Introduction
The acquisition of resistance to apoptosis and the
ability to escape from immunological surveillance are the hallmarks
of tumor cells (1,2). The inhibitors of apoptosis proteins
(IAPs) inhibit apoptosis by interacting with the pro-apoptotic
caspases via their baculoviral IAP repeat (BIR) (3). Previous studies indicated that
downregulating the expression of IAPs is beneficial to the
treatment of cancer and may be a necessary component of the
antitumor activities of conventional treatments such as radiation
and chemotherapy (4–6). Livin is a novel member of the IAP
family; it has two isoforms that are termed livin α (298-amino acid
residues) and livin β (280- amino acid residues). Each isoform
contains a single BIR domain and a RING finger domain (7). In contrast to other members of the IAP
family, such as cIAP-1, cIAP-2, XIAP and NAIP, which are expressed
at low levels in normal adult tissues, livin is not expressed in
normal adult tissues but is overexpressed in primary lung cancer
and other malignant tumor cells (8,9). Livin
expression levels correlate to some degree with the stage and
pathological type of the cancer, and livin expression can induce
tumor cells to become resistant to antineoplastic drugs and are
characteristic of a cancer with a poor prognosis (9–12).
Evidence suggests that interfering with either the expression of
the livin gene or the function of the livin protein could provide a
potential therapeutic avenue to induce apoptosis in tumor cells and
to significantly improve antitumor responses (13–15).
Since anti-livin autoantibodies have been identified
in the serum of the majority of cancer patients, immunologic
tolerance to livin is thought to be very weak (16,17),
making livin a promising candidate target for immunotherapy.
Dendritic cells (DCs) are classic antigen-presenting cells (APCs)
and can efficiently take up and present tumor antigens as well as
provide the co-stimulatory molecules required to activate naive T
lymphocytes (18,19). In addition to antigen modulation and
immune evasion (20), cancer cells
may cause DCs to downregulate their expression of MHC class I
molecules when the two cell types are co-cultured (21). This reduces the ability of the DCs
to activate CD8+ T cells and leads to immune escape. In
the present study, we showed that DCs that have been modified to
express the livin α gene could provide stable presentation of livin
epitopes and could induce T-cell activation. We developed a
cell-transfer therapy based on these observations in which
pre-sensitized tumor-specific effector cells were expanded in
vitro and transplanted into autologous hosts (22,23).
Umbilical cord blood (UCB) is a rich source of hematopoietic stem
cells (24) and is resistant to
replicative senescence and immunosenescence (25). HLA-mismatched cord blood-derived
transplants are associated with a lower incidence and severity of
acute or chronic graft-versus-host disease (GVHD) (26). Thus, UCB cells are an ideal source
of cells for allogeneic antitumor transplants (27). Due to the biological activity of
livin, we used a replication-defective recombinant adenovirus (rAd)
vector encoding the livin α gene to infect UCB-derived DCs to
generate an antigen-specific vaccine that can be applied to cancer
immunotherapy (28–30).
Hariu et al demonstrated that stimulating the
peripheral blood lymphocytes (PBMCs) of HLA-A24+ lung
cancer patients in vitro with livin peptides in the context
of matched HLA molecules induced peptide-specific cytotoxic T
lymphocytes (CTLs) that showed cytotoxicity against
livin-expressing lung cancer cell lines in an HLA-A24-restricted
manner (11). However, the previous
study could not be universally applied in oncotherapy in
individuals with various HLA subtypes as the livin-expressing DC
vaccine generated responses that were restricted to the HLA-A24 MHC
subtype. We previously confirmed that human livin α gene-modified
DC vaccines can induce tumor-specific CTLs and mount significant
anti-Lewis lung cancer responses in mice (28). In addition to CTLs, there are
multiple cell types in the human immune system that can mediate
non-specific antitumor effects, such as macrophages, NK and LAK
cells (31,32). In this study, we expanded on
previous work to show that livin α gene-modified DC vaccines
derived from UCB can activate multiple innate effector cell types
in the human immune system in addition to CTLs to exert antitumor
effects.
Materials and methods
Cell lines and culture media
The lung cancer cell lines A549 and H460
(HLA-A2+/A24+) were established in the
Laboratory Center of the Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology, Wuhan, China. HBE
cells (HLA-A2+/A24−) were kindly provided by
Dr Shanshan Song (Department of Pathophysiology, Tongji Medical
College, Huazhong University of Science and Technology). HEK-293
cells were purchased from AGTC Gene Technology Company Ltd.,
Beijing, China. A549 and H460 cells were maintained in RPMI-1640
while HBE and HEK cells were maintained in DMEM. Both culture media
were supplemented with 10% heat-inactivated fetal bovine serum
(HyClone; Thermo Scientific, Waltham, MA, USA), 2 mM L-glutamine, 1
mM sodium pyruvate, 100 U/ml penicillin and 100 μg/ml streptomycin
(all from Sigma, St. Louis, MO, USA) in a humidified incubator at
37°C with 5% CO2.
Construction of recombinant adenoviral
vector encoding the livin α gene
Replication-defective type-5 adenoviruses (rAd5) in
which the E1 and E3 region were deleted and that encoded enhanced
green fluorescent protein (EGFP) were used as vectors in this
study. Our lab used the shuttle plasmid pDC316-EGFP-cmv-livin α,
which was synthesized by Dr Junping Xie. The helper plasmid
pBHGlox_E1,3Cre was purchased from AGTC Gene Technology Company
Ltd. HEK-293 cells were co-transfected with the shuttle plasmid and
the helper plasmid to produce the recombinant adenovirus.
After three rounds of amplification, the crude
lysates were purified by density gradient centrifugation and
dialysis. The titer of the rAd-livin α was calculated using the 50%
tissue culture infective dose (TCID50) assay.
