Introduction
Breast cancer is the second most common cancer among
Korean women. Numerous studies have been conducted on treatments
for patients with breast cancer, as most treatments with
radiotherapy and chemotherapy fail, and recurrence and metastasis
often occur. Therefore, it is crucial to discover new therapies
than can reduce breast cancer mortality. General conventional
therapy does not kill all tumor cells and a small side population
of cells, which are resistant to radiation and drugs, might be the
origin of recurrence and metastasis. These cells are referred to as
cancer stem cells (CSCs) (1), and
are tumor initiating cells that propagate tumors phenotypically
similar to the parental tumor.
Breast CSCs have been isolated by sorting
CD44+CD24−/low cells to initiate the process
of carcinogenesis in NOD/SCID mice (2). Tumor growth, invasion, and metastasis
depend on the properties of CSCs and their interactions with the
tumor microenvironment (3). The
importance of the tumor niche has been highly recognized. Among
numerous factors to explore tumor microenvironments known to
associate with aggressive CSCs (4),
hypoxia is believed to be crucial and promotes aggressive tumor
phenotypes (5). Oxygen tension is
an important signal to induce the low-oxygen CSC population
associated with maintaining undifferentiated cells (6). The expression levels of
hypoxia-related proteins, including hypoxia-inducible factor-1α
(HIF-1α), mediate the increase in the number of CSCs under hypoxic
conditions (7). Theoretically, CSCs
can be induced by anti-angiogenic therapies under hypoxia,
conferring radioresistance to the CSCs, although this has yet to be
demonstrated in vivo, and its clinical significance remains
unknown.
Therefore, hypoxia-induced stimulation of CSCs might
limit the effectiveness of radiotherapy and chemotherapy. These
studies suggest that radiotherapy and chemotherapy might have to be
combined with cancer stem cell-targeting strategies to improve
patient recovery (7).
Sorafenib is an oral multikinase inhibitor (8,9) that
blocks tumor cell proliferation and angiogenesis by inhibiting a
Raf serine/threonine kinase and vascular endothelial growth factor
(VEGF) receptors (10). Sorafenib
is currently being used in clinics to treat patients with advanced
renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC)
(11,12). Eliminating the
CD44+CD24−/low cell population from breast
cancer cells occurs by inhibiting RAF-β-catenin activation in
vitro(13). In addition,
sorafenib effectively reduces melanoma, breast, colon, and lung
cancer in vivo(10,14).
Sorafenib increases HIF-1α expression in melanoma
cells (15); however, the
anticancer mechanism of sorafenib combined with ionizing radiation
has yet to be investigated. In the present study, we examined the
efficacy of sorafenib and radiation against breast cancer and CSCs
in non-metastatic MCF-7 and metastatic MDA-MB-231 cells. We
demonstrated that a combination of sorafenib and radiation more
effectively eliminates CSCs, which might be reflected by the
inhibition of HIF-1α expression from metastatic MDA-MB-231 cells
rather than non-metastatic MCF-7 cells under hypoxic conditions.
Collectively, our results demonstrated that sorafenib and radiation
can be successfully combined to potentiate anti-CSC and
anti-angiogenesis activities.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-231 and
MCF-7 were obtained from the American Type Culture Collection
(Rockville, MD, USA) and maintained in DMEM (Welgene, Daegu, Korea)
supplemented with sodium pyruvate (1 mM), 10% fetal bovine serum
(FBS; HyClone, Logan, UT, USA), and 2% penicillin/streptomycin
(Gibco, Carlsbad, CA, USA). Cells were cultured at 37°C in a
humidified atmosphere of 5% CO2. Cells were maintained
under hypoxic conditions in a glove box-type anaerobic chamber
(Thermo Forma, Marietta, OH, USA). The hypoxic atmosphere was
<1% O2, 5% CO2, 10% H2, and 85%
N2 with continuous computerized monitoring, indicating a
partial O2 pressure of <15 mm Hg at 37°C.
O2-dependent experiments were performed in both hypoxic
and normoxic incubators. Cells were irradiated with 10 Gy using a
calibrated 137Cs γ-ray source (BioBeam 8000, STS,
Braunschweig, Germany). All media were changed every 3 days.
Antibodies and reagents
Antibody against MMP-2 was obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). HIF-1α antibody was
purchased from Abcam (London, UK). Anti-β-actin antibody was
purchased from Sigma-Aldrich (St. Louis, MO, USA) and was incubated
with specific horseradish peroxidase-conjugated secondary
antibodies (Invitrogen, Carlsbad, CA, USA). Sorafenib was purchased
from LC Laboratories (Woburn, MA, USA) and solubilized in DMSO.
DMSO was used in all experiments as a vehicle control. Mammospheres
were cultured in serum-free MammoCult medium (Stem Cell
Technologies, Vancouver, BC, Canada) containing 0.9%
methylcellulose to prevent cell aggregation. Cells were harvested
at various time points, fixed in 70% ethanol, and stained with 40
μg/ml propidium iodide (PI) in the presence of 50 μg/ml RNase A.
Cell viability was determined using Thiazolyl Blue tetrazolium
bromide (Sigma-Aldrich).
Cell viability assay
MTT assay was conducted using a Thiazolyl Blue
tetrazolium bromide and was based on the conversion of MTT to
MTT-formazan by mitochondria. MCF-7 and MDA-MB-231 cells were
resuspended and plated in 96-well plates at 1×104
cells/200 μl in culture media with 5% FBS. Cells were incubated
with or without drugs for 24–72 h then incubated with MTT (5 mg/ml
in phosphate-buffered saline; PBS) for 3 h. The plate was then
centrifuged at 2,000 rpm for 5 min at 4°C, and the MTT solution was
removed from the wells by aspiration. The formazan crystals were
dissolved in 2 ml of DMSO. The absorbance was recorded on a
Paradigm Detection spectrophotometer (Beckman Coulter, Inc.,
Fullerton, CA, USA) at a wavelength of 540 nm.
