Introduction
Neovascularization is vital for progressive
multiplication, metastasis and the recurrence of malignant tumors.
Since Asahara et al isolated endothelial progenitor cells
(EPCs) from human peripheral blood in 1997 (1), there is a growing body of evidence
supporting the notion that adjacent activated endothelial cell
sprouting is not a unique manner to form new blood vessels. Bone
marrow derived-EPCs (BM-EPCs) have the capacity to stimulate the
initiation and maintenance of angiogenic processes by integrating
into developing vasculature under physiological and pathological
conditions (2,3). EPCs resemble embryonic angioblasts
which characteristically migrate, proliferate and differentiate
into vascular endothelial cells (VECs) (4). In general, BM-EPCs can be identified
as cells that simultaneously express cell surface markers CD133,
CD34, and VEGFR2 (5,6). Broadly, any bone marrow derived cells
(BMDCs) that possess the potential of integrating into vessel walls
by differentiating into VECs can be defined as endothelial
progenitor cells/endothelial precursor cells (EPCs) (7).
HCC is among the most vascularized tumors and the
degree of vascularization correlates directly with prognosis in HCC
(8). However, the actual effect of
antivascular treatment including embolization to HCC has not
reached the expectations. A thorny problem is the rapid development
of new collateral circulation (9).
There must be certain compensatory mechanisms that promote
angiogenesis during therapy. With respect to cellular mechanisms,
growing attention is being paid to the role of BM-EPCs in tumor
angiogenesis (10–12). However, the degree of contribution
by EPC to vasculature vary highly with tumor type in different
studies, from substantial to zero, and the role of EPC, in
particular, remains somewhat controversial (13,14).
Although increased circulating EPC levels have been reported in
patients with HCC (15–17), up to now, there is no report
providing direct evidence that BM-EPCs are involved in the
neovascularization of HCC, and its impact on angiogeneis in HCC
remains uncertain.
Thus we established orthotropic HCC mice, observed
the dynamic changes of circulating BM-EPCs, analyzed when the
mobilization of BMCs starts and how long this process takes,
examined whether BM-EPCs contributed to tumor blood vessels
directly, and calculated the proportion of BM-EPCs in vessels. All
of this information not only is extremely important in order to
evaluate the impact of BM-EPCs on the neovascularization of HCC,
but also may have important value to perfect the current
anti-vascular strategy to patients with HCC.
Materials and methods
Isolation, culture, identification of
GFP+EPCs
GFP+EPCs were obtained according to the
literature (18). Briefly, BM
derived mononuclear cells from GFP transgenic C57BL/6 mice were
collected through density gradient centrifugation and cultured in
fibronectin coated dishes containing EGM-2 (Lonza, USA). After 48
h, non-adherent cells were collected and cultured continually. All
experiments were performed with second passage cells. The tube
formation ability of BM-EPCs was observed within 24-h culture in
matrigel (BD Biosciences, USA) (19). BM-EPC phenotypes were identified by
flow cytometry (FCM) analysis. PE-conjugated anti-CD133,
PECy7-conjugated anti-CD34, and PECy7-conjugated anti-VEGFR2
(eBioscience, USA) were applied. Appropriate
fluorochrome-conjugated isotype was used as control. The
CD133+CD34+ cells and
CD133+VEGFR2+ cells were classified as EPCs
(20,21). The animal research ethics committee
of our hospital approved the protocol. All mice were sourced from
the national genetically engineered mouse resources bank in
China.
Orthotropic HCC model
In order to trace BM cells (BMCs),
GFP+BM-C57BL/6 mice were established by transplanting
whole BMCs of GFP-transgenic mice (22) (Fig.
1A). Four weeks later, FCM analysis was used to confirm full BM
recovery. Orthotropic HCC mice were induced by an intrahepatic
injection of 1~2×105 H22 hepatoma cells (23) (gift from Dr Rutian Li, Institute of
Oncology, Nanjing University, China). The control group received
the same volume of PBS. To further confirm the role of EPCs on
tumor angiogenesis, GFP+EPCs (1–2×106)
cultured in vitro were injected into orthotropic HCC nude
mice via tail vein every 12 h for 3 days from day 7 after modeling