PCR
Polymerase chain reaction (PCR) was used to evaluate
whether the rAd-livin α vector had been generated successfully. We
utilized an empty rAd vector as a negative control and
pDC316-EGFP-cmv-livin α as a positive control. Briefly,
amplification was performed in 25 μl of PCR mixture containing 1 μl
of the template, 0.5 μl of primers (50 μM) and 0.25 μl of Taq DNA
polymerase. The PCR mixture was initially incubated at 94°C for 4
min, followed by 30 cycles of denaturation at 94°C for 30 sec,
annealing at 56°C for 40 sec and extension at 70°C for 50 sec,
followed by a final extension at 72°C for 10 min. PCR products were
analyzed by electrophoresis on a 1% agarose gel.
The forward primer 5′-GCCATGGGGCCTAAAGACAGTG-3′ and
the reverse primer 5′-CTGCTAGGACAGGAAGGTGCG-3′ were used according
to the manufacturer’s protocol for the specific detection of livin
α. The expected size of PCR product for livin α is 897 bp.
Isolation and induction of UCB-derived
DCs in vitro
Heparin anti-coagulated (20 U/ml)
HLA-A2+/A24− and
HLA-A2+/A24+ UCB samples were isolated
following cesarean sections with the approval of the ethics
committee of the Tongji Medical School, Huazhong University of
Science and Technology. All patients provided informed consent
prior to the surgery, which was performed at the Obstetrics
Department, Wuhan Union Hospital, China. The HLA class was
determined using polymerase chain reaction with sequence-specific
primers (PCR-SSP).
We generated UCB-derived DCs in vitro using a
combination of previously described protocols (33–36).
Briefly, mononuclear cells (MNC) were obtained using a
Ficoll-Hypaque (TBD Biotechnology developing center, relative
density 1.077 at 20°C) density gradient. The precursor DCs were
isolated using anti-CD34 microbeads (Miltenyi Biotec, Bergisch
Gladbach, Germany). These cells were cultured in RPMI-1640 medium
supplemented with 20% FBS. The culture media were also supplemented
with 50 ng/ml of GM-CSF (PeproTech Inc., Rocky Hill, NJ, USA), 50
ng/ml of SCF (PeproTech) and 20 ng/ml of IL-4 (PeproTech) to induce
the development of UCB-derived DCs. Half of the culture medium was
replaced with fresh medium containing the appropriate concentration
of cytokines every other day.
The cultured cells were then separated into adherent
cells and non-adherent fractions and the non-adherent cells were
supplemented with 100 U/ml of IL-2 (Peprotech) to induce the
development of effector cells, which were predominantly CTLs.
Preparation of the stimuli and DC
vaccines
On Day 5, DCs were harvested and washed with
RPMI-1640 medium, then pulsed with a variety of tumor-associated
antigens (TAAs), either rAd-livin α or rAd-c at a multiplicity of
infection (MOI) of 200, and 30 μg/106 cells of A549 and
H460 lysates that had undergone 3 cycles of freezing and thawing
between −80 and 37°C and had been passed through a 0.22-μm filter.
The cultures were incubated for 2 h in 0.3 ml of serum-free medium
supplemented with GM-CSF and IL-4 but not SCF, then supplemented
with complete medium. On Day 7, 50 ng/ml GM-CSF, 20 ng/ml IL-4, 10
ng/ml TNF-α (all PeproTech) and 1 μg/ml PGE (Sigma) were added to
the cultures to induce DC maturation and the cells were cultured
for an additional 2 days.
On Day 9, IL-2-induced cells were co-cultured with
mature DCs at a ratio of 10:1 in culture medium supplemented with
IL-2 and IL-12 (PeproTech). The cells were cultured together for an
additional 48 h before the cytotoxic activity of the effector cells
was measured.
Evaluation of the infection efficiency of
restructured adenoviruses in DCs and cell viability
DCs infected rAd at MOI 50, 100, 200 and 400. As the
viral vector was modified to encode EGFP, the efficiency of
infection can be correlated to the proportion of EGFP-positive
cells detected by flow cytometry. Viable cells cannot be stained
with trypan blue and trypan blue staining was used to evaluate cell
viability.
Differentiation and maturation of DC
cells measured by flow cytometry
The expression of DC cell surface molecules (CD1a,
CD80, CD86 and HLA-DR) was analyzed by flow cytometry. The DC cells
were divided into precursor DC cells, mature DC cells, and DC cells
infected with either rAd-control or rAd-livin α. Cells were
harvested and incubated with monoclonal antibodies (anti-human CD86
Alexa Fluor 488, anti-human CD80 PE-Cy5, anti-human CD1a PE and
anti-human HLA-DR APC) (eBioscience Inc., San Diego, CA, USA) at
4°C for 30 min. The lysates were centrifuged at 1,000 rpm for 6
min, the supernatant was then discarded and the cells were washed
with PBS. The cells were centrifuged again at 1,000 rpm for 6 min,
then fixed in 4% paraformaldehyde and analyzed by flow
cytometry.
Western blot analysis
We used western blots to analyze livin expression in
H460, HBE and A549 cells, in rAd-c-infected and rAd-livin
α-infected DCs and in MNCs. Amino acids 264–280 of the short
isoform or amino acids 281–298 of the long isoform of human livin
(Abcam, Cambridge, MA, USA) were used as positive controls. Cells
were lysed in lysis buffer (12.5 mM HEPES, 200 mM KCl, 5 mM EDTA,
50 mM NaF, 0.5% NP-40, 0.5 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml
aprotinin and 2 mM Na3VO4). The protein
content of the supernatant was determined using the Bio-Rad Protein
Assay kit. Whole cell extract (50 μg) was denatured in SDS sample
buffer and loaded onto a 12% SDS-PAGE gel.