Cell proliferation and apoptosis
analysis
Live cell numbers were counted with the ADAM-MC
automated cell counter (Digital Bio, NanoEnTek Inc., Seoul, Korea).
After a 72-h treatment, the cell counter measured total cell
numbers, dead cell numbers, and cell viability using two sensitive
fluorescence dye staining solutions, AccuStain Solution T (PI/lysis
solution) and AccuStain Solution N (PI/PBS). AccuStain Solution T
permeabilizes the plasma membrane and stains the nucleus, which
allows total cell enumeration measurements, whereas AccuStain
Solution N exclusively stains non-viable cells. A 532-nm optic
laser was automatically focused on the cell suspension contained in
a disposable microchip, and the cell analysis was conducted with a
CDD camera. The Annexin V/PE Apoptosis Detection kit (BD
Biosciences, Bedford, MA, USA) was used to assess Annexin
V-positive cells. Briefly, fresh cell preparations were incubated
with 1X Annexin binding buffer and Annexin V/PE (2.5
μg/ml)-conjugated primary antibody and 7-AAD (5 μl) for 15 min on
ice. Following incubation, PI (10 μg/ml) was added to the
suspension, and the cells were analyzed by FACSAria (BD
Biosciences, San Jose, CA, USA).
Cell cycle analysis
Cells were treated with 5 μg/ml sorafenib or 10 Gy
radiation for 24 h and harvested. The cells were trypsinized and
resuspended in 3 ml PBS. Cells were centrifuged and washed in 3 ml
PBS. Following fixation in 70% ethanol for 16 h at −20°C, the cells
were stained with PI (40 μg/ml) and RNAse A (50 μg/ml) prior to
analysis. The stained cells were subjected to cell cycle analysis
using FACSAria.
FACS sorting and analysis
Cells (1×106 cells) were incubated with
anti-CD44-APC and anti-CD24-FITC antibodies (BD Biosciences) at 2
μg/ml (1:10) in the dark, on ice, for 15 min (MDA-MB-231) or 1 h
(MCF-7), washed twice with cold PBS, then resuspended in PBS (0.5
ml). CD44+CD24− and
CD44−CD24+ cells were identified and isolated
from MDA-MB-231 and MCF-7 cells using a FACSAria instrument.
Mammosphere formation
Cells were suspended at MCF-7 (5,000 cells/ml) and
MDA-MB-231 (600 cells/ml) in primary mammosphere formation and
MCF-7 (1,000 cells/ml) and MDA-MB-231 (300 cells/ml) in secondary
mammosphere formation in complete Mammocult media (Stem Cell
Technologies) and plated in triplicate wells on a 24-well ultra-low
attachment culture plate (Corning Inc., Corning, NY, USA). Cells
were incubated at 37°C for 7–10 days. The number of mammospheres
was imaged by inverted microscopy (Nikon Eclipse TS 100, Tokyo,
Japan) and mammosphere diameters were determined using Image-Pro
Plus 7.0 software. The primary mammosphere formation culture was
exposed to sorafenib (5 μg/ml) and radiation (10 Gy), whereas the
second passage was cultured in the absence of sorafenib and
radiation. Mammospheres were collected at the second passage by
gentle centrifugation (800 rpm) and dissociated enzymatically (10
min in 0.05% trypsin, 0.53 mM EDTA) and mechanically. The
dissociated cells were plated in a 24-well ultra-low attachment
plate and cultured for 7–10 days.
Immunocytochemistry
Collected cell fractions from FACS were counted and
cytospun onto glass slides at 1,000 cells per spot with a Cytospin
4 cytospinner (Thermo Scientific, Waltham, MA, USA). Cells were
fixed in 4% paraformaldehyde and stained overnight at 4°C with
primary antibodies directed against HIF-1α (1:500) followed by
secondary antibodies (1:1000 Texas Red conjugated anti-mouse and
anti-rabbit H+L IgG; Vector Laboratories, Burlingame, CA, USA) for
1 h at room temperature. Nuclei were counterstained with
4′,6-diamidino-2-phenylindole, and images were captured with a
fluorescent microscope (Nikon Eclipse 80i).
Western blotting
Cells were harvested with ice-cold PBS, and
re-suspended in lysis buffer containing (mM) 20 Tris-HCl (pH 7.5),
150 NaCl, 1 Na2EDTA, 1 EGTA, 1% Triton, 2,5 sodium
pyrophosphate, 1 β-glycerophosphate, 1
Na3VO4, 1 μg/ml leupeptin and 1 mM
phenylmethanesulfonyl fluoride. Extracts were diluted in a mix of
LDS sample buffer and heated at 95°C for 5 min. Samples were
electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels
(Invitrogen) and transferred onto nitrocellulose membranes (GE
Healthcare Life Sciences, Piscataway, NJ, USA). The blots were
saturated in TBS-T buffer (20 mM Tris, 137 mM NaCl, 0.05% Tween-20,
pH 7.6), containing 3% BSA for 1 h at room temperature, incubated
overnight at 4°C with primary antibodies: anti-VEGF (1:500; Santa
Cruz Biotechnology Inc.), anti-HIF-1α (1:500; Abcam), anti-β-actin
(1:5,000; Sigma-Aldrich) and subsequently incubated with specific
horseradish peroxidase-conjugated secondary antibodies. The
immunoreactive proteins were detected using enhanced
chemiluminescence (Thermo Scientific, Rockford, IL, USA).
Immunoblots were quantified using the ImageMaster densitometry
program.
Statistical analysis
The paired Student’s t-test and Microsoft Excel were
used for the MTT assays, cell proliferation, mammosphere formation,
and FACS data, which were conducted in triplicate and repeated
three times. Percent inhibition of the western blot data was
determined from the ratio of band density. p-value <0.05 was
considered to indicate a statistically significant difference.
Results
Sorafenib and radiation inhibit
proliferation and induce breast cancer cell apoptosis
To investigate the effect of sorafenib and radiation
on MDA-MB-231 and MCF-7 cells, we first examined the potential to
inhibit cell growth by sorafenib and radiation using MTT and
apoptosis assays. Sorafenib and radiation treatment resulted in a
dose-dependent inhibition of cell viability, with an
IC50 of 5 μg/ml for sorafenib and 10 Gy of radiation in
both cell lines (Fig. 1A).
