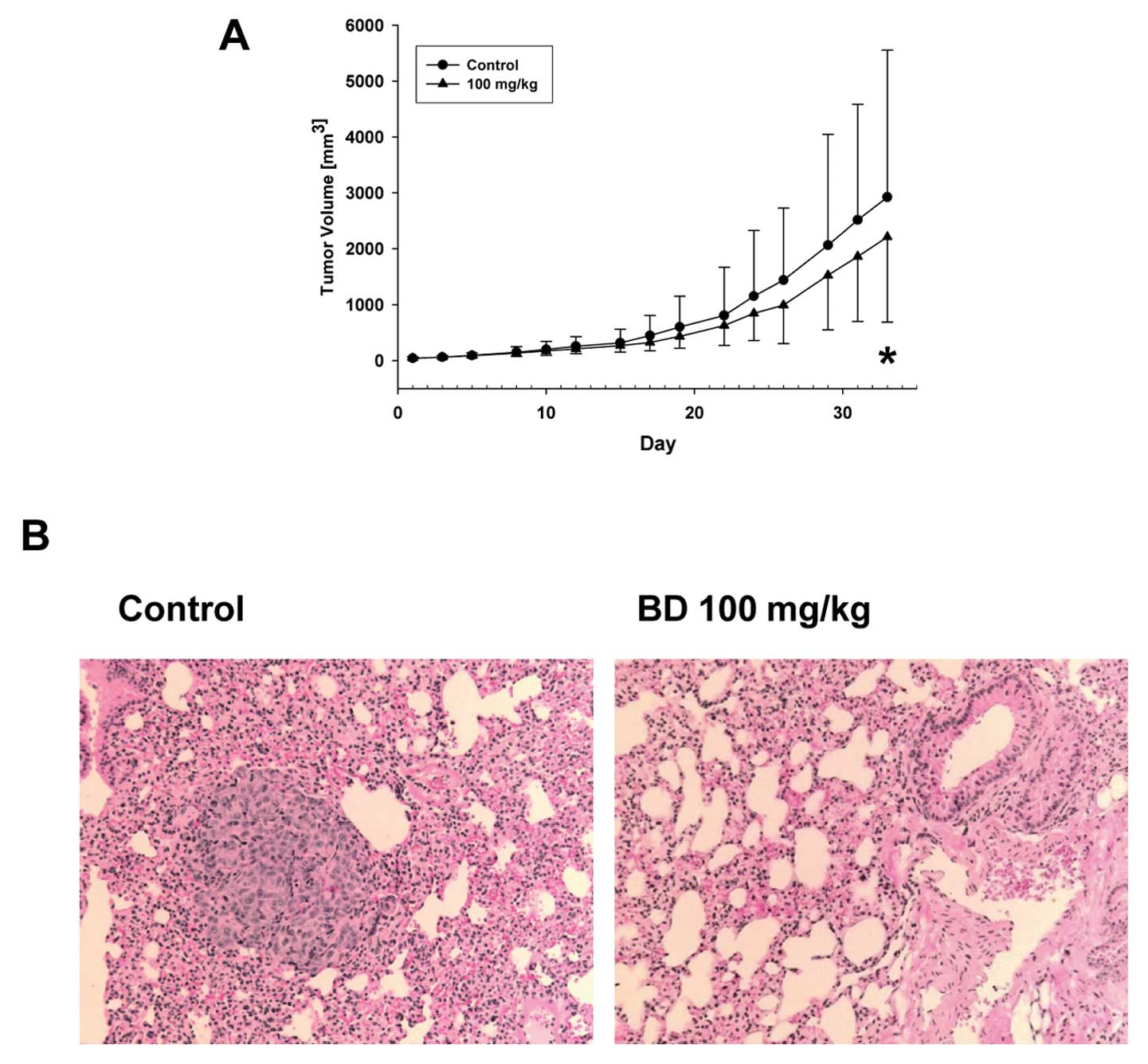
volumes for the first 2 weeks of the treatment. After that the
tumors in the BD treatment group were smaller, and we detected a
significant difference in the change in tumor volume over time
between control and BD treatment groups (p=0.002) (Fig. 2A).