Following electrophoresis, the proteins were
transferred to PVDF membranes. The membranes were blocked with 5%
non-fat milk in Tween-20 in TBS (TBST, 15 mM Tris-HCl, pH 7.5, 200
mM NaCl and 0.1% Tween-20) for 2 h at room temperature.
Subsequently, the membranes were incubated overnight with a rabbit
anti-livin polyclonal antibody (Abcam) at 4°C. The primary
antibodies were detected using a horse-radish peroxidase
(HRP)-conjugated secondary antibody (Santa Cruz Biotechnology,
Santa Cruz, CA, USA). The signal was detected by treating the
membrane with ECL reagent and exposing it to X-ray film. Protein
loading was assessed by GAPDH.
IFN-γ ELISPOT assays
An IFN-γ ELISPOT assay was performed to detect
livin-specific effector T cells (37). According to the manufacturer’s
instructions, UCB-derived effector cells were resuspended in
Lympho-Spot™ serum free medium at a final concentration of
1.0×106 cells/ml.
Lympho-Spot serum free medium (200 μl/well) was
added to 48-well PVDF plates (DKW, China) that had been pre-coated
with anti-IFN-γ antibody. After 10 min, the serum free medium was
replaced and the effector cells were moved to suspension cultures
in plates (1.5×105 cells/well). The cells were then
stimulated with a final concentration of 10 μg/ml of either a livin
polypeptide (KWFPSCQFLL) or a livin-unrelated polypeptide
(RYLRDQQLLGI), both of which have previously been demonstrated to
be restricted to HLA-A24 (11).
Lympho-Spot serum free medium alone was used as a negative control
and PHA (plant hemagglutinin, 2 μg/ml) was used as a positive
control. The PVDF plates were placed in a humidified incubator at
37°C for 20 h. The media were then removed from the plates and the
cells were lysed with cold ddH2O. After washing the
plates, each well was incubated first with biotinylated anti-IFN-γ
antibody (100 μl/well) and then with streptavidin-HRP (100 μl/well)
at 37°C for 1 h. Then, the PVDF plates were treated with AEC (100
μl/well) at room temperature in the dark for 25 min. After washing
with ddH2O, the number of spot-forming cells (SFC) was
analyzed using a Biosys-Bioreader. The results are expressed as
SFC/105 effector cells = (SFC number/well) ×
105/(number of cells/well).
Cytotoxicity assay
The cytotoxic activity of the effector cells was
measured by flow cytometry as previously described (38–41).
Briefly, the target cells were resuspended in PBS supplemented with
0.1% BSA at a final concentration of 2×106 cells/ml. The
target cells were incubated in 1 μM CFSE at 37°C for 10 min. Then,
5 volumes of ice-cold culture media were added to quench the
staining. The CFSE-stained target cells were centrifuged,
resuspended and mixed with the effector cells at effector/target
ratios of 5:1, 10:1 and 20:1. The cells were co-cultured in a
humidified incubator at 37°C with 5% CO2.
After 4 h, the cells were resuspended, incubated
with 5 μl of Annexin V-APC and 5 μl of PI and examined by flow
cytometry. Annexin V staining was assessed on CFSE-positive cells
to measure target cell death. Annexin V−/PI+
cells were defined as necrotic and were excluded from the analysis.
The percentage of specific lysis was calculated as follows: (%
experimental lysis - % basal lysis)/(% maximum lysis - % basal
lysis) × 100.
Statistical analysis
Values are expressed as the means ± SE. Statistical
analysis was performed using the Student’s t-test or one way
analysis of variance (ANOVA). Values of P≤0.05 were considered to
indicate statistically significant differences.
Results
Verification of adenovirus encoding the
livin α gene
Seven days after co-transfecting HEK-293 cells
(Fig. 1A) with the shuttle plasmid
pDC316-EGFP-cmv-livin α and the backbone plasmid pBHGlox_E1,3Cre,
the cells acquired an appearance typical of cytopathic effects
(CPE) and viral plaques were visible under microscopic examination
(Fig. 1B). Moreover, the cells
appeared beaded and to have undergone botryoid changes, and the
majority of the cells had detached from the surface of the plate.
The expression of green fluorescent protein was visualized using a
fluorescent microscope (Fig. 1C),
and the results indicated that the recombination adenovirus had
successfully infected the target cells and that the adenovirus
encoded genes were being expressed.
 | Figure 1Construction and identification of
rAd-livin α. (A) HEK-293 cells before co-transfection
(magnification, ×200). (B) HEK-293 cells show typical CPE after
co-transfection, (magnification, ×200). (C) GFP expression was
detected after the infection of HEK-293 cells with rAd-livin α
(magnification, ×200). (D) Identification of rAd-livin α by PCR.
Lane M, DNA ladder marker; lane 1, empty adenovirus as a negative
control; lane 2, pDC316-EGFP-cmv-livin α as a positive control;
lane 3, rAd-livin α. (E) Western blot analysis of livin protein
expression in NMCs, rAd-c-infected DCs, rAd-livin α-infected DCs,
A549, H460 and HBE cells. |
The target gene detected by PCR was consistent with
the expected gene livin α (897 bp) (Fig. 1D). The titer of the rAd-livin α was
2×109 TCID50/ml, and the titer of the
rAd-control was 2×1011 TCID50/ml, as
determined by the TCID50 method.