Furthermore, the effect of combining sorafenib and radiation was
evaluated by Annexin V/PE apoptosis assay after 72 h of treatment.
Cells negative for 7-AAD and positive for Annexin V were regarded
as cells early in apoptosis (Annexin V+, 7-AAD−); 7-AAD-positive
cells, which bind Annexin V, were defined as late apoptotic cells
(Annexin V+, 7-AAD+); 7-AAD-positive cells that did not bind
Annexin V were considered necrotic cells (Annexin V−, 7-AAD+). The
combined treatment of sorafenib and radiation in MDA-MB-231 cells
significantly increased the antitumor effect of radiation alone or
sorafenib alone at 21% O2 (normoxia). Late apoptotic
cells were observed in the combined treatment group (30.1±3.5%)
compared with apoptotic cells in the sorafenib (2.9±0.5%) and
radiation (9.8±0.9%) treatment groups at 1% O2 (hypoxia)
(Fig. 1B). However, the combined
treatment increased subsequent necrosis (42.5±2.5%) and it
moderately increased late apoptosis (34.5±1.2%) in comparison with
sorafenib or radiation treatment alone at 1% O2
(Fig. 1B). Hypoxia may have induced
more necrosis through the effect of combined treatment in both cell
lines, unlike normoxia. Similarly, early apoptotic MCF-7 cells
increased under normoxia rather than under hypoxia but apoptotic
cells were not clearly observed in the combined treatment group as
compared with apoptotic cells in the sorafenib and radiation
treatment groups under normoxia or hypoxia (Fig. 1B). These data suggest a potential
synergistic effect of the combined treatment on MDA-MB-231 cells
but not on MCF-7 cells. Similar results were observed in athymic
nude mice bearing subcutaneously transplanted MDA-MB-231 cells
(data not shown).
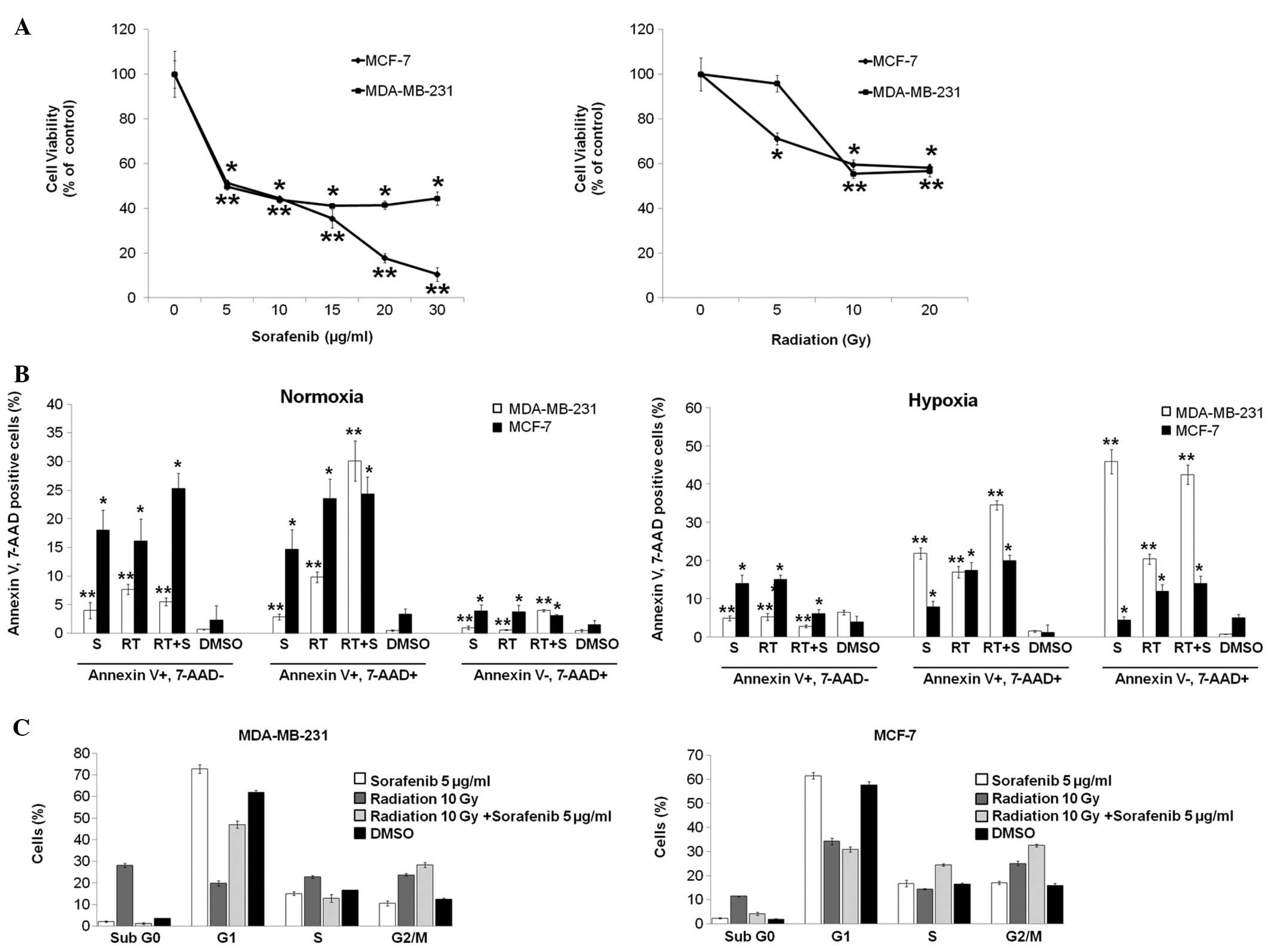 | Figure 1Combination of radiation and
sorafenib induces synergistic apoptosis and G2/M cell cycle arrest
in breast cancer cells. (A) MDA-MB-231 and MCF-7 cells
(1×104 cells/well) were seeded in 96-well microplates
and treated with increasing doses of radiation and concentrations
of sorafenib as indicated. After 72 h (radiation) and 48 h
(sorafenib) of incubation, respectively, cell viability was
assessed by the MTT assay, and the concentration that induced 50%
growth inhibition (IC50) was determined to be of ~10 Gy
radiation and 5 μg/ml sorafenib for both cell lines. (B) Cells were
treated with radiation (RT, 10 Gy), sorafenib (S, 5 μg/ml),
radiation + sorafenib (RT + S, 10 Gy + 5 μg/ml) or DMSO. After 72
h, the percentage of Annexin V/PE-positive cells was quantified
using flow assisted cell sorting. (C) Cells were treated with
radiation (RT, 10 Gy), sorafenib (S, 5 μg/ml), radiation +
sorafenib (RT + S, 10 Gy + 5 μg/ml) or DMSO for 24 h and analyzed
by flow cytometry for the cell cycle analysis. Mean percentages ±
standard deviation (SD) of cells in each cycle phase are shown.