(24). At day 14, mice liver, lung,
kidney, pancreas, and stomach were examined to observe the
distribution of injected GFP+EPCs.
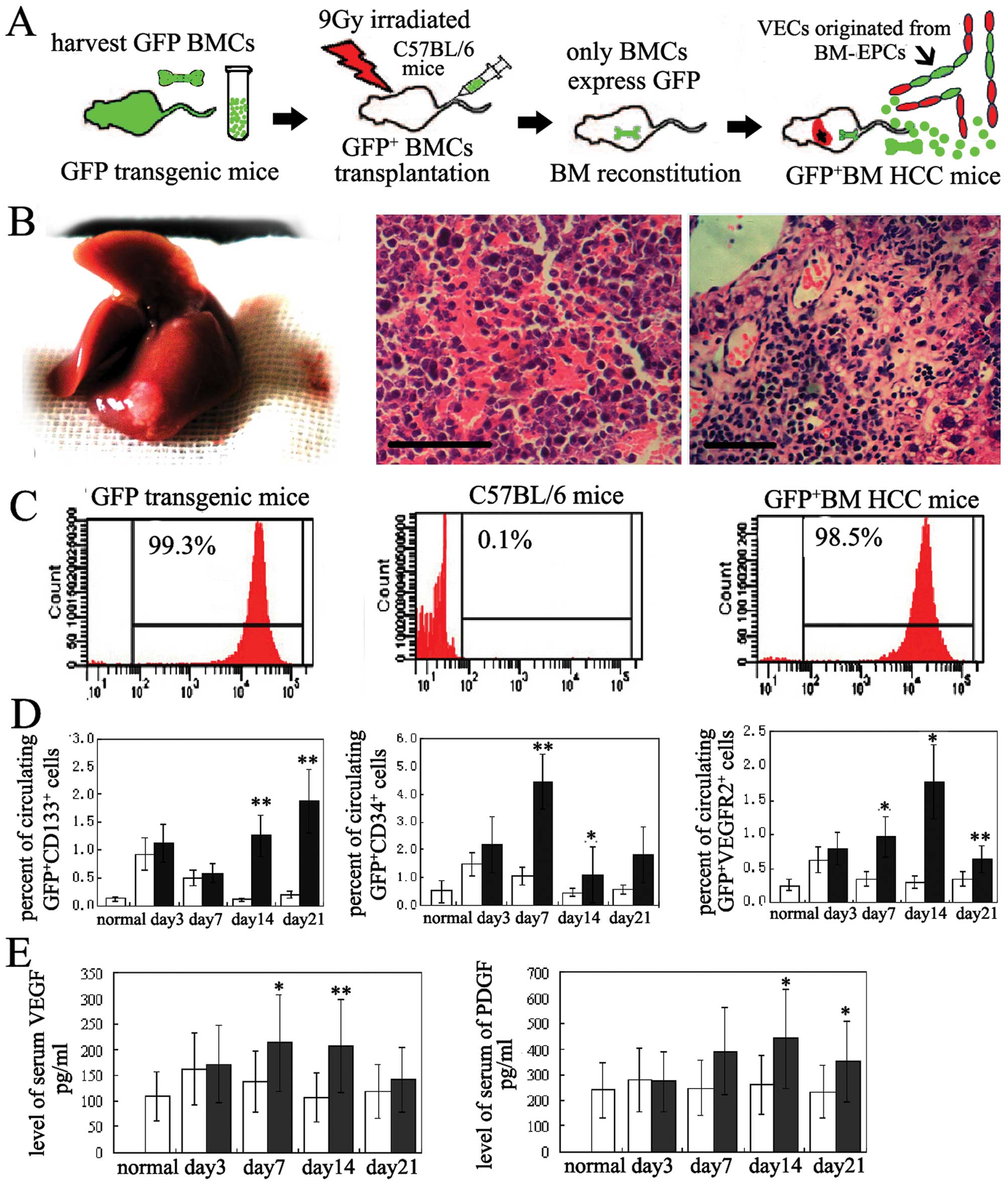 | Figure 1BM-EPCs were mobilzed into ciculation
in orthotropic HCC mice. (A) A schematic and flow chart shows how
GFP+-BM orthotropic HCC mice were established to assess
the contribution of BM-EPCs to HCC induced neovascularization. (B)
GFP+BM orthotopic HCC mice with classic pathological
characteristics. 1, The gross change of liver in
GFP+BM-orthotopic HCC mice, 2, Intratumor hemorrhage. 3,
Intratumor neovasculariztion, bar, 10 μm (paraffin, H&E, 2 μm).
(C) FCM analysis of GFP positive rate of BMCs in GFP transgenic
mice, normal mice, and GFP+BM HCC mice, respectively. (D
and E) Dynamic change of circulating BM-EPCs and serum VEGF, PDGF
in HCC mice vs sham-operation group. The number of circulating
GFP+CD133+, GFP+CD34+,
GFP+VEGFR2+ cells, and the levels of serum
VEGF, PDGF in HCC group increased significantly compared with those
in sham-operation group. *P<0.05;
**P<0.01; normal, normal mice; n=8. ■, HCC mice
group; □, sham-operation group. |
Flow cytometry
Peripheral blood (300 μl) was obtained on days 3, 7,
14, and 21 after building orthotropic HCC mice. Mononuclear cells
were separated and incubated for 30 min at 4°C using PE-conjugated
anti-CD133, PE-conjugated anti-CD34, and PE-conjugated anti-VEGFR2
(eBioscience, USA). Appropriate fluorochrome-conjugated isotypes
were used as controls.
ELISA
Serum vascular endothelial growth factor (VEGF) and
platelet-derived growth factor (PDGF) in HCC mice were tested using
an ELISA kit (Abcam) following the collection of sera at the same
time-points as stated above.
Immunofluorescence
By day 14 after establishing the
GFP+BM-orthotropic HCC model, 2 μm frozen sections of
mouse livers were cut and then incubated with primary antibodies
overnight at 4°C: rat anti-mouse CD31 (1:800, eBioscience, USA).
After washing with PBS, slices were incubated with secondary
antibodies: Alexa Fluor 488-conjugated rabbit anti-rat IgG antibody
(1:1000, Molecular Probes, USA). DAPI was used to dry the nucleus.
To quantify the proportion of BM-EPCs in vessels, on days 7 and 21
after modeling, anti-CD31 immunofluorescence stained sections were
analyzed by counting the number of GFP+ VECs and total
VECs in 15 random high-power fields (21). The quantitative contribution of
BM-EPCs to vessels was expressed as a percentage.
Immunohistochemistry
Sections of mouse livers (2 μm) were cut as
described above and then incubated with primary antibodies
overnight at 4°C: rabbit anti-mouse ICAM1 (1:50, Protein Tech
Group, USA), VCAM1 (1:200, Santa Cruz, USA), and VEGF (1:1000,
Abcam). A secondary antibody was labeled using horseradish
peroxidase. Positive reactions were visualized using
diaminobenzidine solution followed by counterstaining with
hematoxylin. Negative controls were obtained by substituting the
primary antibodies with PBS.
Western blot analysis
Proteins (100 μg) from each sample were subjected to
10% SDS-PAGE. Target protein levels were measured by immunoblotting
with the corresponding antibodies: rabbit anti-mouse actin (1:800,
Beyotime, China), rabbit anti-mouse ICAM1 (1:1000, Santa Cruz),
rabbit anti-mouse VEGF (1:1000) and goat anti-mouse VCAM1 (1:1000,
Abcam). They were then incubated for 30 min with secondary
antibodies. Bands were visualized using enhanced chemiluminescence
(Thermo, USA).
Real-time PCR
The levels of expression of BM-EPCs marker gene in
tumor tissues (TF) and tumor-free tissues (TT) were determined
using real-time PCR. RNA was extracted from the livers with TRIzol
(Invitrogen, USA). The sequences of primers were synthesized by
Takara (Takara, Japan): CD133 (5′-AAC GTG GTC CAG CCG AAT G-3′,
5′-TCC CAG GAT GGC GCA GAT A-3′), CD34 (5′-ACC CAC CGA GCC ATA TGC
TTA C-3′, 5′-GAT ACC CTG GGC CAA CCT CA-3′), VEGFR2, (5′ AGG GTG
GTC CAG CCG AAT G-3′, 5′TCC CAG GAT GGC GCA GAT A-3′) and β-actin
(5′-CAT CCG TAA AGA CCT CTA TGC CAA C-3′, 5′-ATG GAG CCA CCG ATC
CAC A-3′). PCR reactions were performed using a SYBR Premix Ex Taq
Kit (Takara, Japan) and the Stratagene QPCR System (Bioscience,
USA). Each sample was measured in duplicate. The relative levels of
expression were determined by DDCt normalized to endogenous
controls (β-actin).