Livin is expressed in tumor cell lines
and in DC cells infected by rAd-livin α, but not in HBE cell lines
or mononuclear cells
We determined the expression of endogenous protein
livin in A549, H460, HBE, rAd-c DC, rAd-livin α DC and MNC by
western blot analysis. We confirmed that the livin protein is
expressed in A549 and H460 cells and rAd-livin α-infected DCs, but
not in HBE cells, rAd-c-infected DCs or MNCs (Fig. 1E).
Optimal MOI for recombinant adenovirus
infection of DC cells
DC cells were infected with rAd-livin α after 7 days
of culture. The expression levels of EGFP were determined by flow
cytometry after 48 h. We determined that the optimal MOI value was
200, at which 88% of the cells expressed EGFP and 69% of the cells
were viable.
Expression of DC surface molecules is
increased when DCs mature or are infected with adenovirus
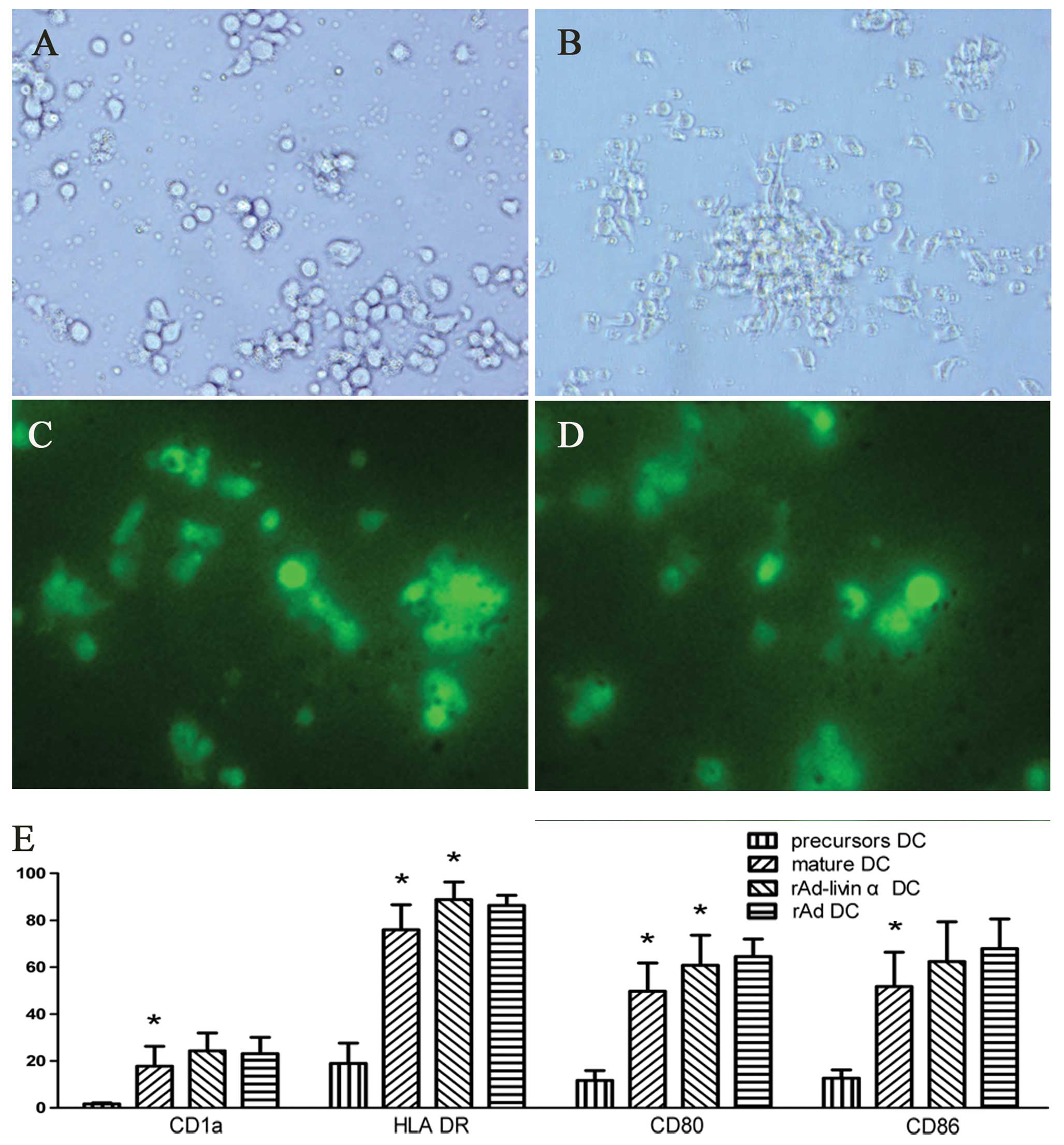
DC precursors were isolated using anti-CD34
microbeads. Freshly isolated precursor cells were small, round,
transparent and non-adherent (Fig.
2A). After 3 days of culture with cytokines, the majority of
the cells developed multiple surface protrusions of various
lengths. The cultured cells appeared to proliferate and to increase
in size and granularity and became either adherent or
semi-adherent. Over time, the cultured DC cells developed longer
protrusions and a more irregular morphology. After being cultured
with TNF-α for 3 days, the majority of DC cells became
non-adherent, scattered uniformly throughout the medium, and
acquired a morphology typical of mature DCs (Fig. 2B). DCs infected with either rAd-c or
rAd-livin α fluoresced green when viewed using a fluorescent
microscope (Fig. 2C and D).
The expression of CD1a, CD80, CD86 and HLA-DR are
important markers of immunocompetence and maturity in DCs. As shown
in Fig. 2E, DC precursors expressed
low levels of CD80, CD86 and HLA-DR. The expression levels of these
surface markers increased significantly when these cells were
cultured in the presence of cytokines and were further increased by
infection with adenovirus although this difference did not reach
significance for CD1a and CD86. The mature DCs exhibited the same
phenotype regardless of whether they were infected with rAd-livin α
or rAd-c. These results suggest that infection with rAd can promote
the maturation of UCB-derived DCs.