Data are means of three separate experiments
*p<0.001, **p<0.01. |
To determine the effect of sorafenib and radiation
on cell cycle distribution, cells were synchronized by 24 h
stabilization prior to the different treatments. After 24 h of
treatment, G2/M arrest was observed in a flow cytometry cell cycle
analysis with the combined treatment resulting in 28±1.1%
(MDA-MB-231) and 32.5±0.6% (MCF-7) of the cell population compared
with the control group and the single treatments (Fig. 1C).
Combination of sorafenib and radiation
inhibits breast CSCs in vitro
To evaluate whether the combination of sorafenib and
radiation treatment could suppress breast CSCs in vitro
under normoxic and hypoxic conditions, both cell lines were treated
with 5 μg/ml of sorafenib alone, 10 Gy of radiation alone, or a
combined treatment for 72 h. We assessed expression of the
prospective CSC markers CD44-APC and CD24-FITC by flow cytometry.
The basal cell line, MDA-MB-231 cells, showed a significantly
decreased percentage of CD44+CD24−/low cells
during co-treatment with sorafenib and radiation (42±1.5%) compared
to that in the sorafenib alone (72±1.6%) and radiation alone
(59±4%) groups under hypoxic conditions, whereas no significant
difference was observed under normoxic conditions (Fig. 2A).
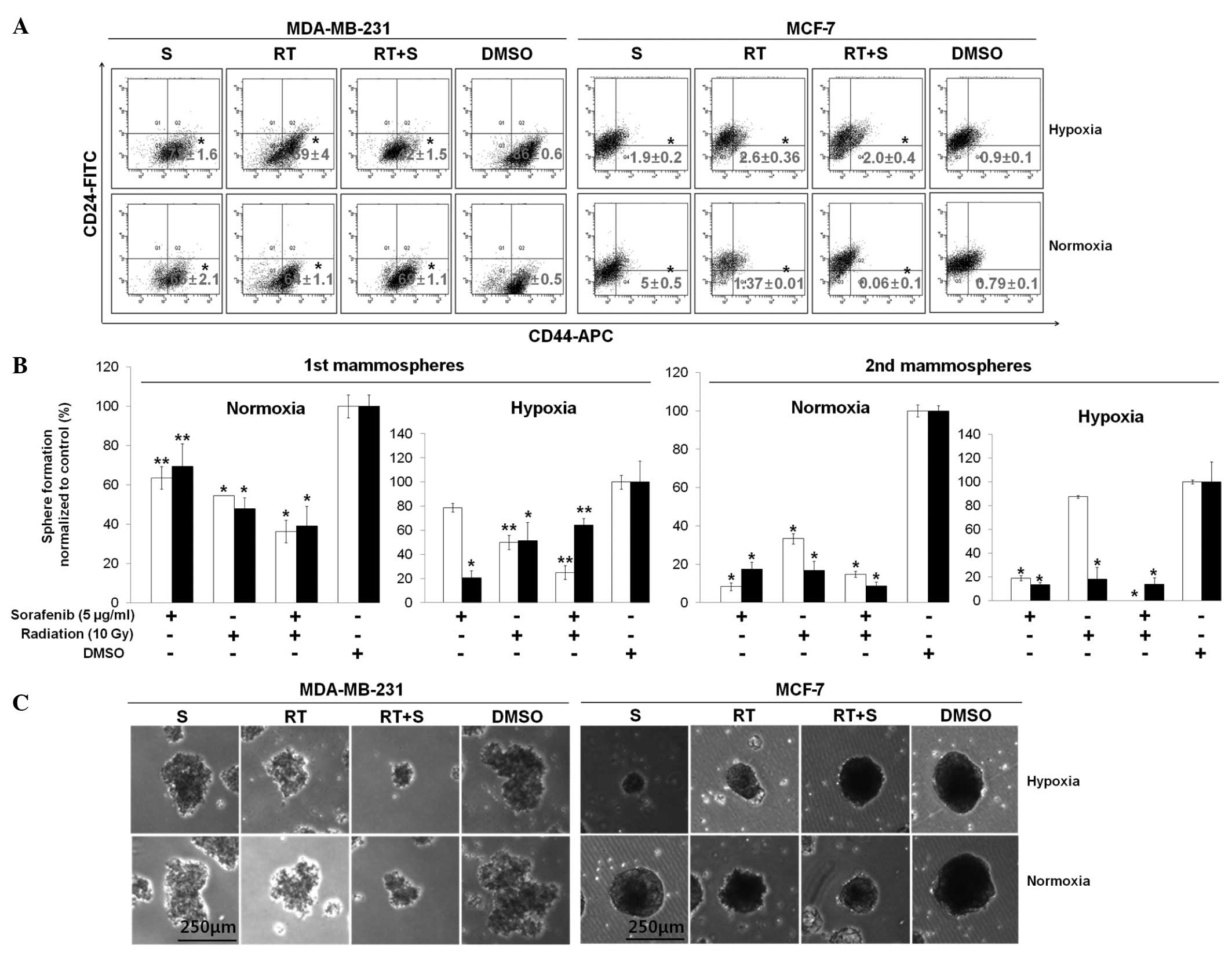 | Figure 2Combination of sorafenib and
radiation inhibits the CD44+CD24−/low cell
population and mammosphere formation. (A) MDA-MB-231 and MCF-7
cells were treated with or without sorafenib (S, 5 μg/ml) combined
with radiation (RT, 10 Gy) under 21% O2 (normoxia) or 1%
O2 (hypoxia). After 72 h, a flow assisted cell sorting
analysis was conducted using specific surface markers for basal
(CD44-APC) and luminal (CD24-FITC) epithelial cells and the
percentage of cells (CD44+CD24−/low) of each
cell line was evaluated in three independent experiments. (B) Cells
were seeded to form primary mammospheres at a density of MCF-7
(5,000 cells/ml) and MDA-MB-231 (600 cells/ml) and secondary
mammospheres at a density of MCF-7 (1,000 cells/ml) and MDA-MB-231
(300 cells/ml). Additionally, primary mammospheres were incubated
with radiation (RT, 10 Gy), sorafenib (S, 5 μg/ml), radiation +
sorafenib (RT + S, 10 Gy + 5 μg/ml) or DMSO for 7–10 days, whereas
secondary mammospheres were not treated with radiation or sorafenib
under hypoxia or normoxia for 7–10 days. White and black bars
indicate MDA-MB-231 and MCF-7 cells, respectively. Data are means
of three separate experiments; bar, standard deviation (SD).