Statistical analysis
Numeric data were presented as mean ± SD. t-tests
and ANOVA were used to analyze the normal distribution data. All
statistical analysis was performed using SPSS 15.0. Values of
P<0.05 were considered significant.
Results
The success of establishing the
GFP-labeled BM-orthotropic HCC model
The pathological examinations demonstrated that
GFP+BM-orthotropic HCC mice retained classic
clinicopathological characteristics (Fig. 1B). The high GFP+ rate of
circulating nucleated cells in the HCC model (>95%) (Fig. 1C) suggested that nearly all BMCs
express GFP stably, guaranteeing success in distinguishing BM-EPCs
derived VECs from pre-existed VECs (Fig. 1A).
BM-EPCs were mobilized into
circulation
In general BM-EPCs are believed to originate from
hematopoietic stem cells and express stem cell antigens CD133,
CD34, and VEGFR2 (5,6). While CD133, CD34 are expressed also in
certain tumor cells, such as hepatic cancer stem cells (25,26).
Thus, to a certain degree, the number of circulating
GFP+CD133+, GFP+CD34+
and GFP+VEGFR2+ cells can reflect the
mobilized degree of BM-EPCs. FCM analysis showed that by day 7
after modeling, the mean percentages of circulating
GFP+CD34+ and
GFP+VEGFR2+ cells in HCC mice were 0.97±0.29
and 4.46±3.47%, respectively, which were much higher than those in
control mice (0.35±0.14 and 1.04±1.32%). By day 14, the mean
percentages of circulating GFP+CD133+,
GFP+CD34+ and
GFP+VEGFR2+ cells in HCC mice were 1.26±0.70,
1.77±1.35 and 1.08±0.59%, respectively, which increased
dramatically compared with those in control mice (0.12±0.15,
0.31±0.24, and 0.46±0.34%). By day 21, mean percentages of
circulating GFP+CD133+,
GFP+VEGFR2+ cells (1.88±0.98 and 1.8±0.88%)
were also elevated relative to control mice (0.20±0.23 and
0.55±0.19%) (Fig. 1D). The ELISA
results indicated that compared with a transient increase in the
control, serum VEGF and PDGF maintained at a higher level were
obviously induced by HCC (Fig.
1E).
BM-EPCs were recruited and incorporated
into vascular endothelium
The distribution of BM-EPCs was assessed based on
the distribution of the specific surface antigens (CD133, CD34, and
VEGFR2), combined with GFP (the tracer of BMCs) in the serial
sections. Consecutive immunohistochemistry data showed that the
BM-EPCs surface markers CD133, CD34, VEGFR2, and GFP antigens were
mostly concentrated on TT compared to TF (Fig. 2A). Quantity PCR showed the levels of
CD133, CD34 and VEGFR2 genes in TT were also much higher than that
in TF (Fig. 2B). Based on
fluorescence microscopy, these positive cells were from BM as
evidenced by GFP in serial sections. At high magnifications, we
found that some VECs co-expressed CD133, VEGFR2 and GFP, which is
direct evidence that BM-EPCs melted into vascular endothelium by
differentiating into VECs in situ (Fig. 2C).
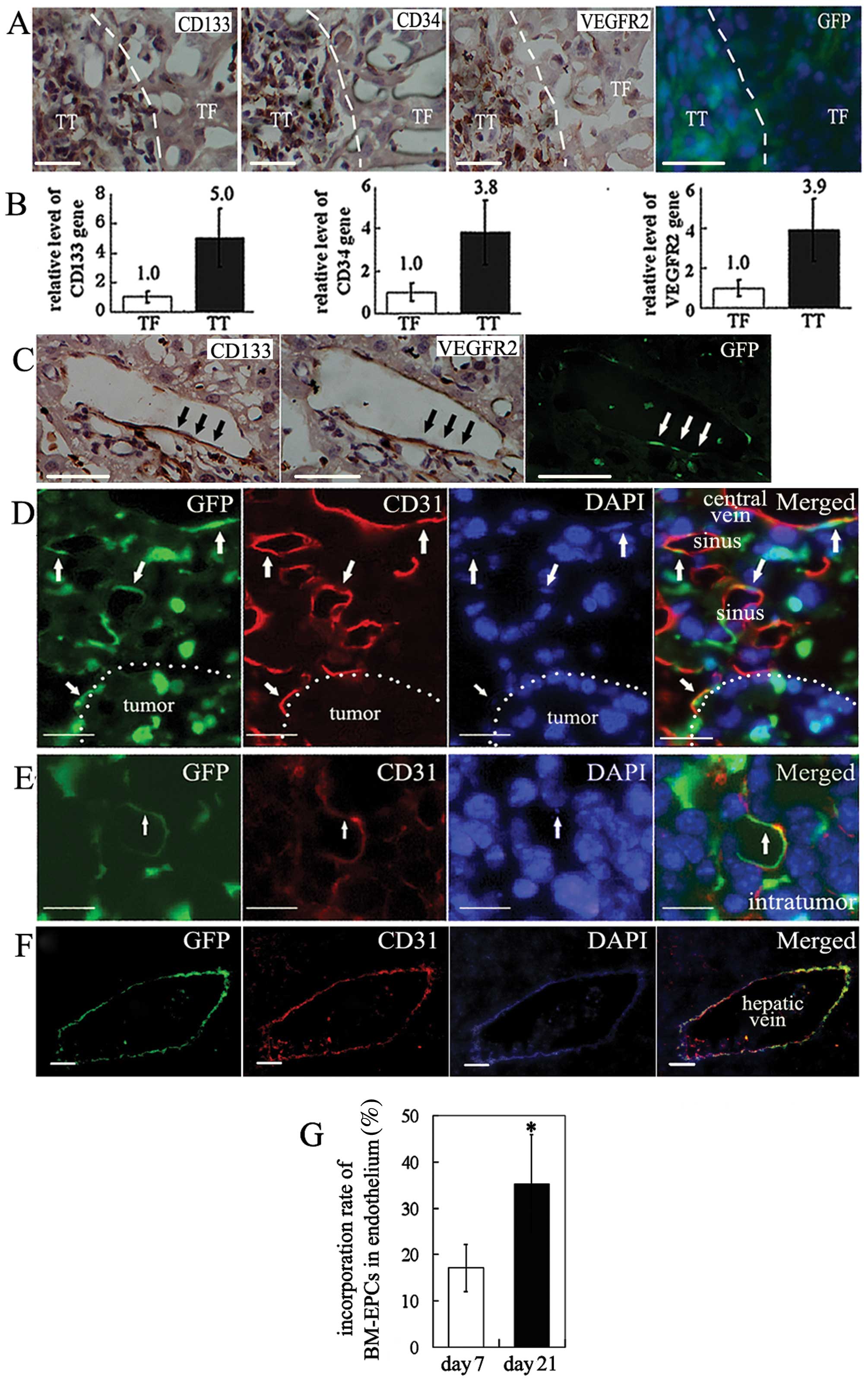 | Figure 2BM-EPCs were recruited and contribute
to neovascularization in HCC. (A) Distribution of EPC marker
phenotype CD133, CD34 and VEGFR2 antigens in TT and TF in three of
four consecutive sections of HCC. Dotted lines represent the