Since we used an orthotopic model of breast cancer
where tumors form from the human breast cancer cells and
metastasize to lungs, we evaluated the incidence of metastasis and
metastasis multiplicity number in the control and BD treatment
groups. In the control group we detect breast-to-lung cancer
metastases in 10 of 15 animals (67%) whereas in the BD group only 3
of 15 animals (20%) developed breast to lung cancer metastasis
(Fig. 2B). Therefore, the 100 mg/kg
of BD significantly decreased incidence of breast-to-lung cancer
metastasis by 70% (p=0.025) demonstrating preventive effect of BD
against breast-to-lung cancer metastasis (Table II). Moreover, BD also significantly
suppressed the amount of lung metastases from 2.8 (0.0, 48) to 0
(0.0, 14.2) (Table II).
 | Table IIBD prevents breast-to-lung cancer
metastasis. |
Table II
BD prevents breast-to-lung cancer
metastasis.
| Treatment | Metastasis
incidence (animals with metastases, %) | Metastasis
multiplicity (metastases per animal) |
|---|
| Control | 10/15 (67) | 2.8 (0.0,
48.0) |
| BD (100 mg/kg) | 3/15 (20)a | 0.0 (0.0,
14.2)b |
Effect of BD on the gene expression in
tumors
We have recently demonstrated that BD inhibits
invasive behavior and expression of uPA and CXCR4 in MDA-MB-231
cells (6). Because cell
invasiveness in vitro reflects metastatic properties in
vivo we hypothesized that the inhibition of breast-to-lung
cancer metastasis by BD is associated with the downregulation of
expression of uPA and CXCR4 in primary tumors. To evaluate the
effect of BD on the expression of PLAU (uPA protein) and
CXCR4 genes in breast tumors, we isolated RNA and performed
quantitative RT-PCR in control and BD treated mice as described in
Materials and methods. In agreement with our in vitro study
(6), BD treatment significantly
downregulated expression of PLAU (p=0.026) and CXCR4
(p=0.002) in breast tumors (Fig.
3). Moreover, PLAU and CXCR4 expression in
primary tumors was significantly increased in animals with
breast-to-lung cancer metastasis (Table III). In addition, we have
evaluated expression of other genes associated with breast-to-lung
cancer metastasis: ezrin (EZR) (19), HRAS(20), S100A4(21), CDKN1A (protein p21) (22) and HTATIP2 (protein TIP30)
(23). However, BD treatment did
not change expression of these genes in the primary tumors
(Fig. 3).
 | Table IIIPLAU and CXCR4
expression based on metastasis status. |
Table III
PLAU and CXCR4
expression based on metastasis status.
| Gene | No metastases
n=17 | Metastases
n=13 |
|---|
| PLAU | 0.4 (0.3, 0.9) | 1.1 (0.8,
2.2)a |
| CXCR4 | 0.1 (0.0, 0.6) | 1.7 (0.9,
2.3)a |
Discussion
Since cancer metastases are the major reason for the
mortality of cancer patients, prevention of metastases will
significantly extend life of cancer patients. Here we showed that
dietary supplement BD markedly prevented breast-to-lung cancer
metastases in an animal model of metastatic human breast cancer.
Although we observed a modest but significant inhibition in tumor
volume over time between control and BD treatment groups, this
effect was not so dramatic. Nevertheless, BD treatment prevented
breast-to-lung cancer metastases by 70%. The in vivo
systemic effect of BD, after an oral application of BD, can protect
against breast-to-lung cancer metastasis as we are demonstrating
here.
BD is a polybotanical compound and some of its
isolated components suppressed cancer metastases in vivo.
For example, dietary administration of curcumin decreased the
incidence of breast-to-lung cancer metastasis in nude mice
(24). Apigenin, biologically
active compound from Scutellaria barbata prevented
hepatocyte growth factor induced lung metastasis of breast cancer
cells (25). Protein-bound
polysaccharide isolated from Coriolus versicolor, PSK
(Krestin) inhibited lung metastasis in mice (26), and when combined with chemotherapy,
PSK significantly prolonged survival of patients with metastatic
gastric cancer (27). Isolated
polysaccharides or an extract from Phellinus linteus
suppressed pulmonary metastasis of melanoma cells in mice (28,29).
In addition, triterpenoid fraction from Ganoderma lucidum
inhibited liver metastasis (30),
and G. lucidum in diet or i.p. injection of isolated
ganoderic acid T suppressed lung metastasis, respectively (31,32).
Finally, an oral administration of DIM markedly inhibited lung
metastasis of murine cancer cells in mice (33). Therefore, in agreement with our
in vitro study with BD (6)
combined anti-metastatic effects of isolated components in BD could
result in the prevention of breast-to-lung cancer metastasis in
vivo.