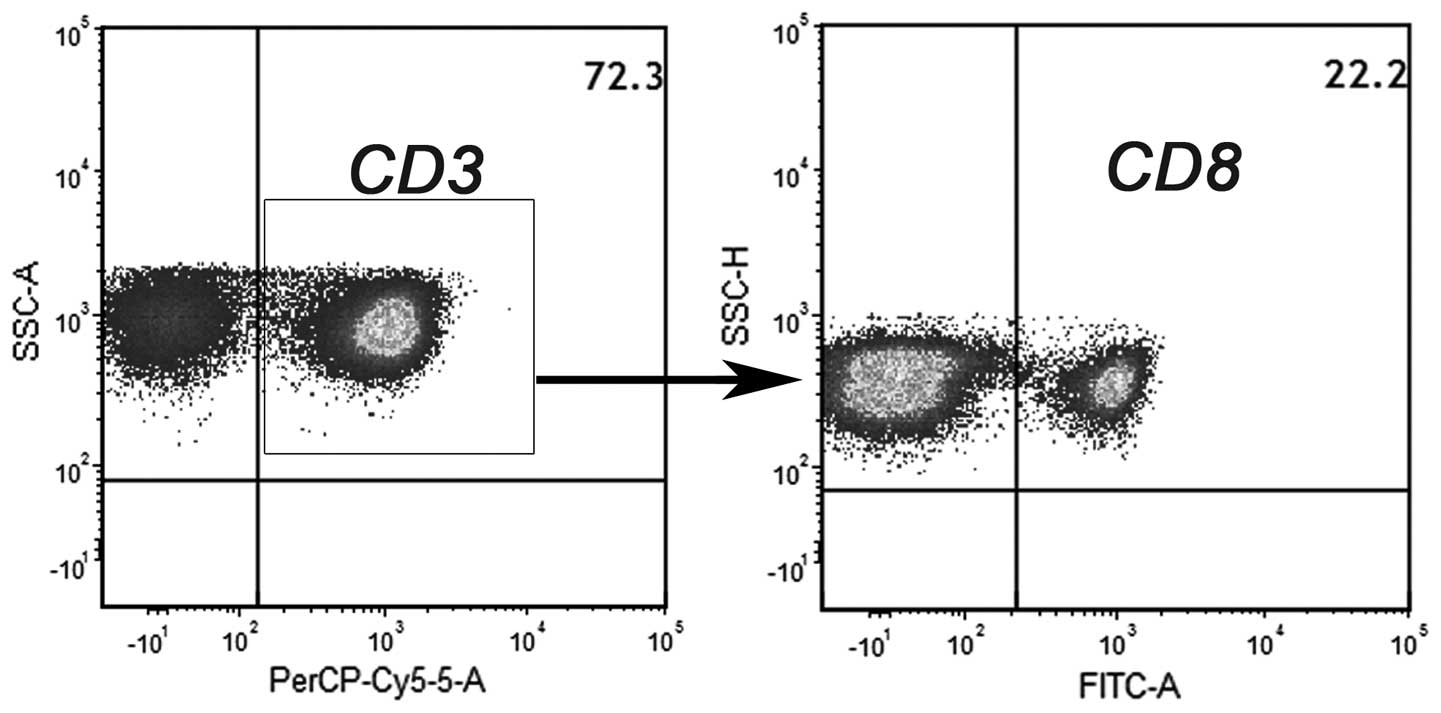
Percentage of CD4 and CD8 T-cell
populations in the effector cells
After culturing the cells in the presence of IL-2,
non-adherent NMCs were used as effector cells. Flow cytometric
analysis revealed that 72% of the effector cells were
CD3+ lymphocytes. Further characterization of the T-cell
subsets demonstrated that 78% of the CD3+ population was
CD3+CD8− (CD4 T cells) and 22% of the
CD3+ population was CD3+CD8+ (CD8
T cells) (Fig. 3).
Effector cells activated by rAd-livin
α-infected DCs respond specifically to livin polypeptides in an
HLA-restricted manner
We assessed the response of T lymphocytes to livin
polypeptides using ELISPOT assays to detect IFN-γ production.
Effector cells that had been stimulated by HLA-24+
rAd-livin α-infected DCs and were then exposed to HLA-24 presenting
livin polypeptide generated a statistically greater number of IFN-γ
spots than either cells stimulated by rAd-c-infected DCs or the
blank control (Fig. 4B). By
contrast, HLA-24− lymphocytes incubated with the peptide
did not produce a significant number of IFN-γ spots. Furthermore,
when the effector cells were exposed to irrelevant peptides, there
were very few IFN-γ spots regardless of the HLA type, as shown in
the bottom row of Fig. 4A.
rAd-livin α-infection of DCs enhances
tumor-specific CTL cytotoxicity and decreases autoimmunity
HLA-A2+ UCB-derived DCs were pulsed with
different forms of antigen from either A549 or H460 cells that had
undergone multiple freeze-thaw cycles or rAd-c or rAd-livin α to
study whether rAd-livin α-infected DCs could enhance tumor-specific
cytotoxicity against livin protein-expressing tumor cells (A549,
H460) without inducing autoimmune responses against livin protein
negative cells (HBE). Cytotoxicity against A549, H460 and HBE cells
was examined by flow cytometry by staining for Annexin V/PI. The
data are presented in Fig. 5.
Effector cells stimulated with rAd-livin α-infected DCs mediated
more cytotoxicity than effector cells stimulated with rAd-c DC or
the blank control at effector/target ratios of 5:1, 10:1 and 20:1.