*p<0.001, **p<0.05. (C) Representative
images from primary mammospheres. |
Conversely, the luminal cell line MCF-7 showed a
reduced percentage of CD44+CD24−/low cells
during co-treatment with sorafenib and radiation (0.06±0.1%) rather
than sorafenib alone (5±0.5%) and radiation alone (1.37±0.01%)
under normoxic conditions, whereas no significant difference was
observed under hypoxic conditions (Fig.
2A). Thus, our data suggest that the synergistic efficacy of
co-treatment with sorafenib and radiation resulted in a potential
combined treatment effect for specific targeting of
CD44+CD24−/low cells from breast cancer cells
in vitro.
A small population of breast cancer cells and
mammary stem cell-like/progenitor cells are enriched in floating
spherical clusters of cells (16).
These properties, based on self-renewal ability, exhibit serial
mammosphere formation and differentiation into multiple lineages
(16). To confirm whether the
combination of sorafenib and radiation could more effectively
suppress mammosphere formation than single treatments such as
reduction of CD44+CD24−/low cells from
co-treated breast cancer cells, cells were cultured in radiation
(10 Gy), sorafenib (5 μg/ml), radiation + sorafenib (10 Gy + 5
μg/ml) or DMSO in ultra-low attachment plates and then cultured to
the secondary passage in the absence of sorafenib and radiation
under normoxic and hypoxic conditions. The combination of sorafenib
and radiation inhibited the formation of primary mammospheres under
both normoxic and hypoxic conditions (Fig. 2B), whereas these treatments
increased the formation of primary mammospheres in MCF-7 cells
under hypoxic conditions. The percentage of secondary mammospheres
formed decreased significantly in MDA-MB-231 cells under the
hypoxic conditions but not under normoxia (p<0.001) (Fig. 2B). The number of mammospheres was
reduced by 1.5–87.5-fold (MDA-MB-231 cells) and 1-2.1-fold (MCF-7