Urokinase plasminogen activator (uPA; PLAU
gene) is one of the major proteins involved in the invasive
behavior (adhesion, migration and invasion) of cancer cells and
cancer metastasis (34,35). Moreover, mice with knockout
PLAU gene demonstrated slower growth and fewer metastases of
human xenografted breast cancer cells in immunodeficient mice
(36). Overexpression of the
chemokine receptor CXCR4 was originally detected in human
breast cancer cells, malignant breast tumors and metastases
(37). Inhibiting CXCR4 expression
in breast cancer cells, using different strategies (e.g., siRNA
silencing, phenotypic CXCR4 knockout, peptide inhibitor of
protein kinease-α), also suppressed breast cancer metastasis
(38–40). Therefore, targeting PLAU and
CXCR4 expression with natural compounds should result in the
suppression of breast to lung cancer metastasis. Indeed, here we
show that 70% of the inhibition of breast-to-lung cancer metastases
is associated with the downregulation of expression of PLAU
and CXCR4 in primary tumors in mice treated with BD.
Moreover, the relative expression of PLAU and CXCR4
in primary tumors is a predictive marker of breast-to-lung cancer
metastasis because we have identified significant increase of these
genes in animals with metastases.
As mentioned above, the effect of BD on the growth
of primary tumors was significant albeit modest. However, we
observed a dominant effect in the inhibition of breast-to-lung
cancer metastases. Since BD mainly prevents metastases, combination
therapy with other agents directly targeting tumor growth and/or
surgical tumor resection could be considered for the alternative
treatment of invasive breast cancers.
In our study we induced primary tumors and
breast-to-lung cancer metastasis with MDA-MB-231 cells. These cells
are characterized as basal-like/triple-negative breast cancer
cells; lacking expression of estrogen receptor-α (ER), progesterone
receptor (PR) and ErbB2/neu (HER2), which represent a highly
aggressive breast cancer subtype, that is resistant to treatment
and is associated with poor prognosis (41). Therefore, BD inhibits breast-to-lung
cancer metastases in a model based on breast cancer cells which do
not respond to the targeted receptor treatments (e.g., trastumazab
and hormonal treatments) (42),
suggesting BD as a natural dietary agent for the treatment of
triple-negative therapy resistant breast cancer cells.
In conclusion, our data demonstrate that the novel
dietary supplement BreastDefend (BD): i) is not toxic in
vivo at the concentration 100 mg/kg of body weight, and ii)
inhibits growth of breast tumors and breast-to-lung cancer
metastases in mice. Our data suggest that BD specifically targets
expression of PLAU and CXCR4 in triple-negative and
highly aggressive breast tumors in vivo. In conclusion, BD
may be considered as novel polybotanical preparation for the
prevention and alternative therapy of metastatic breast cancer.
Acknowledgements
This study was supported by a research grant from
EcoNugenics, Inc., Santa Rosa, CA. The authors would like to thank
Barry Wilk for his contribution to this study and Saravanan
Kanabasbai for the help with qRT-PCR.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
3
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velicer CM and Ulrich CM: Vitamin and
mineral supplement use among US adults after cancer diagnosis: a
systematic review. J Clin Oncol. 26:665–673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller MF, Bellizzi KM, Sufian M, Ambs AH,
Goldstein MS and Ballard-Barbash R: Dietary supplement use in
individuals living with cancer and other chronic conditions: a
population-based study. J Am Diet Assoc. 108:483–494. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang J, Wojnowski R, Jedinak A and Sliva
D: Suppression of proliferation and invasive behavior of human
metastatic breast cancer cells by dietary supplement BreastDefend.
Integr Cancer Ther. 10:192–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho CY, Kim CF, Leung KN, Fung KP, Tse TF,
Chan H and Lau CB: Differential anti-tumor activity of Coriolus
versicolor (Yunzhi) extract through p53- and/or Bcl-2-dependent
apoptotic pathway in human breast cancer cells. Cancer Biol Ther.
4:638–644. 2005.PubMed/NCBI
|
|
8
|
Jiang J, Slivova V and Sliva D:
Ganoderma lucidum inhibits proliferation of human breast
cancer cells by downregulation of estrogen receptor and NF-kappaB
signaling. Int J Oncol. 29:695–703. 2006.
|
|
9
|
Thyagarajan A, Jiang J, Hopf A, Adamec J
and Sliva D: Inhibition of oxidative stress-induced invasiveness of
cancer cells by Ganoderma lucidum is mediated through the
suppression of interleukin-8 secretion. Int J Mol Med. 18:657–664.