clusters of positively stained cells in immunohistochemistry
sections. In serial sections, the distribution of BMCs expressing
GFP and the expression of EPC marker antigen are consistent. The
BMCs labeled by GFP appear green with the nuclei stained blue by
DAPI. Positive staining appears brown (bar, 20 μm). (B) Relative
level of CD133, CD34 and VEGFR2 gene in TT and TF (n=7). (C)
CD133+, VEGFR2+ cells incorporated into
vascular endothelium of HCC liver. Arrows indicate
CD133+VEGFR2+ cells (bar, 15 μm). (D) BM-EPCs
infiltration to peritumoral hepatic sinus and central veins (bar,
10 μm). (E) BM-EPCs infiltration to intratumoral vessels (bar, 5
μm). (F) BM-EPCs infiltration to hepatic veins (bar, 40 μm). Mature
ECs expressing marker CD31 are stained red. BMCs tagged by GFP are
stained green. Nuclei stained with DAPI appear blue. Arrows
indicate CD31+GFP+VECs. (G) The incorporation
rate of EPCs in HCC blood vessels at day 21 versus that at day 7.
*P<0.01, n=15. |
BM-EPCs contribute to the formation of
different type vessels in HCC
It is very difficult to quantitatively analyze the
incorporation rate of classic endothelial progenitor cells into the
endothelium because the stem cells would be undetectable after the
loss of their surface markers during differentiation. Based on the
broad sensing of BM-EPCs (7,21), the
GFP combined with the EC-specific antigen CD31 (18) makes it easy to identify whether the
BM-EPCs incorporate into the vascular endothelium in the
GFP+BM HCC mice (Fig.
1A). In this study, we found that the
CD31+GFP+ cells existed not only in the
peripheral vessels but also in the intratumoral vessels and in
vessel walls of different sizes, such as sinusoids, central veins
and hepatic veins (Fig. 2D–F).
These CD31+GFP+ cells offer direct evidence
that the BM-EPCs contribute to the HCC-induced neovascularization.
Additionally, more BM-EPCs incorporated into the vascular
endothelium in the HCC tissue during tumor progression, as observed
by the higher rate of incorporation of BM-EPCs in all VECs, at day
21 (35.3±21.2%) than at day 7 (17.1±8.9%) (Fig. 2G). This indicated that BM-EPCs are
needed and play an important role in HCC angiogenesis.
Injected GFP+EPCs specifically
home to tumor and incorporate into blood vessels
In culture, GFP+EPCs gradually attached
onto fibronectin-coated plates, and adopted the shape of
cobblestones, a characteristic morphology of EPCs. Two weeks later,
these cells proliferated rapidly and could form tube-like
structures on matrigel, a functional feature of EPCs (Fig. 3B). FCM analysis revealed 85% of
cultured cells were CD133+VEGFR2+ cells or
CD133+CD34+ cells (Fig. 3C), which indicated success in
obtaining GFP+EPCs. At day 7 after GFP+EPC
injection, GFP positive cells were found scarcely distributed in
the organs examined, lungs, kidneys, pancreas, stomach, except for
within the liver tumor mass, where injected GFP+EPCs
were highly concentrated (Fig. 3D).
At high magnification, the GFP+CD31+ cells
were found in tumor vascular endothelium, which is direct,
intuitive evidence that BM-EPCs contributed to the formation of new
tumor vessels (Fig. 3E and F).