cells) (p<0.05) but also the size of both primary (Fig. 2B and C) and secondary mammospheres
(data not shown) decreased compared to those in single treatments.
These results demonstrate that the combined treatment of sorafenib
and radiation was able to preferentially target breast CSCs.
Combination of sorafenib and radiation
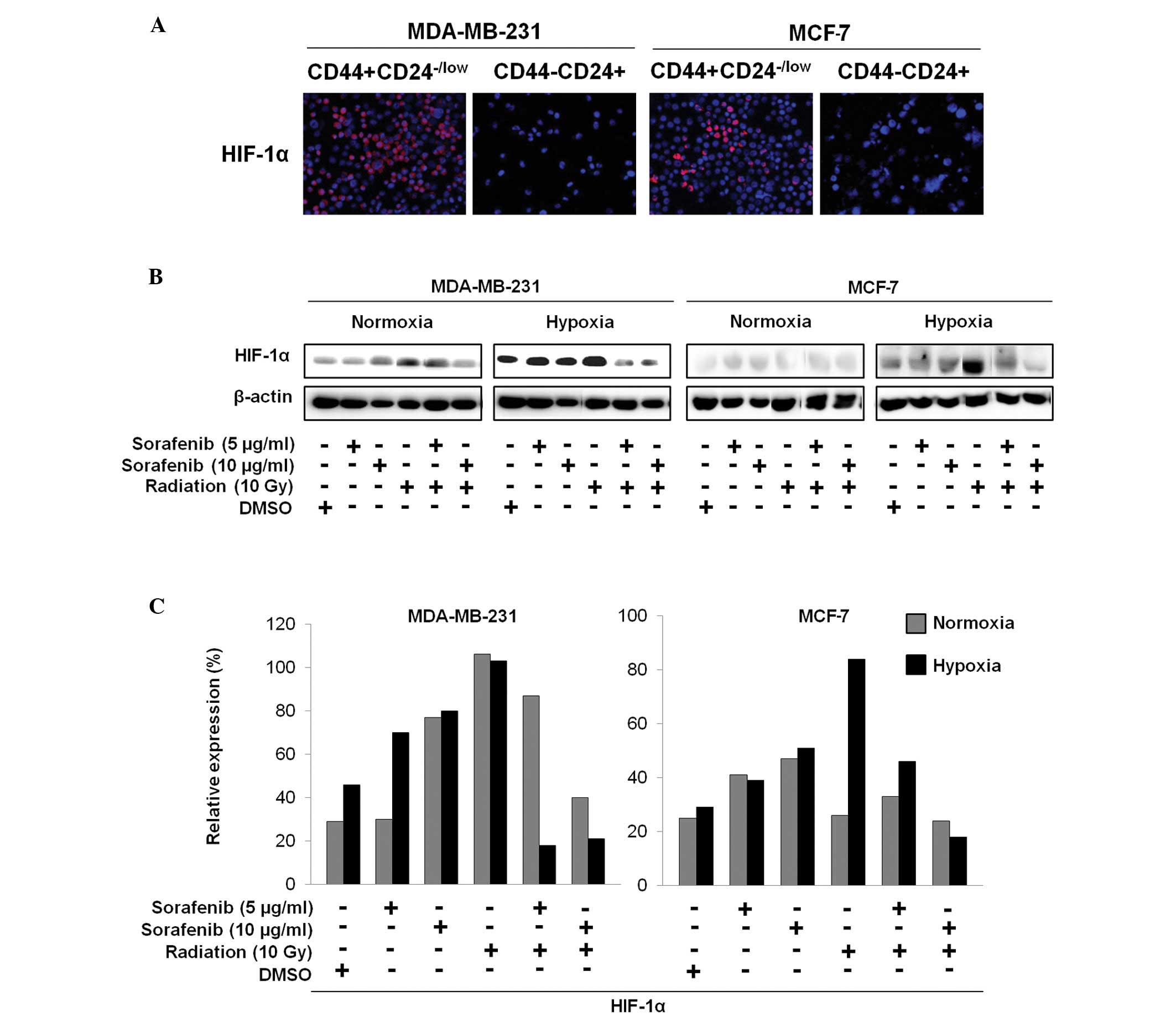
inhibits HIF-1α expression associated with highly activated
CD44+CD24−/low cells
The sorted CD44+CD24−/low
cells and CD44−CD24+ cells were further
examined by immunocytochemistry to explore whether increased HIF-1α
expression in CSCs is associated with targeting of CSCs by the
combination of sorafenib and radiation (Fig. 3A). Additionally, after treatment
with radiation (10 Gy), sorafenib (5 and 10 μg/ml), radiation +
sorafenib (10 Gy + 5 μg/ml and 10 Gy + 10 μg/ml) or DMSO for 72 h,
immunoblotting of breast cancer cells under hypoxic and normoxic
conditions showed different HIF-1α expression patterns (Fig. 3B and C). HIF-1α was aberrantly
expressed in the sorted CD44+CD24−/low cells
but not in the sorted CD44−CD24+ cells
(Fig. 3A). As shown in Fig. 3B and C, the combination of sorafenib
and radiation appeared to significantly reduce HIF-1α expression in
MDA-MB-231 cells under hypoxia but was slightly decreased in MCF-7
cells under hypoxia compared to that from sorafenib or radiation
alone, whereas these effects resulted in no difference under
normoxia (Fig. 3B and C).
Therefore, we suggest that inhibition of HIF-1α by sorafenib and
radiation may eliminate breast CSCs under hypoxic conditions.
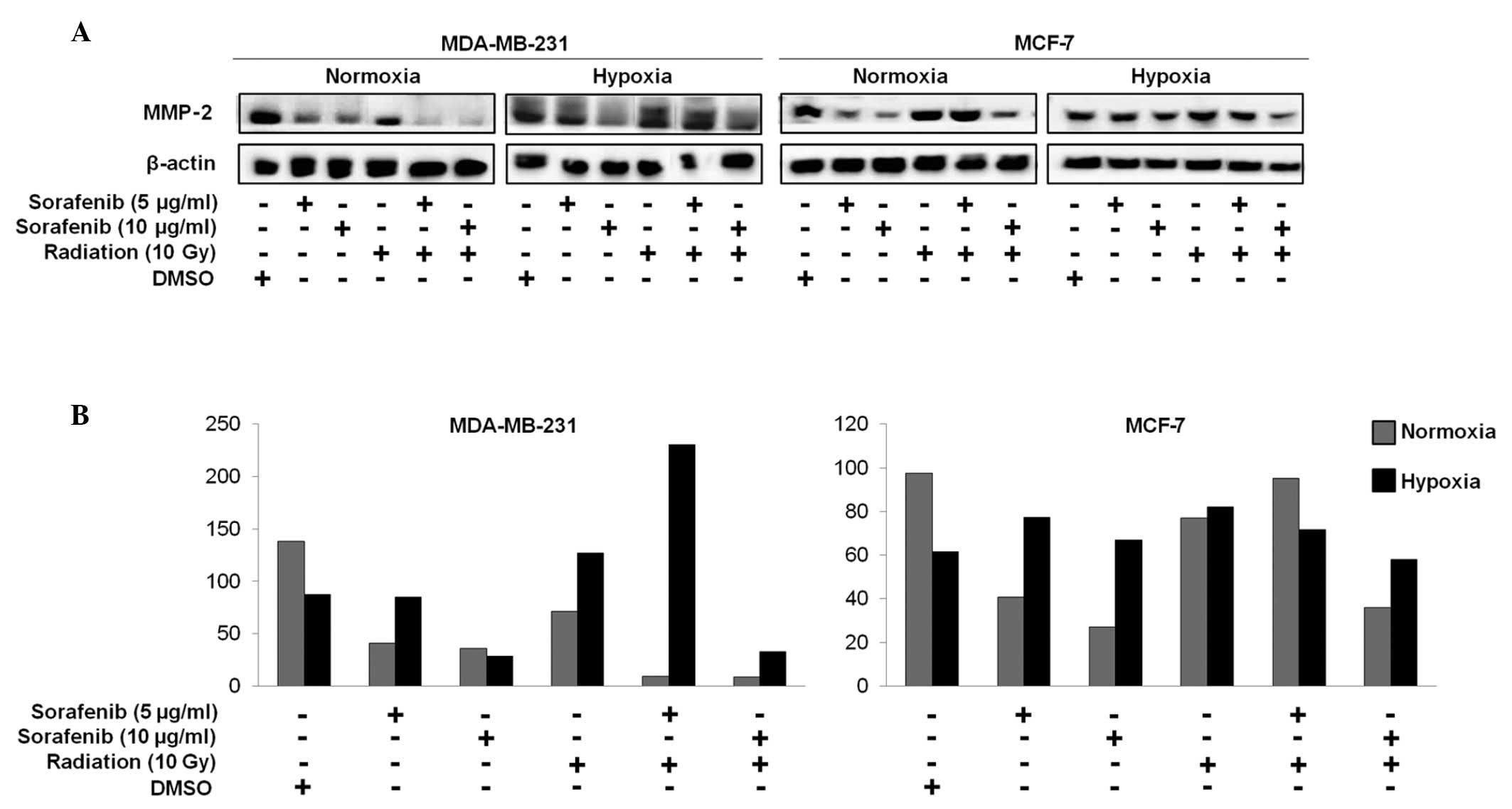
Combination of sorafenib and radiation
effectively inhibits matrix metalloproteinase-2 (MMP-2) activity in
metastatic MDA-MB-231 cells
MMP-2 is well recognized for its role in
tumorigenesis. Studies have shown that inhibiting MMP-2 activity
reduces the metastatic potential of malignant cells and MMP-2
downregulation leads to decreased tumor cell invasion (17–21).