2006.PubMed/NCBI
|
|
10
|
Sliva D: Suppression of cancer
invasiveness by dietary compounds. Mini Rev Med Chem. 8:677–688.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bui-Xuan NH, Tang PM, Wong CK and Fung KP:
Photo-activated pheophorbide-a, an active component of Scutellaria
barbata, enhances apoptosis via the suppression of ERK-mediated
autophagy in the estrogen receptor-negative human breast
adenocarcinoma cells MDA-MB-231. J Ethnopharmacol. 131:95–103.
2010. View Article : Google Scholar
|
|
12
|
Ye MN and Chen HF: Effects of Astragalus
injection on proliferation of basal-like breast cancer cell line
MDA-MB-468. Zhong Xi Yi Jie He Xue Bao. 6:399–404. 2008.PubMed/NCBI
|
|
13
|
Shao ZM, Shen ZZ, Liu CH, Sartippour MR,
Go VL, Heber D and Nguyen M: Curcumin exerts multiple suppressive
effects on human breast carcinoma cells. Int J Cancer. 98:234–240.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bachmeier B, Nerlich AG, Iancu CM, Cilli
M, Schleicher E, Vené R, Dell’Eva R, et al: The chemopreventive
polyphenol Curcumin prevents hematogenous breast cancer metastases
in immunodeficient mice. Cell Physiol Biochem. 19:137–152. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schlachterman A, Valle F, Wall KM, Azios
NG, Castillo L, Morell L, Washington AV, et al: Combined
resveratrol, quercetin, and catechin treatment reduces breast tumor
growth in a nude mouse model. Transl Oncol. 1:19–27. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castillo-Pichardo L, Martínez-Montemayor
MM, Martínez JE, Wall KM, Cubano LA and Dharmawardhane S:
Inhibition of mammary tumor growth and metastases to bone and liver
by dietary grape polyphenols. Clin Exp Metastasis. 26:505–516.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu EL, Chen N, Westbrook A, Wang F, Zhang
R, Taylor RT and Hankinson O: CXCR4 and CXCL12 down-regulation: a
novel mechanism for the chemoprotection of 3,3′-diindolylmethane
for breast and ovarian cancers. Cancer Lett. 265:113–123.
2008.PubMed/NCBI
|
|
18
|
Ahmad A, Kong D, Wang Z, Sarkar SH,
Banerjee S and Sarkar FH: Down-regulation of uPA and uPAR by
3,3′-diindolylmethane contributes to the inhibition of cell growth
and migration of breast cancer cells. J Cell Biochem. 108:916–925.
2009.
|
|
19
|
Elliott BE, Meens JA, SenGupta SK, Louvard
D and Arpin M: The membrane cytoskeletal crosslinker ezrin is
required for metastasis of breast carcinoma cells. Breast Cancer
Res. 7:R365–R373. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gelmann EP, Thompson EW and Sommers CL:
Invasive and metastatic properties of MCF-7 cells and
rasH-transfected MCF-7 cell lines. Int J Cancer. 50:665–669. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue C, Plieth D, Venkov C, Xu C and
Neilson EG: The gatekeeper effect of epithelial-mesenchymal
transition regulates the frequency of breast cancer metastasis.
Cancer Res. 63:3386–3394. 2003.PubMed/NCBI
|
|
22
|
Vanzulli SI, Soldati R, Meiss R, Colombo
L, Molinolo AA and Lanari C: Estrogen or antiprogestin treatment
induces complete regression of pulmonary and axillary metastases in
an experimental model of breast cancer progression. Carcinogenesis.
26:1055–1063. 2005. View Article : Google Scholar
|
|
23
|
Zhao J, Ni H, Ma Y, Dong L, Dai J, Zhao F,
Yan X, et al: TIP30/CC3 expression in breast carcinoma: relation to
metastasis, clinicopathologic parameters, and P53 expression. Hum
Pathol. 38:293–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aggarwal BB, Shishodia S, Takada Y,
Banerjee S, Newman RA, Bueso-Ramos CE and Price JE: Curcumin
suppresses the paclitaxel-induced nuclear factor-kappaB pathway in
breast cancer cells and inhibits lung metastasis of human breast
cancer in nude mice. Clin Cancer Res. 11:7490–7498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee WJ, Chen WK, Wang CJ, Lin WL and Tseng
TH: Apigenin inhibits HGF-promoted invasive growth and metastasis
involving blocking PI3K/Akt pathway and beta 4 integrin function in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
226:178–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishihara Y, Iijima H and Matsunaga K:
Contribution of cytokines on the suppression of lung metastasis.