Effector cells stimulated with rAd-livin α-infected DCs or with
proteins that had undergone multiple freeze-thaw cycles mediated
similar levels of cytotoxicity but effector cells stimulated with
rAd-livin α-infected DCs mediated less non-specific cytotoxicity
than those stimulated with proteins from A549 or H460 cells that
had undergone multiple freeze-thaw cycles.
Discussion
A variety of immune cells and immune molecules
participate in antitumor immune responses. CD8+ CTLs and
CD4+ Th1 cells play key roles in antitumor activity
(42). We propose using DCs loaded
with tumor antigen as a DC vaccine to induce or enhance antitumor
immune responses. CTLs require not only appropriately presented
antigen and co-stimulatory signals from APCs for their activation,
but also co-stimulatory signals from activated CD4+ T
cells. In addition, a few CD4+ T cells show MHC
II-restricted cytotoxicity towards cancer cells (43–46).
Due to these immune mechanisms and the in vivo immune
environment, we chose non-adherent UCB-derived MNCs cultured with
IL-2 as effector cells instead of CD8+ T lymphocytes
sorted from MNCs. The effector cell population was composed
predominantly of CD4+ and CD8+ T lymphocytes,
with a minor contribution from other immune cells, which mounted
weak specific cytotoxic responses against the targets.
When DC vaccines are administered either
simultaneously with or sequentially in combination with other
anticancer agents, they can induce synergistic antitumor activity
while minimizing the toxicity to normal hematopoietic or other
organs (47–49). There are several methods to load DCs
with tumor antigens. DC cells can either be pulsed with
tumor-associated antigens (TAAs) or tumor cell lysates or be
modified to express TAA genes (30,50–52).
Tumor proteins are known to be involved in the degradation and
recycling of MHC molecules and tumor-associated polypeptides are
only weakly immunogenic in the context of HLA restriction. Tumor
cell extracts also contain typical host antigens and may induce
autoimmune response when used as vaccines (53). In the present study we showed that
although DC cells pulsed with lysates from A549 or H460 cells can
activate lymphocytes and induce a strong cytotoxic response against
A549 or H460 cells, they also induce the significant non-specific
killing of HBE cells. This indicates that unmodified proteins,
polypeptides or tumor cell extracts may not be optimal for making
DC vaccines. A key goal in cancer immunotherapy is to present TAA
in an appropriate form that can be widely applied to induce
anti-tumor immunity.
It is not sufficient that the peptide epitope be
recognized by an allo-reactive CTL; it is important to target tumor
antigens that play a key role in the growth and metabolism of
malignant tumors. As the livin protein contributes directly to the
phenotype of malignant tumors, it is a rational target for novel
therapeutic strategies. As livin α contains virtually all the
antigenic epitopes of livin β, we infected DCs with rAd-livin α.
Our strategy allows the DCs to express the livin protein
endogenously, process it and present it in the context of MHC I to
activate CTLs. Furthermore, we observed that infection with rAd can
promote DC maturation and enhance their expression of
co-stimulatory molecules (28,46,54).
T lymphocytes specifically recognize antigen
peptides presented by APCs in the context of MHC I molecules and
become activated and secrete cytokines (55). The IFN-γ ELISPOT assay shows that
homologous effector cells sensitized by rAd-livin α-infected DCs
respond specifically to livin polypeptide presented in the context
of the appropriate HLA molecules.
HLA-A24−/HLA-A2+ T cells that had been
sensitized by rAd-livin α-infected DCs were able to recognize and
kill A549 and H460 cells. We hypothesize that rAd-livin α-infected
DCs expressing the complete livin gene present a wide variety of
molecular epitopes in the context of MHC I, so the rAd-livin
α-infected DCs may also present other HLA-restricted livin antigens
in either HLA-A2 or other HLA subtypes. Thus, rAd-livin α-infected
DCs could be widely applied to the treatment of cancer, regardless
of HLA subtype.
The cytotoxicity test demonstrated that all target
cells were susceptible to a low level of non-specific killing by
effector cells. In addition to activated CTLs, there are other
types of effector cells that can exert nontumor antigen-specific or
MHC-restricted cytolytic activity against the tumors, which include
macrophages, NK cells and LAK cells. The relatively small number of
apoptotic cells observed in the control wells indicates that the
majority of cytolytic activity is mediated by the CTLs and Th1
cells, rather than by the NK cells or the macrophages. As shown in
Fig. 4, homologous effector cells
sensitized by rAd-livin α-infected DCs show significant
cytotoxicity against A549 and H460 cells, but only moderate
cytotoxicity against HBE cells. Effector cells stimulated with
rAd-livin α-infected DC demonstrated the same level of cytotoxicity
against HBE cells as the control, indicating that effector cells
alone are mildly non-specifically cytotoxic to HBE cells.
The present study demonstrated for the first time
that UCB-derived DCs infected with rAd-livin α can sensitize
autologous T lymphocytes to effectively kill livin-expressing tumor
cells without inducing autoimmunity against normal tissue. This
antitumor strategy shows great potential, but needs to be studied
further in vivo. In particular, the antitumor effect of
UCB-derived rAd-livin α-infected DC vaccines in allogenic cancer
patients warrants further study.
Acknowledgements
This study was supported by the Hubei Key Laboratory
of Biological Targeted Therapy.
References
|
1
|
Cramer DW and Finn OJ: Epidemiologic
perspective on immune-surveillance in cancer. Curr Opin Immunol.
23:265–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kruyt FAE and Schuringa JJ: Apoptosis and
cancer stem cells: implications for apoptosis targeted therapy.