We performed immunoblotting studies to investigate
whether a decrease in MMP-2 could be correlated with the efficacy
of combined effect of sorafenib and radiation in breast cancer.
MMP-2 expression in MDA-MB-231 cells decreased in response to the
combined treatment under normoxia but not under hypoxia, whereas no
differences were observed in MCF-7 cells (Fig. 4A and B).
Discussion
The anticancer efficacy of chemotherapy and
radiotherapy has been evaluated in various types of cancer.
However, although patients with breast cancer are treated by
chemotherapy or radiotherapy, CSCs, a side population of the bulk
tumor responsible for initiating and self-renewing tumor, cause
relapse and metastasis and eventually give rise to new tumors
(22). Therefore, we determined if
the combination of sorafenib and radiation induced anti-CSC
activity under hypoxia in breast cancer cells. The anticancer
efficacy of sorafenib combined with radiation can be partially
determined by cell cycle arrest. Sorafenib, which leads to a G1
block by inhibiting the mitogen activated protein kinase pathway,
causes no obvious arrest in the majority of asynchronous cell
lines, but it causes cell cycle arrest in some cell lines (23). In MDA-MB-231 and MCF-7 cells, we
showed that sorafenib alone might not affect cell cycle arrest but
that a combination with radiation induces G2/M arrest.
Additionally, a high concentration of sorafenib is associated with
inducing apoptosis via the mitochondrial intrinsic pathway and
inhibits cell proliferation (24).
We showed that sorafenib in combination with radiation
synergistically induced apoptosis (early and late apoptosis) in
MDA-MB-231 cells (5.5 and 30.1%) and MCF-7 cells (25.3 and 24.3%)
compared to sorafenib alone (4 and 2.9%) and radiation alone (7.7
and 9.8%) in MDA-MB-231 cells, and sorafenib alone (18 and 14.7%)
and radiation alone (16.1 and 23.5%) in MCF-7 cells under normoxia.
Sorafenib may be associated with the induction of necrosis rather
than apoptotic cell death, and sorafenib in combination with
radiation increased late apoptosis in MDA-MB-231 cells under
hypoxic conditions. Cancer cells more resistant to hypoxia show
that low O2 tension induces apoptosis as well as
necrosis and completely prevents apoptosis by Bcl-2 and Bcl-X
(25). However, apoptosis and
necrosis were not observed in MCF-7 cells in response to the
combined sorafenib and radiation treatment under hypoxia in
vitro.
Sorafenib has been clinically used in treatment for
RCC, HCC, and thyroid cancer (12,26,27).
Sorafenib alone is insufficient for inhibiting tumors in a
colorectal carcinoma (HT29/tk-luc)-bearing animal model
compared with radiation alone (28). Instead of treatment of sorafenib
alone or radiation alone, a combination with sorafenib and
radiation may be successfully used for treating advanced RCC
(29). Similar results were also
observed in metastatic MDA-MB-231 cell-bearing athymic nude mice
(data not shown). Human breast cancer cells develop an increased
CSC population under hypoxic conditions (7). Hypoxia-induced HIF-1α reduces
migration potential and sphere formation in glioma cells and
expansion of CD133+ CSCs in glioblastoma (30,31).
Sorafenib eliminates EZH-induced BTIC expansion,
decreases the number of CD44+CD24−/low cells,
and blocks the formation of precancerous mammospheres in human
breast cancer cells (13). These
observations are consistent with our result that a combination of
sorafenib and radiation produced synergistic inhibition of
CD44+CD24−/low cells in basal breast cancer
cells (MDA-MB-231 cells) under hypoxic conditions. Furthermore, we
demonstrated that the combination of sorafenib and radiation
significantly suppressed mammosphere formation in MDA-MB-231 cells
but not in MCF-7 cells under both hypoxic and normoxic conditions.
The mechanism of suppressing breast CSCs by the combination of
sorafenib and radiation is unknown, however, our results suggest
that the combination of sorafenib and radiation is not cytotoxic to
non-CSCs, and preferentially cytotoxic to CSCs. Accumulating
evidence indicates that HIF-1α is significantly correlated with the
rate of CSCs that express CD44+CD24−/low in
the early stage of breast cancer (32). Thus, HIF-1α may be a strong
candidate for breast CSC-targeting with the combination of
sorafenib and radiation.
This is the first in vitro study to
demonstrate the efficacy of a combination of sorafenib and
radiation for treating breast cancer cells and CSCs. The
combination of sorafenib and radiation is a potentially novel
strategy to inhibit breast CSCs by reducing HIF-1α and MMP-2
expression. However, these findings remain to be demonstrated in
preclinical and clinical evaluations for breast cancer therapy.
Nevertheless, our results clearly show for the first time that the
combination of sorafenib and radiation is potentially efficacious
for inhibiting breast cancer stem cells in vitro.