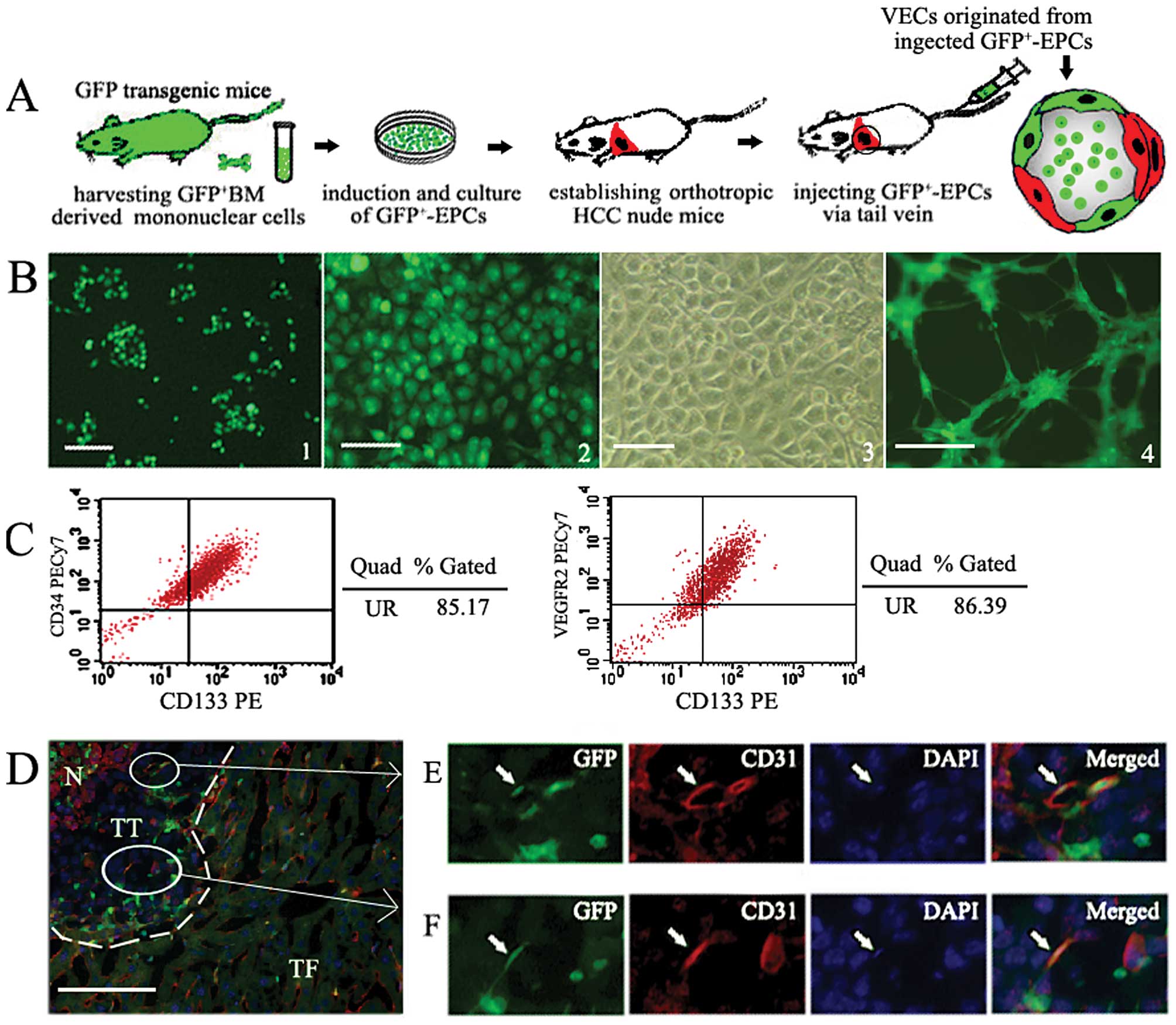 | Figure 3Injected GFP+ BM-EPCs
specifically home to tumor and incorporate into tumor vessels. (A)
A schematic and flow chart shows BM-EPCs cultured in vitro
were injected into orthotropic HCC nude mice in order to further
confirm that BM-EPCs were specifically recruited and incorporate
into endothelium of tumor vessels. (B) Isolation, culture and
identification of GFP+EPCs. 1,
GFP+-mononuclear cells obtained form GFP transgenic mice
cultured in the first week. 2, The photograph of second passage
GFP+EPCs cultured in EGM-2, observed under fluorescent
inverse microscopy. 3, A representative phase-contrast photograph
of second passage GFP+EPC, identified as a
well-circumscribed monolayer of cobblestone-appearing cells. 4,
GFP+EPCs form capillary-like structures within 24-h
culture in matrigel (bar, 200 μm). (C) Flow cytometric analysis of
GFP+EPCs cultured in vitro. (D) Injected
GFP+EPCs were recruited into HCC tissue. Dotted lines
show the tumor position. N, tumor necrosis area. (E and F) The
higher magnification of the area denoted by a rectangle in (D). At
high magnification, the GFP+CD31+ cells were
found in vascular endothelium in HCC tissue. Mature VECs expressing
marker CD31 are stained red. BMCs tagged by GFP are stained green.
Nuclei stained with DAPI appear blue. Arrows indicate VECs
co-expressing CD31 and GFP (frozen section, 2 μm, bar, 25 μm). |
Up-regulated expression of ICAM1, VCAM1,
and VEGF in HCC tissue
Immunohistochemistry and western blot results showed
that the expression of ICAM1, VCAM1, and VEGF in TT was much higher
than it was in TF (Fig. 4).
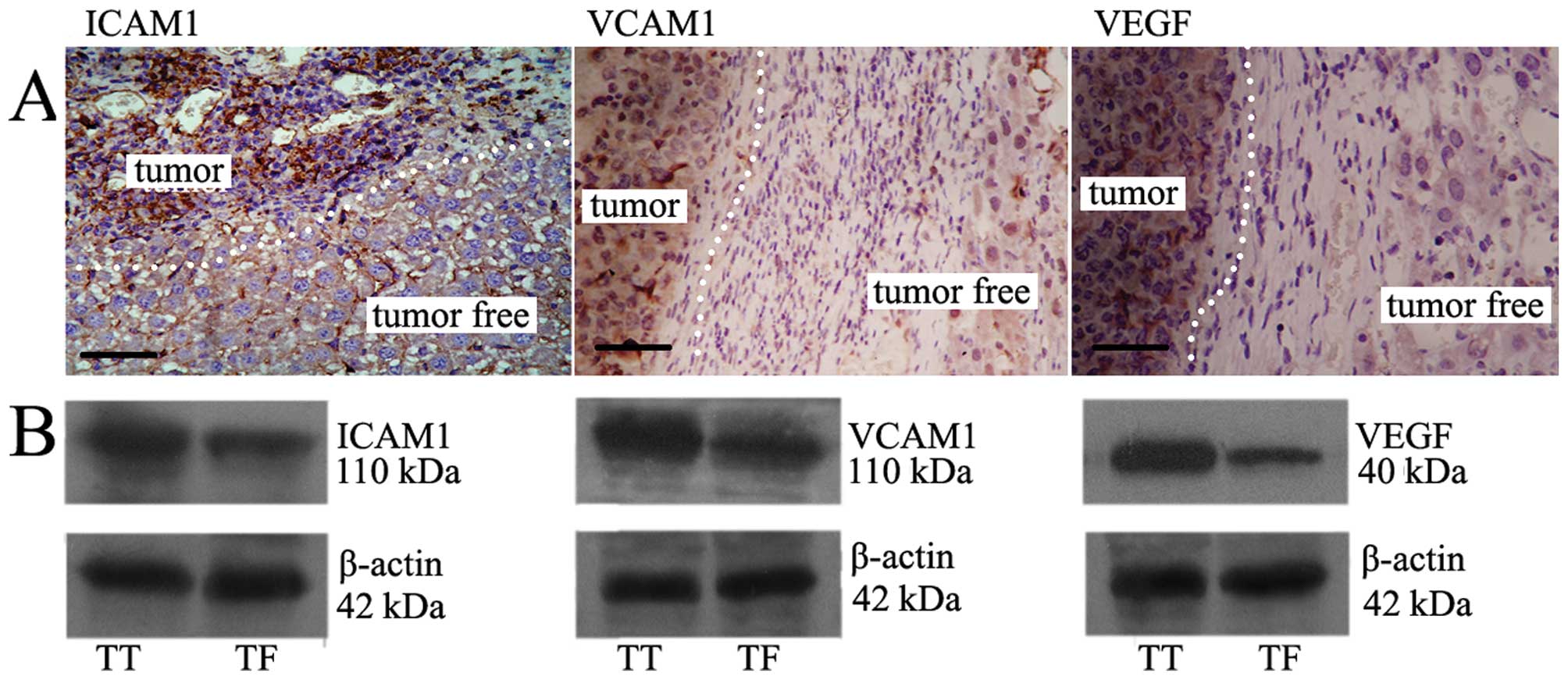 | Figure 4The expression of ICAM1, VCAM1, VEGF
in TT vs TF. (A) Immunohistochemistry shows the expression of
ICAM1, VCAM1, VEGF in TT was much more than that in TF. Dotted
lines show the tumor position. Brown particles, positive products
of ICAM1, VCAM1, VEGF protein, respectively. Bar, 20 μm (paraffin,
2 μm). (B) Western blotting demonstrated that expression of ICAM1,
VCAM1, VEGF in TT was higher than that in TF. The upper bands are
the protein, respectively; the lower are the internal standard. |
Discussion
Neovascularization is a crucial factor for HCC to
grow and metastasize. The role of BM-EPCs on tumor angiogenesis is
still debated (13,14). Even though the mobilization of
BM-EPCs has been demonstrated in HCC (15,16),
the precise role of BM-EPCs in HCC angiogenesis is not well
understood. Exploring the role of BM-EPCs on HCC
neovascularization, on the one hand, is to indentify whether
BM-EPCs contribute to the HCC-induced neovascularization in theory,
on the other hand may have implications for renovation of current
therapeutic strategy to patients with HCC in practice.
Previous clinical study showed that BM-EPCs mainly
concentrated on adjacent non-malignant liver tissue rather than HCC
tissue (17). Though the exact
distribution of mobilized BM-EPCs in target organ is still in
dispute (27,28), we have to accept the notion that
recruitment into target tissues is a prerequisite for the
effectiveness of BM-EPCs. Moreover, according to the theory that
further tumor growth is accompanied by the formation of tumor
vessels, HCC tissue should contain more BM-EPCs, in accordance with