Biochem Pharmacol. 80:423–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaux DL and Silke J: IAPs, RINGs and
ubiquitylation. Nat Rev Mol Cell Biol. 6:287–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li XF, Gong RY, Wang M, et al: Sorafenib
down-regulates c-IAP expression post-transcriptionally in hepatic
carcinoma cells to suppress apoptosis. Biochem Biophys Res Commun.
418:531–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ndubaku C, Varfolomeev E, Wang L, et al:
Antagonism of c-IAP and XIAP proteins is required for efficient
induction of cell death by small-molecule IAP antagonists. ACS Chem
Biol. 4:557–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berger R, Jennewein C, Marschall V, et al:
NF-kappaB is required for Smac mimetic-mediated sensitization of
glioblastoma cells for gamma-irradiation-induced apoptosis. Mol
Cancer Ther. 10:1867–1875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashhab Y, Alian A, Polliack A, Panet A and
Ben Yehuda D: Two splicing variants of a new inhibitor of apoptosis
gene with different biological properties and tissue distribution
pattern. FEBS Lett. 495:56–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wagener N, Crnkovic-Mertens I, Vetter C,
et al: Expression of inhibitor of apoptosis protein Livin in renal
cell carcinoma and non-tumorous adult kidney. Br J Cancer.
97:1271–1276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Mesallamy HO, Hegab HM and Kamal AM:
Expression of inhibitor of apoptosis protein (IAP) livin/BIRC7 in
acute leukemia in adults: correlation with prognostic factors and
outcome. Leuk Res. 35:1616–1622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stewart DJ: Tumor and host factors that
may limit efficacy of chemotherapy in non-small cell and small cell
lung cancer. Crit Rev Oncol Hematol. 75:173–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hariu H, Hirohashi Y, Torigoe T, et al:
Aberrant expression and potency as a cancer immunotherapy target of
inhibitor of apoptosis protein family, Livin/ML-IAP in lung cancer.
Clin Cancer Res. 11:1000–1009. 2005.PubMed/NCBI
|
|
12
|
Chen N, Gong J, Chen X, et al: Caspases
and inhibitor of apoptosis proteins in cutaneous and mucosal
melanoma: expression profile and clinicopathologic significance.
Hum Pathol. 40:950–956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang TS, Ding QQ, Guo RH, et al:
Expression of livin in gastric cancer and induction of apoptosis in
SGC-7901 cells by shRNA-mediated silencing of livin gene. Biomed
Pharmacother. 64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crnkovic-Mertens I, Wagener N, Semzow J,
et al: Targeted inhibition of Livin resensitizes renal cancer cells
towards apoptosis. Cell Mol Life Sci. 64:1137–1144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crnkovic-Mertens I, Hoppe-Seyler F and
Butz K: Induction of apoptosis in tumor cells by siRNA-mediated
silencing of the livin/ML-IAP/KIAP gene. Oncogene. 22:8330–8336.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yagihashi A, Asanuma K, Tsuji N, et al:
Detection of anti-livin antibody in gastrointestinal cancer
patients. Clin Chem. 49:1206–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yagihashi A, Asanuma K, Kobayashi D, et
al: Detection of autoantibodies to livin and survivin in Sera from
lung cancer patients. Lung Cancer. 48:217–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engelhardt JJ, Boldajipour B, Beemiller P,
et al: Marginating dendritic cells of the tumor microenvironment
cross-present tumor antigens and stably engage tumor-specific T
cells. Cancer Cell. 21:402–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steinman RM and Banchereau J: Taking
dendritic cells into medicine. Nature. 449:419–426. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Atanackovic D, Luetkens T, Kloth B, et al:
Cancer-testis antigen expression and its epigenetic modulation in
acute myeloid leukemia. Am J Hematol. 86:918–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo MJ, Kim GR, Son YM, et al:
Interactions of dendritic cells with cancer cells and modulation of
surface molecules affect functional properties of CD8+ T
cells. Mol Immunol. 48:1744–1752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kakimi K, Nakajima J and Wada H: Active
specific immunotherapy and cell-transfer therapy for the treatment
of non-small cell lung cancer. Lung Cancer. 65:1–8. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brenner MK and Heslop HE: Adoptive T cell
therapy of cancer. Curr Opin Immunol. 22:251–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han P, Hodge G, Story C and Xu X:
Phenotypic analysis of functional T-lymphocyte subtypes and natural
killer cells in human cord blood: relevance to umbilical cord blood
transplantation. Br J Haematol. 89:733–740. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Germenis AE and Karanikas V: Cord blood as
a source of non-senescent lymphocytes for tumor immunotherapy. J
Reprod Immunol. 85:47–50. 2010.PubMed/NCBI
|
|
26
|
Brown JA and Boussiotis VA: Umbilical cord
blood transplantation: basic biology and clinical challenges to
immune reconstitution. Clin Immunol. 127:286–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brunstein CG, Gutman JA, Weisdorf DJ, et
al: Allogeneic hematopoietic cell transplantation for hematologic
malignancy: relative risks and benefits of double umbilical cord
blood. Blood. 116:4693–4699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie J, Xiong L, Tao X, et al: Antitumor
effects of murine bone marrow-derived dendritic cells infected with
xenogeneic livin alpha recombinant adenoviral vectors against Lewis
lung carcinoma. Lung Cancer. 68:338–345. 2010. View Article : Google Scholar
|
|
29
|
Antonia SJ, Mirza N, Fricke I, et al:
Combination of p53 cancer vaccine with chemotherapy in patients
with extensive stage small cell lung cancer. Clin Cancer Res.
12:878–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson RJ and Schneider J: Plasmid DNA
and viral vector-based vaccines for the treatment of cancer.