Acknowledgements
This study was supported by the National R&D
Program through the Dongnam Institute of Radiological and Medical
Sciences (DIRAMS) funded by the Ministry of Education, Science, and
Technology (50593-2012, 50595-2012).
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cell, cancer and cancer stem cell. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Generali D, Buffa FM, Berruti A, et al:
Phosphorylated ERalpha, HIF-1alpha, and MAPK signaling as
predictors of primary endocrine treatment response and resistance
in patients with breast cancer. J Clin Oncol. 27:227–234. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu M and Polyak K: Molecular
characterization of the tumour microenvironment in breast cancer.
Eur J Cancer. 44:2760–2765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heddleston JM, Zhizhong L, McLendon RE,
Hjelmeland AB and Rich JN: The hypoxic microenvironment maintains
glioblastoma stem cells and promotes reprogramming towards a cancer
stem cell phenotype. Cell Cycle. 8:3274–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xing F, Okuda H, Watabe M, et al:
Hypoxia-induced Jagged2 promotes breast cancer metastasis and
self-renewal of cancer stem-like cells. Oncogene. 30:4075–4086.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conley SJ, Gheordunescu E, Kakarala P, et
al: Antiangiogenic agents increase breast cancer stem cells via the
generation of tumor hypoxia. Proc Natl Acad Sci USA. 109:2784–2789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iyer R, Fetterly G, Lugade A and Thanavala
Y: Sorafenib: a clinical and pharmacologic review. Expert Opin
Pharmacother. 11:1943–1955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar
|
|
11
|
Siegel AB, Olsen SK, Magun A and Brown RS:
Sorafenib: Where do we go from here? Hepatology. 52:360–369. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J
Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang CJ, Yang JY, Xia W, et al: EZH2
promotes expansion of breast tumor initiating cells through
activation of RAF1-β-catenin signaling. Cancer Cell. 19:86–100.
2011.PubMed/NCBI
|
|
14
|
Sharma A, Trivedi NR, Zimmerman MA,
Tuveson DA, Smith CD and Robertson GP: Mutant V599 EB-Raf regulates
growth and vascular development of malignant melanoma tumors.
Cancer Res. 65:2412–2421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar SM, Yu H, Edwards R, et al: Mutant
V600E BRAF increases hypoxia inducible factor-1alpha expression in
melanoma. Cancer Res. 67:3177–3184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dontu G, Abdallah WM, Foley JM, et al: In
vitro propagation and transcriptional profiling of human mammary
stem/progenitor cells. Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ata N, Oku T, Hattori M, Fujii H, Nakajima
M and Saiki I: Inhibition by galloylglucose (GG6-10) of tumor
invasion through extracellular matrix and gelatinase-mediated
degradation of type IV collagens by metastatic tumor cells. Oncol
Res. 8:503–511. 1996.
|
|
18
|
Elkin M, Reich R, Nagler A, et al:
Inhibition of matrix metalloproteinase-2 expression and bladder
carcinoma metastasis by halofuginone. Clin Cancer Res. 5:1982–1988.
1999.PubMed/NCBI
|
|
19
|
Zervos EE, Shafii AE, Haq M and Rosemurgy
AS: Matrix metalloproteinase inhibition suppresses MMP-2 activity
and activation of PANC-1 cells in vitro. J Surg Res. 84:162–167.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Denkert C, Siegert A, Leclere A, Turzynski
A and Hauptmann S: An inhibitor of stress-activated MAP-kinase
reduces invasion and MMP-2 expression of malignant melanoma cells.
Clin Exp Metastasis. 19:79–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kargozaran H, Yuan SY, Breslin JW, et al:
A role for endothelial-derived matrix metalloproteinase-2 in breast
cancer cell transmigration across the endothelial-basement membrane
barrier. Clin Exp Metastasis. 24:495–502. 2007. View Article : Google Scholar
|
|
22
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 10:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Plastaras JP, Kim SH, Liu YY, et al: Cell
cycle dependent and schedule-dependent antitumor effects of
sorafenib combined with radiation. Cancer Res. 67:9443–9454. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonelli MA, Fumarola C, Alfieri RR, et al:
Synergistic activity of letrozole and sorafenib on breast cancer
cells. Breast Cancer Res Treat. 124:79–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu S, Eguchi Y, Kamiike W, et al:
Induction of apoptosis as well as necrosis by hypoxia and
predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer
Res. 56:2161–2166. 1996.PubMed/NCBI
|
|
26
|
Gupta-Abramson V, Troxel AB, Nellore A, et
al: Phase II trial of sorafenib in advanced thyroid cancer. J Clin
Oncol. 26:4714–4719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuo YC, Lin WC, Chiang IT, et al:
Sorafenib sensitizes human colorectal carcinoma to radiation via
suppression of NF-κB expression in vitro and in vivo. Biomed
Pharmacother. 66:12–20. 2012.PubMed/NCBI
|
|
29
|
Kasibhatla M, Steinberg P, Meyer J,
Ernstoff MS and George DJ: Radiation therapy and sorafenib:
Radiation therapy and sorafenib: clinical data and rationale for
the combination in metastatic renal cell carcinoma. Clin Genitourin
Cancer. 5:291–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soeda A, Park M, Lee D, et al: Hypoxia
promotes expansion of the CD133-positive glioma stem cells through
activation of HIF-1α. Oncogene. 28:3949–3959. 2009.PubMed/NCBI
|
|
31
|
Méndez O, Zavadil J, Esencay M, et al:
Knock down of HIF-1α in glioma cells reduces migration in vitro and
invasion in vivo and impairs their ability to form tumor spheres.
Mol Cancer. 9:1332010.
|
|
32
|
Wang Z, Shi Q, Wang Z, et al:
Clinicopathologic correlation of cancer stem cell markers CD44,
CD24, VEGF and HIF-1α in ductal carcinoma in situ and invasive
ductal carcinoma of breast: an immunohistochemistry-based pilot
study. Pathol Res Pract. 207:505–513. 2011.PubMed/NCBI
|