HCC neovasculature development. The image data from serial sections
substantiate our hypothesis. The marker antigens CD133, CD34,
VEGFR2, and GFP that are the hallmark of BM-EPCs, were mainly
concentrated in HCC tissues (Fig.
2A), as measured by quantitative PCR (Fig. 2B). Moreover, also injected BM-EPCs
homed to TT with high specificity (Fig.
3D). To understand the homing mechanism, the expression of cell
adhesion molecules ICAM1, VCM1 and VEGF in TT and TF was examined.
We found increased serum VEGF, PDGF (Fig. 1E) and that VEGF, ICAM1 and VCAM1
were predominately expressed in TT (Fig. 4). ICAM-1 is considered the main
cellular adhesive molecule and VEGF is the most essential
mobilization factor (29,30). We reasonably inferred that with HCC
proliferation, the BM-EPCs were mobilized into circulation by BM
mobilization factors released from HCC and subsequently entered
into liver via the blood stream. The cells finally were arrested on
HCC tissues specifically due to the induction of a high local
content of adhesive molecules. The main reasons for the conflicting
conclusions may stem from the following: a) HCC mice lack a
background of chronic hepatitis and/or cirrhosis-precancerous
diseases with angiogenesis (31,32),
b) HCC in the present study is as a result of implanted tumors
rather than from spontaneous tumors, c) rapid tumor proliferation
results in augmented blood supply within a short time or d)
different types or stages of cancer may have led to the different
conclusions. Despite these speculations, our results may reflect to
some degree the natural link between HCC growth and BM-EPCs. Two
effects of recruited BM-EPCs on neovascularization were found.
First, BM-EPCs cells were incorporated into vascular endothelium
directly, and second, proangiogenic factors were secreted (33,34).
The current debate as to when during progressive
tumor growth BM-EPCs are mobilized and recruited into the tumor
continues. Some believed that the vasculogenesis of BM-EPCs is a
late event (35), whereas others
have taken the view that BM-EPCs were incorporated for a brief
period during early phases (<2 weeks) of tumor growth. The
longer-term tumor vascular endothelium was derived from nearby host
vessels (36). However, in our
study, the contribution of BM-EPCs to tumor vessels was detectable
by day 7, by which point the proportion of BM-EPCs in all VECs had
already reached 17%. Thus, the recruitment of mobilized BM-EPCs to
HCC tissues must arise before this time-point. Obviously, the
mobilization of BM-EPCs began much earlier. Moreover, the
proportion of BM-EPCs in vessels gradually increased to 35% by day
21 along with tumor growth (Fig.
2G). We believe that the contribution of BM-EPCs to
neovascularization is not the sole feature of advanced tumors but
rather an integral part of tumor development that becomes activated
during the ‘angiogenic switch’ and is required for early,
premalignant lesions to progress to frank tumors. In fact, the
premalignant changes of HCC such as cirrhosis or hepatitis virus
infection have tangible angiogenesis (31,32).
After tumor formation, neovascularization becomes the outstanding
characteristic of HCC and determines tumor progression and patient
prognosis.