Vaccine. 25:B24–B34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ljunggren HG and Malmberg KJ: Prospects
for the use of NK cells in immunotherapy of human cancer. Nat Rev
Immunol. 7:329–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guiducci C, Vicari AP, Sangaletti S,
Trinchieri G and Colombo MP: Redirecting in vivo elicited tumor
infiltrating macrophages and dendritic cells towards tumor
rejection. Cancer Res. 65:3437–3446. 2005.PubMed/NCBI
|
|
33
|
Xu RL, Tang Y, Ogburn PL, et al:
Implication of delayed TNF-alpha exposure on dendritic cell
maturation and expansion from cryopreserved cord blood
CD34+ hematopoietic progenitors. J Immunol Methods.
293:169–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Safdar A, Decker WK, Li S, et al: De novo
T-lymphocyte responses against baculovirus-derived recombinant
influenzavirus hemagglutinin generated by a naive umbilical cord
blood model of dendritic cell vaccination. Vaccine. 27:1479–1484.
2009. View Article : Google Scholar
|
|
35
|
Mainali ES and Tew JG: Dexamethasone
selectively inhibits differentiation of cord blood stem cell
derived-dendritic cell (DC) precursors into immature DCs. Cell
Immunol. 232:127–136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gansuvd B, Hagihara M, Higuchi A, et al:
Umbilical cord blood dendritic cells are a rich source of soluble
HLA-DR: synergistic effect of exosomes and dendritic cells on
autologous or allogeneic T-Cell proliferation. Hum Immunol.
64:427–439. 2003.PubMed/NCBI
|
|
37
|
Tang XD, Wan Y, Chen L, et al:
H-2Kb-restricted CTL epitopes from mouse heparanase elicit an
antitumor immune response in vivo. Cancer Res. 68:1529–1537. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oku M, Okumi M, Sahara H, et al: Porcine
CFSE mixed lymphocyte reaction and PKH-26 cell-mediated lympholysis
assays. Transpl Immunol. 20:78–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo F, Song X, Zhang Y and Chu Y: Low-dose
curcumin leads to the inhibition of tumor growth via enhancing
CTL-mediated antitumor immunity. Int Immunopharmacol. 11:1234–1240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsieh CH, Huang YC, Tsai TH and Chen YJ:
Cantharidin modulates development of human monocyte-derived
dendritic cells. Toxicol In Vitro. 25:1740–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cholujova D, Jakubikova J, Kubes M, et al:
Comparative study of four fluorescent probes for evaluation of
natural killer cell cytotoxicity assays. Immunobiology.
213:629–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Disis ML, Bernhard H and Jaffee EM: Use of
tumour-responsive T cells as cancer treatment. Lancet. 373:673–683.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seki N, Brooks AD, Carter CR, et al:
Tumor-specific CTL kill murine renal cancer cells using both
perforin and Fas ligand-mediated lysis in vitro, but cause tumor
regression in vivo in the absence of perforin. J Immunol.
168:3484–3492. 2002. View Article : Google Scholar
|
|
44
|
Caldwell SA, Ryan MH, McDuffie E and
Abrams SI: The Fas/Fas ligand pathway is important for optimal
tumor regression in a mouse model of CTL adoptive immunotherapy of
experimental CMS4 lung metastases. J Immunol. 171:2402–2412. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Begley J and Ribas A: Targeted therapies
to improve tumor immunotherapy. Clin Cancer Res. 14:4385–4391.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Smith SG, Patel PM, Selby PJ and Jackson
AM: The response of human dendritic cells to recombinant
adenovirus, recombinant Mycobacterium bovis Bacillus Calmette
Guerin and biolistic methods of antigen delivery: different
induction of contact-dependant and soluble signals. Immunol Lett.
76:79–88. 2001. View Article : Google Scholar
|
|
47
|
Yee C: Adoptive T cell therapy: addressing
challenges in cancer immunotherapy. J Transl Med. 3:172005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xing W, Wu S, Yuan X, et al: The
anti-tumor effect of human monocyte-derived dendritic cells loaded
with HSV-TK/GCV induced dying cells. Cell Immunol. 254:135–141.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wojas-Krawczyk K, Krawczyk P, Buczkowski
J, et al: Immunotherapy of lung adenocarcinoma patient with
Peptide-pulsed dendritic cells: a case report. Arch Immunol Ther
Exp. 60:69–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Prasad S, Cody V, Saucier-Sawyer JK, et
al: Polymer nanoparticles containing tumor lysates as antigen
delivery vehicles for dendritic cell-based antitumor immunotherapy.
Nanomedicine. 7:1–10. 2011. View Article : Google Scholar
|
|
51
|
Lucas S and Coulie PG: About human tumor
antigens to be used in immunotherapy. Semin Immunol. 20:301–307.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Beebe M, Qin MS, Moi M, et al: Formulation
and characterization of a ten-peptide single-vial vaccine, EP-2101,
designed to induce cytotoxic T-lymphocyte responses for cancer
immunotherapy. Hum Vaccin. 4:210–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Spiotto MT, Fu YX and Schreiber H: Tumor
immunity meets autoimmunity: antigen levels and dendritic cell
maturation. Curr Opin Immunol. 15:725–730. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang JY, Cao DY, Liu WC, Zhang HM, Teng ZH
and Ren J: Dendritic cell generated from CD34+
hematopoietic progenitors can be transfected with adenovirus
containing gene of HBsAg and induce antigen-specific cytotoxic T
cell responses. Cell Immunol. 240:14–21. 2006.PubMed/NCBI
|
|
55
|
Dianzani U, Chiocchetti A and Ramenghi U:
Role of inherited defects decreasing Fas function in autoimmunity.
Life Sci. 72:2803–2824. 2003. View Article : Google Scholar : PubMed/NCBI
|