Biotherapy. 11:267–275. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maehara Y, Tsujitani S, Saeki H, Oki E,
Yoshinaga K, Emi Y, Morita M, et al: Biological mechanism and
clinical effect of protein-bound polysaccharide K
(KRESTIN(®)): review of development and future
perspectives. Surg Today. 42:8–28. 2012. View Article : Google Scholar
|
|
28
|
Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID,
Yang KH and Kim HM: The inhibitory effect of polysaccharides
isolated from Phellinus linteus on tumor growth and metastasis.
Immunopharmacology. 41:157–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HJ, Lee HJ, Lim ES, Ahn KS, Shim BS,
Kim HM, Gong SJ, et al: Cambodian Phellinus linteus inhibits
experimental metastasis of melanoma cells in mice via regulation of
urokinase type plasminogen activator. Biol Pharm Bull. 28:27–31.
2005.PubMed/NCBI
|
|
30
|
Kimura Y, Taniguchi M and Baba K:
Antitumor and antimetastatic effects on liver of triterpenoid
fractions of Ganoderma lucidum: mechanism of action and
isolation of an active substance. Anticancer Res. 22:3309–3318.
2002.PubMed/NCBI
|
|
31
|
Nonaka Y, Ishibashi H, Nakai M, Shibata H,
Kiso Y and Abe S: Effects of the antlered form of Ganoderma
lucidum on tumor growth and metastasis in
cyclophosphamide-treated mice. Biosci Biotechnol Biochem.
72:1399–1408. 2008.PubMed/NCBI
|
|
32
|
Chen NH, Liu JW and Zhong JJ: Ganoderic
acid T inhibits tumor invasion in vitro and in vivo through
inhibition of MMP expression. Pharmacol Rep. 62:150–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim EJ, Shin M, Park H, Hong JE, Shin HK,
Kim J, Kwon DY, et al: Oral administration of 3,3′-diindolylmethane
inhibits lung metastasis of 4T1 murine mammary carcinoma cells in
BALB/c mice. J Nutr. 139:2373–2379. 2009.
|
|
34
|
Sliva D, Jedinak A, Kawasaki J, Harvey K
and Slivova V: Phellinus linteus suppresses growth,
angiogenesis and invasive behaviour of breast cancer cells through
the inhibition of AKT signalling. Br J Cancer. 98:1348–1356. 2008.
View Article : Google Scholar
|
|
35
|
Han B, Nakamura M, Mori I, Nakamura Y and
Kakudo K: Urokinase-type plasminogen activator system and breast
cancer (review). Oncol Rep. 14:105–112. 2005.PubMed/NCBI
|
|
36
|
Frandsen TL, Holst-Hansen C, Nielsen BS,
Christensen IJ, Nyengaard JR, Carmeliet P and Brünner N: Direct
evidence of the importance of stromal urokinase plasminogen
activator (uPA) in the growth of an experimental human breast
cancer using a combined uPA gene-disrupted and immunodeficient
xenograft model. Cancer Res. 61:532–537. 2001.
|
|
37
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, et al: Involvement of chemokine
receptors in breast cancer metastasis. Nature. 410:50–56.
2001.PubMed/NCBI
|
|
38
|
Liang Z, Yoon Y, Votaw J, Goodman MM,
Williams L and Shim H: Silencing of CXCR4 blocks breast cancer
metastasis. Cancer Res. 65:967–671. 2005.PubMed/NCBI
|
|
39
|
Ma WF, Du J, Fu LP, Fang R, Chen HY and
Cai SH: Phenotypic knockout of CXCR4 by a novel recombinant protein
TAT/54R/KDEL inhibits tumors metastasis. Mol Cancer Res.
7:1613–1621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim J, Thorne SH, Sun L, Huang B and
Mochly-Rosen D: Sustained inhibition of PKCα reduces intravasation
and lung seeding during mammary tumor metastasis in an in vivo
mouse model. Oncogene. 30:323–333. 2011.
|
|
41
|
Giricz O, Calvo V, Pero SC, Krag DN,
Sparano JA and Kenny PA: GRB7 is required for triple-negative
breast cancer cell invasion and survival. Breast Cancer Res Treat.
Oct 18–2011.(Epub ahead of print).
|
|
42
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|