Although Asahara et al (2) demonstrated the presence of circulating
BM-EPCs, the role of BM-EPCs in neovasculariztion has remained
controversial. Some even demonstrated that EPCs were not required
for tumor growth at all (14,37).
While in HCC, BM-EPCs were specifically recruited into TT and truly
incorporated into peritumoral and intratumoral vessels, involving
different types of vasculatures such as sinus, central veins,
microvasculature, and large vessels (Fig. 2D–F). Moreover, the BM-EPCs injected
via vein were also specifically rested on TT and contributed to the
formation of tumor new vessels (Fig.
3D). The BM-EPCs may have also maintained HCC
neovascularization, because their proportion in vessels increased
gradually along tumor growth (Fig.
2G). In HCC mice, we found that BM-EPCs mainly integrated into
the network of pre-existing vessels rather than forming an entirely
new vascular endothelium. Increased vascular caliber was usually
detectable earlier than the morphological signs of tumors (38). We infer that there might exist two
patterns of how BM-EPCs contribute to tumor neovascularization: one
is dilatation in diameter and the other is an extension in length.
In large vessels, mobilized BM-EPCs supplied endothelial cells in
order to meet the demand to enlarge the inner diameter to increase
blood flow. In microvasculature, BM-EPCs can make capillaries
longer and wider. Obviously, with the deposition of matrices,
contribution of pericytes (39),
and dilatation in diameter, these capillaries would soon become
new, larger vessels that carry more blood to support tumor rapid
proliferation.
The dependence of tumor growth on angiogenesis has
been generally acknowledged, however, the actual effect of
antivascular treatment including embolization to patients with HCC
remains limited. A thorny problem is the rapid development of new
collateral circulation (9). It has
been reported that treated tumors dramatically increase in serum
VEGF (40,41) and circulating BM-EPCs (42,43).
Obviously, the further aggravation of tumor metabolic acidosis,
hypoxia, and necrosis that result from ischemia would in turn lead
to more soluble factors being released into blood. Subsequently,
more BM-EPCs would be mobilized. Therefore, even though the tumor
vasculature could be damaged, BM-EPCs provide an alternative source
of VECs that contribute to formation of new vessels in order to
compensate for this blood supply loss. BM-EPCs seem to play an
important part in the compensatory cellular and molecular
mechanisms that inhibit the efficiency of present treatment
strategies. Thus, we should seriously consider inhibiting the
mobilization and recruitment of BM-EPCs as a therapeutic target in
HCC treatment.
In concusion, the presented data indicated that
BM-EPCs were recruited specifically and contributed to
neovascularization in HCC. This process began at an early stage,
and may continue throughout the whole process of HCC growth.
Moreover, the injected BM-EPCs homed to tumors with significantly
higher specificity and also contribute to the formation of tumor
vessels. These findings suggest that BM-EPCs are needed and play an
important role in angiogenesis in HCC. Thus BM-EPCs might serve as
biomarkers for predicting the progression or recurrence of HCC, and
blocking the BM-EPCs mediated angiogenesis may help improve the
efficacy of current therapies for HCC patients.
Acknowledgements
We thank Fan Xiangshan, Chen Jun, Xie Lili for
excellent technical assistance and Cui Fengming for help in
carrying out the animal experiments. This study was supported by
National Natural Science Fund (30972904) and 135 Key Clinical
Center of Institutes of Health in Jiangsu Province (SK200215).
References
|
1
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asahara T, Masuda H, Takahashi T, et al:
Bone marrow origin of endothelial progenitor cells responsible for
postnatal vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–218. 1999. View Article : Google Scholar
|
|
3
|
Mund JA and Case J: The role of
circulating endothelial progenitor cells in tumor angiogenesis.
Curr Stem Cell Res Ther. 6:115–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
George AL, Bangalore-Prakash P, Rajoria S,
et al: Endothelial progenitor cell biology in disease and tissue
regeneration. J Hematol Oncol. 24:242011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoder MC: Defining human endothelial
progenitor cells. J Thromb Haemost. 7:49–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doyle B, Metharom P and Caplice NM:
Endothelial progenitor cells. Endothelium. 13:403–410. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chao H and Hirschi KK: Hemato-vascular
origins of endothelial progenitor cells? Microvasc Res. 79:169–173.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu AX, Duda DG, Sahani DV, et al: HCC and
angiogenesis: possible targets and future directions. Nat Rev Clin
Oncol. 8:292–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HC, Chung JW, Lee W, et al:
Recognizing extrahepatic collateral vessels that supply
hepatocellular carcinoma to avoid complications of transcatheter
arterial chemoembolization. Radiographics. 25:S25–S39. 2005.
View Article : Google Scholar
|
|
10
|
Lyden D, Hattori K, Dias S, et al:
Impaired recruitment of bone-marrow-derived endothelial and
hematopoietic precursor cells blocks tumor angiogenesis and growth.
Nat Med. 7:1194–1201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mellick AS, Plummer PN, Nolan DJ, et al:
Using the transcription factor inhibitor of DNA binding 1 to
selectively target endothelial progenitor cells offers novel
strategies to inhibit tumor angiogenesis and growth. Cancer Res.
70:7273–7282. 2010. View Article : Google Scholar
|
|
12
|
Rigolin GM, Maffei R, Rizzotto L, et al:
Circulating endothelial cells in patients with chronic lymphocytic
leukemia: clinical-prognostic and biologic significance. Cancer.
116:1926–1937. 2010. View Article : Google Scholar
|
|
13
|
Hagensen MK, Raarup MK, Mortensen MB, et
al: Circulating endothelial progenitor cells do not contribute to
regeneration of endothelium after murine arterial injury.
Cardiovasc Res. 93:223–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wickersheim A, Kerber M, de Miguel LS, et
al: Endothelial progenitor cells do not contribute to tumor
endothelium in primary and metastatic tumors. Int J Cancer.
125:1771–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sieghart W, Fellner S, Reiberger T, et al:
Differential role of circulating endothelial progenitor cells in
cirrhotic patients with or without hepatocellular carcinoma. Dig
Liver Dis. 41:902–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ho JW, Pang RW, Lau C, et al: Significance
of circulating endothelial progenitor cells in hepatocellular
carcinoma. Hepatology. 44:836–843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu D, Sun X, Qiu Y, et al: Identification
and clinical significance of mobilized endothelial progenitor cells
in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer
Res. 13:3814–3824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sangidorj O, Yang SH, Jang HR, et al: Bone
marrow-derived endothelial progenitor cells confer renal protection
in a murine chronic renal failure model. Am J Physiol Renal
Physiol. 299:F325–F335. 2010. View Article : Google Scholar
|
|
19
|
Hu J, Dong A, Fernandez-Ruiz V, et al:
Blockade of Wnt signaling inhibits angiogenesis and tumor growth in
hepatocellular carcinoma. Cancer Res. 69:6951–6959. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peichev M, Naiyer AJ, Pereira D, et al:
Expression of VEGFR-2 and AC133 by circulating human
CD34+ cells identifies a population of functional
endothelial precursors. Blood. 95:952–958. 2000.PubMed/NCBI
|
|
21
|
Massa M, Rosti V, Ramajoli I, et al:
Circulating CD34+, CD133+, and vascular
endothelial growth factor receptor 2-positive endothelial
progenitor cells in myelofibrosis with myeloid metaplasia. J Clin
Oncol. 23:5688–5695. 2005.
|
|
22
|
Suriano R, Chaudhuri D, Johnson RS, et al:
17Beta-estradiol mobilizes bone marrow-derived endothelial
progenitor cells to tumors. Cancer Res. 68:6038–6042. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmitz V, Tirado-Ledo L, Tiemann K, et
al: Establishment of an orthotopic tumor model for hepatocellular
carcinoma and non-invasive in vivo tumour imaging by high
resolution ultrasound in mice. J Hepatol. 40:787–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn JB, Rha SY, Shin SJ, et al:
Circulating endothelial progenitor cells (EPC) for tumor
vasculogenesis in gastric cancer patients. Cancer Lett.
288:124–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding W, Mouzaki M, You H, et al:
CD133+ liver cancer stem cells from methionine adenosyl
transferase 1A-deficient mice demonstrate resistance to
transforming growth factor (TGF)-beta-induced apoptosis.
Hepatology. 49:1277–1286. 2009.
|
|
26
|
Zhu Z, Hao X, Yan M, et al: Cancer
stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular
carcinoma. Int J Cancer. 126:2067–2078. 2010.PubMed/NCBI
|
|
27
|
Arbab AS, Pandit SD, Anderson SA, et al:
Magnetic resonance imaging and confocal microscopy studies of
magnetically labeled endothelial progenitor cells trafficking to
sites of tumor angiogenesis. Stem Cells. 24:671–687. 2006.
View Article : Google Scholar
|
|
28
|
Shirakawa K, Furuhata S, Watanabe I, et
al: Induction of vasculogenesis in breast cancer models. Br J
Cancer. 87:1454–1461. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Croll SD, Ransohoff RM, Cai N, et al:
VEGF-mediated inflammation precedes angiogenesis in adult brain.
Exp Neurol. 187:388–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gehling UM, Ergun S, Schumacher U, et al:
In vitro differentiation of endothelial cells from AC133-positive
progenitor cells. Blood. 95:3106–3112. 2000.PubMed/NCBI
|
|
31
|
Messerini L, Novelli L and Comin CE:
Microvessel density and clinicopathological characteristics in
hepatitis C virus and hepatitis B virus related hepatocellular
carcinoma. J Clin Pathol. 57:867–871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pinzani M and Vizzutti F: Fibrosis and
cirrhosis reversibility: clinical features and implications. Clin
Liver Dis. 12:901–913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moon MH, Kim SY, Kim YJ, et al: Human
adipose tissue-derived mesenchymal stem cells improve postnatal
neovascularization in a mouse model of hindlimb ischemia. Cell
Physiol Biochem. 17:279–290. 2006. View Article : Google Scholar
|
|
34
|
Thomas J, O’Neill IV, Brian R, et al:
Mobilization of bone marrow-derived cells enhances the angiogenic
response to hypoxia without transdifferentiation into endothelial
cells. Circ Res. 97:1027–1035. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hämmerling GJ and Ganss R: Vascular
integration of endothelial progenitors during multistep tumor
progression. Cell Cycle. 5:509–511. 2006.PubMed/NCBI
|
|
36
|
Nolan DJ, Ciarrocchi A, Mellick AS, et al:
Bone marrow-derived endothelial progenitor cells are a major
determinant of nascent tumor neovascularization. Genes Dev.
12:1546–1558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He Y, Rajantie I, Ilmonen M, et al:
Preexisting lymphatic endothelium but not endothelial progenitor
cells are essential for tumor lymphangiogenesis and lymphatic
metastasis. Cancer Res. 64:3737–3740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ryschich E, Schmidt J, Hammerling GJ, et
al: Transformation of the microvascular system during multistage
tumorigenesis. Int J Cancer. 97:719–725. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dar A, Domev H, Ben-Yosef O, et al:
Multipotent vasculogenic pericytes from human pluripotent stem
cells promote recovery of murine ischemic limb. Circulation.
125:87–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Poon RT, Lau C, Yu WC, et al: High serum
levels of vascular endothelial growth factor predict poor response
to transarterial chemoembolization in hepatocellular carcinoma: a
prospective study. Oncol Rep. 11:1077–1084. 2004.
|
|
41
|
Wang B, Xu H, Gao ZQ, et al: Increased
expression of vascular endothelial growth factor in hepatocellular
carcinoma after transcatheter arterial chemoembolization. Acta
Radiol. 49:523–529. 2008. View Article : Google Scholar
|
|
42
|
Shaked Y, Henke E, Roodhart JM, et al:
Rapid chemotherapy-induced acute endothelial progenitor cell
mobilization: implications for antiangiogenic drugs as
chemosensitizing agents. Cancer Cell. 14:263–273. 2008. View Article : Google Scholar
|
|
43
|
Pircher A, Kähler CM, Skvortsov S, et al:
Increased numbers of endothelial progenitor cells in peripheral
blood and tumor specimens in non-small cell lung cancer: a
methodological challenge and an ongoing debate on the clinical
relevance. Oncol Rep. 19:345–352. 2008.
|


















